Abstract
Cell division cycle 42 (CDC42) regulates the progression of leukemia via mediating proliferation and immune evasion of malignant cells. The study aimed to investigate the correlation of CDC42 with clinical features, treatment response, event-free survival (EFS) and overall survival (OS) in adult Philadelphia chromosome negative acute lymphoblastic leukemia (Ph− ALL) patients. CDC42 expression in bone marrow mononuclear cells was detected in 78 adult Ph− ALL patients and 10 donors using real-time reverse transcriptase-polymerase chain reaction. CDC42 was increased in adult Ph− ALL patients compared with donors (p < .001). Besides, elevated CDC42 was linked with pro-B ALL or early-T ALL (p = .038) and white blood cell (WBC) elevation at diagnosis (p = .025). Fifty (64.1%) and 23 (29.5%) patients had complete remission (CR) at 1 month and minimal residual disease (MRD) after CR, respectively. CDC42 was inversely associated with CR at 1 month (p = .034), but not MRD after CR (p = .066). Concerning survival, patients with CDC42 ≥ 3.310 (cut by median value in patients) showed a shortened EFS (p = .006) and OS (p = .036) compared to those with CDC42 < 3.310. In detail, patients with CDC42 ≥ 3.310 and CDC42 < 3.310 had 5-year EFS rate of 29.9% and 45.4%, and 5-year OS rate of 39.4% and 63.6%, correspondingly. Further multivariate Cox’s regression analyses revealed that CDC42 ≥ 3.310 was independently related to shorter EFS (hazard ratio = 2.933, p = .005). Elevated CDC42 is related with pro-B ALL or early-T ALL, WBC elevation at diagnosis, unfavorable treatment response and worse survival in adult Ph− ALL patients.
Introduction
Acute lymphoblastic leukemia (ALL) is one of the most common acute hematologic malignancies, whose annual incidence increased from 49,070 in 1990 to 64,190 in 2017 around the world [Citation1]. ALL is caused by the dysregulated growth of clonal lymphoid cells, among which pre-B cells are the most commonly affected, followed by T cells and mature B cells [Citation2,Citation3]. Philadelphia chromosome refers to the reciprocal translocation between chromosome 9 and 22, and approximately 75% of adult ALL patients are Philadelphia chromosome negative (Ph−) [Citation4,Citation5]. Currently, chemotherapy, targeted therapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT) and chimeric antigen receptor-T therapy have been applied in clinical management of adult Ph− ALL [Citation6,Citation7]. Unfortunately, different from pediatric ALL patients with a relatively good treatment outcomes, adult ALL patients benefit less and suffer from a dismal survival [Citation8–10]. Therefore, exploring markers with the ability for predicting treatment response and survival of adult Ph− ALL patients is helpful for the promotion of individualized therapy in these patients.
Cell division cycle 42 (CDC42), a Ras homologous guanosine-triphosphate hydrolase (Rho GTPase), mediates cancer progression by regulating stemness, proliferation, migration, and apoptosis of tumor cells [Citation11,Citation12]. Regarding its role in hematological malignancy, the available evidence suggests that CDC42 facilitates proliferation of leukemia cells and mediates their evasion from immune surveillance, leading to a more aggressive disease status [Citation13,Citation14]. For example, a study discloses that upregulated CDC42 expression promotes uncontrolled growth and accumulation of leukemia cells via regulating cell cycle transition and cell division [Citation13]. Another study illustrates that CDC42 regulates the polarization of actin cytoskeleton in leukemia cells, thereby preventing their interaction with natural killer (NK) cells and reducing the cytotoxicity of NK cells [Citation14]. Unlike the confirmed prognostic value of CDC42 in solid tumors [Citation15–17], only one study has identified CDC42 as a potential biomarker for overall survival (OS) in acute myeloid leukemia patients [Citation18], and reports on the correlation of CDC42 with survival in adult ALL patients are limited. Therefore, this study aimed to investigate the association of CDC42 with disease features, as well as its prognostic value in adult Ph− ALL patients.
Methods
Patients
This retrospective study included 78 adult ALL patients treated at our hospital between April 2017 and December 2020. The inclusion criteria were: (a) diagnosed as primary Ph− ALL; (b) aged 18–60 years; (c) had accessible pre-treatment bone marrow tissue specimens; (d) had at least one efficiency evaluation or follow-up data. And Ph− ALL patients who had solid tumors at the time of diagnosis were excluded. A total of 10 healthy bone marrow donors were selected as controls. The inclusion criteria were: (a) physical examination within one month before entering the study was completely normal; (b) aged 18 years or older; (c) had accessible bone marrow tissue specimens. The exclusion criteria were the same as the adult Ph− ALL patients. The study was conducted with the consent of the Ethics Committee. Written informed consent was signed by the patient or his/her family.
Data and specimen collection
Clinical characteristics were obtained from the hospital’s electronic system. Bone marrow mononuclear cells (BMMC) were extracted from the bone marrow tissue specimens of patients and donors which were obtained from the hospital. CDC42 expression in BMMC was detected by real-time reverse transcriptase-polymerase chain reaction (RT-qPCR).
CDC42 detection
First, total RNA was extracted from bone marrow tissue specimens by using a Trizol reagent (Thermo Fisher, Waltham, MA). Second, cDNA was obtained by reverse transcription using the PrimeScript™ RT Master Mix kit (Takara, Kusatsu, Japan). Finally, cDNA was used for RT-qPCR to detect the expression of CDC42. GAPDH was selected as the internal reference. The expression of CDC42 was calculated by the 2−ΔΔCt method. The experimental procedure was carried out by a completely uninformed researcher and was strictly carried out according to the instructions. CDC42 primer sequence: 5′-GTTGTTGTGGGTGATGGTGC-3′ (forward), and 5′-TCCCCACCAATCATAACTGT-3′ (reverse). GAPDH primer sequences: 5′-GGGTCATCATCTCTGCACCT-3′ (forward), and 5′-GGTCATAAGTCCCTCCACGA-3′ (reverse) [Citation19].
To better analyze the relationship between CDC42 expression and the prognosis of the adult Ph− ALL patients, the median value (3.310) of the CDC42 expression in the adult Ph− ALL patients was used as a cut-off value for subsequent analysis.
Treatment
Based on the patient’s condition and their willingness, as well as the doctor’s recommendations, different treatment protocols were chosen. According to the Chinese Guidelines for Diagnosis and Treatment of Acute Lymphoblastic Leukemia (2016) [Citation20] and the Guidelines of Chinese Society of Clinical Oncology (CSCO) hematological malignancies, treatment protocols were mainly involved as follows: the CALLG-2008 protocol (Chinese Acute Lymphoblastic Leukemia Group), the VDP protocol (vincristine, daunorubicin and prednisone), the VDLP protocol (vincristine, daunorubicin, l-asparaginase and prednisone), the VDCLP protocol (vincristine, daunorubicin, cyclophosphamide, l-asparaginase and prednisone) and the Hyper-CVAD protocol (hyper-fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone).
Evaluations
The efficiency evaluation and the follow-up data were obtained from the hospital’s electronic information base. The complete remission (CR) was assessed one month after the treatment; specifically, the adult Ph− ALL patients who were treated with VDP protocol, VDLP protocol, VDCLP protocol and Hyper-CVAD protocol were assessed on day 28, the adult Ph− ALL patients who were treated with CALLG-2008 protocol were assessed on day 33. Patients who reached the CR, the minimal residual disease (MRD) of them would be evaluated next. Based on the follow-up data, the final follow-up date was February 2023. Then, event-free survival (EFS) and OS were calculated.
Statistical analysis
SPSS 21.0 (IBM Corp., Armonk, NY) software was used for data analysis in this study. Continuous variables were shown as median and interquartile range (IQR) or median and range, and categorical variables were expressed as numbers (%). Mann–Whitney’s U-test was used for comparative analysis. The Kaplan–Meier curves showed EFS and OS for patients with different CDC42 expressions (with the median value as the cut-off value), and the analysis method was the log-rank test. Univariate and stepwise forward multivariate Cox’s regression analyses were used to assess factors associated with EFS and OS. Significant differences were shown when the p value was <.05.
Results
Clinical features of adult Ph− ALL patients
This study enrolled 78 adult Ph− ALL patients, with a median (range) age of 34.5 (19.0–59.0) years. Among them, there were 46 (59.0%) male patients. A respective of 16 (20.5%) and 62 (79.5%) patients were assessed as T-cell ALL and B-cell ALL, respectively. The number of patients with pro-B ALL or early-T ALL was 9 (11.5%). Besides, there were 23 (29.5%), 59 (75.6%) and 68 (87.2%) patients with white blood cell (WBC) elevation at diagnosis, hemoglobin (HGB) <100 g/L and platelet (PLT) <100 × 109/L, accordingly. The median (range) bone marrow blast was 74.0 (31.0–94.0) %. In addition, 21 (26.9%) patients were recognized as cytogenetic abnormalities (). Besides, there were 10 donors with a median (range) of 45.0 (32.3–51.5) years, among whom six (60.0%) donors were male (Supplementary Table 1).
Table 1. Clinical characteristics of adult Ph− ALL patients.
Comparison of CDC42 between adult Ph− ALL patients and donors
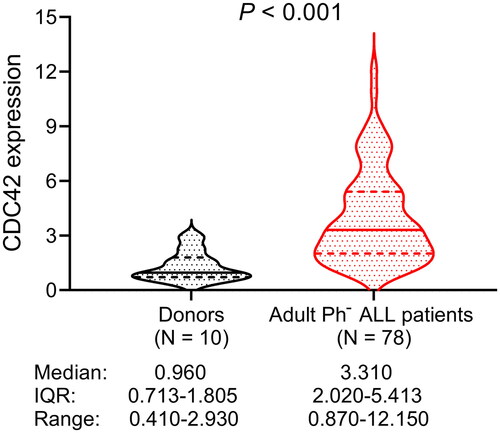
CDC42 was elevated in adult Ph− ALL patients compared with donors (p < .001). In detail, the median (IQR) CDC42 of adult Ph− ALL patients was 3.310 (2.020–5.413), ranging from 0.870 to 12.150. The median (IQR) CDC42 of donors was 0.960 (0.713–1.805), ranging from 0.410 to 2.930 ().
Association of CDC42 with clinical features of adult Ph− ALL patients
Increased CDC42 was linked with pro-B ALL or early-T ALL (p = .038) and WBC elevation at diagnosis (p = .025) in adult Ph− ALL patients. However, CDC42 was not correlated with other clinical characteristics including age, gender, disease type, HGB <100 g/L, PLT <100 × 109/L, bone marrow blasts ≥74% and cytogenetic abnormalities (all p > .050) ().
Table 2. Correlation between CDC42 expression and clinical characteristics of adult Ph− ALL patients.
Clinical outcomes of adult Ph− ALL patients
Fifty (64.1%) adult Ph− ALL patients achieved CR at 1 month. Furthermore, 23 (29.5%) patients were assessed as MRD after CR. Thirty-two (41.0%) patients received allo-HSCT. The median follow-up time was 1.7 years, ranging from 0.1 to 5.0 years. Additionally, the median EFS was 3.5 years, while the median OS was not reached. Besides, the 1-year, 3-year and 5-year accumulating EFS rates were 73.4%, 52.9% and 35.3%, accordingly. The 1-year, 3-year and 5-year accumulating OS rates were 81.5%, 69.1% and 51.0%, correspondingly (Supplementary Table 2).
Linkage of CDC42 with CR and MRD in adult Ph− ALL patients
CDC42 was negatively associated with CR at 1 month in adult Ph− ALL patients (p = .034). In detail, the median (IQR) CDC42 in patients with and without CR at 1 month were 2.970 (1.620–5.028) and 3.755 (2.600–6.385), respectively. However, CDC42 was not linked with MRD after CR (p = .066). Specifically, the median (IQR) CDC42 in patients with and without MRD after CR were 3.290 (2.120–6.000) and 2.160 (1.470–4.410), correspondingly ().
Table 3. Correlation between CDC42 expression and CR/MRD of adult Ph− ALL patients.
Comparison of EFS and OS between adult Ph− ALL patients with CDC42 < 3.310 and ≥3.310
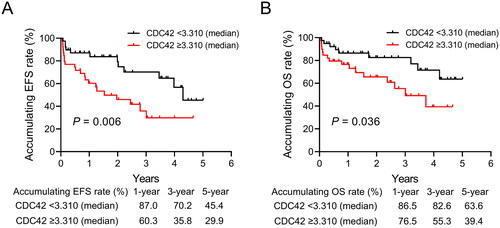
EFS was shorter in adult Ph− ALL patients with CDC42 ≥ 3.310 compared to those with CDC42 < 3.310 (p = .006). In detail, the 1-year, 3-year and 5-year accumulating EFS rates in Ph− ALL patients with CDC42 ≥ 3.310 were 60.3%, 35.8% and 29.9%, respectively; while the rates of those with CDC42 < 3.310 were 87.0%, 70.2% and 45.4%, accordingly (). Besides, patients with CDC42 ≥ 3.310 displayed a shortened OS compared with those with CDC42 < 3.310 (p = .036). Specifically, the 1-year, 3-year and 5-year accumulating OS rates of Ph− ALL patients with CDC42 ≥ 3.310 were 76.5%, 55.3% and 39.4%, correspondingly; whereas the rates in those with CDC42 < 3.310 were 86.5%, 82.6% and 63.6%, respectively ().
Subgroup analyses
In patients with CR, EFS was shortened in patients with CDC42 ≥ 3.310 compared to those with CDC42 < 3.310 (p = .040) (Supplementary Figure 1A), while OS was not different between patients with CDC42 ≥ 3.310 and <3.310 (p = .541) (Supplementary Figure 1B). In patients with MRD after CR, EFS was shorter in patients with CDC42 ≥ 3.310 than those with CDC42 < 3.310 (p = .040) (Supplementary Figure 1C), and no difference was seen in OS between patients with CDC42 ≥ 3.310 and <3.310 (p = .640) (Supplementary Figure 1D). Concerning patients without CR, neither EFS (p = .144) (Supplementary Figure 1E) nor OS (p = .133) (Supplementary Figure 1F) was varied between patients with CDC42 ≥ 3.310 and <3.310.
Factors affecting EFS in adult Ph− ALL patients
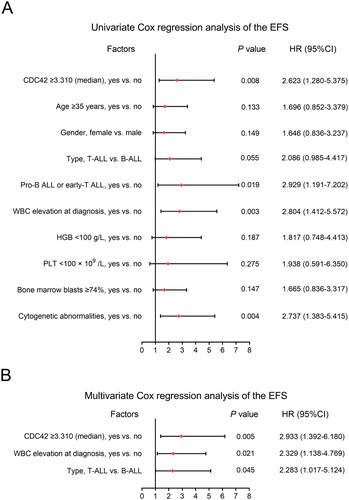
CDC42 ≥ 3.310 (yes vs. no) (hazard ratio (HR) = 2.623, p = .008), pro-B ALL or early-T ALL (yes vs. no) (HR = 2.929, p = .019), WBC elevation at diagnosis (yes vs. no) (HR = 2.804, p = .003) and cytogenetic abnormalities (yes vs. no) (HR = 2.737, p = .004) were related to poor EFS in adult Ph− ALL patients (). According to multivariate Cox’s regression analyses, CDC42 ≥ 3.310 (yes vs. no) (HR = 2.933, p = .005), WBC elevation at diagnosis (yes vs. no) (HR = 2.329, p = .021) and disease type (T-cell ALL vs. B-cell ALL) (HR = 2.283, p = .045) were independently associated with shorter EFS ().
Factors affecting OS in adult Ph− ALL patients
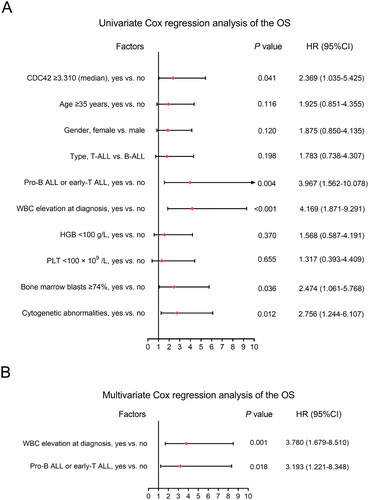
CDC42 ≥ 3.310 (yes vs. no) (HR = 2.369, p = .041), pro-B ALL or early-T ALL (yes vs. no) (HR: 3.967, p = .004), WBC elevation at diagnosis (yes vs. no) (HR = 4.169, p < .001), bone marrow blasts ≥ 74% (yes vs. no) (HR = 2.474, p = .036) and cytogenetic abnormalities (yes vs. no) (HR = 2.756, p = .012) were correlated with shortened OS in adult Ph− ALL patients (). While after adjustment, only WBC elevation at diagnosis (yes vs. no) (HR = 3.780, p = .001) and pro-B ALL or early-T ALL (yes vs. no) (HR: 3.193, p = .018) were independently correlated with worse OS ().
Discussion
Previous studies provide evidence that there is a dysregulation of Rho GTPases (including Rac1, Rho and CDC42, etc.) in hematological malignancy [Citation13,Citation21]. For instance, a study notices that CDC42 expression is elevated in acute myeloid leukemia compared with controls based on the BloodSpot database [Citation13]. Another study observes that Rac1 is overexpressed in ALL patients compared with normal donors [Citation21]. However, no public database has revealed the CDC42 in Ph− ALL patients. The present study identified that CDC42 was increased in adult Ph− ALL patients compared to donors. The potential explanations could be those: (1) CDC42 facilitated cell polarity and asymmetric division, inhibiting the apoptosis of leukemia cells [Citation13]. (2) CDC42 exerted a function of enhancing proliferation of ALL cells, thus, its elevation indicated the increased number of malignant cells [Citation13]. Therefore, there was an elevated CDC42 in adult Ph− ALL patients compared with normal bone marrow donors.
Apart from the aberrant expression of CDC42, the current study also disclosed that CDC42 was positively correlated with pro-B cell ALL or early-T ALL and WBC elevation at diagnosis. The probable reasons might be those: (1) pro-B cell ALL or early-T ALL represented more malignant immunological subtypes of ALL, characterized by the aggressive growth of leukemia cells [Citation22,Citation23]; moreover, increased CDC42 was correlated with the growth of leukemia cells [Citation13,Citation14]. Thus, elevated CDC42 was linked with pro-B cell ALL or early-T ALL. (2) Increased CDC42 modulated dysregulated proliferation of leukemia cells, and potentially facilitated egressing of immature WBCs from bone marrow to peripheral blood in ALL [Citation11,Citation13,Citation24]. Hence, CDC42 was positively linked with WBC elevation at diagnosis in adult Ph− ALL patients.
Concerning the correlation of CDC42 with treatment response, the present study observed that CDC42 was inversely correlated with CR at 1 month in adult Ph− ALL patients. The potential explanations could be those: (1) CDC42 activated p21-activated kinases to induce drug resistance in ALL, leading to worse treatment response [Citation25,Citation26]. (2) Increased CDC42 contributed to immune escape of leukemia cells from cytotoxicity of NK cells via remodeling actin cytoskeleton, resulting in insufficient treatment response in adult Ph− ALL patients [Citation14]. Therefore, elevated CDC42 was related to unpleasing treatment response in adult Ph− ALL patients.
Previous studies have suggested the potential of Rho GTPases as a prognostic marker in patients with leukemia due to their modulatory function in cancer progression [Citation27–29]. A study discloses that RhoH (a member of Rho GTPases) has an ability for predicting prognosis of acute myeloid leukemia patients [Citation28]. Another study reveals that elevated RhoF (another member of Rho GTPases) expression is independently associated with worse OS in acute myeloid leukemia [Citation27]. As a core member of Rho GTPases, CDC42 is hypothesized as a marker for predicting survival of adult Ph− ALL patients. The current study identified that higher CDC42 was correlated with shortened EFS (further confirmed by multivariate Cox’s regression analyses) and OS in adult Ph− ALL patients. The potential explanations might be those: (1) increased CDC42 contributed to resistance toward treatment in ALL patients, resulting in poor prognosis of them [Citation28]. (2) Elevated CDC42 promoted tumor progression, increasing the potential of central nervous system infiltration in ALL, which led to shortened survival of patients [Citation30]. Therefore, CDC42 ≥ 3.310 predicted a worse survival profile of adult Ph− ALL patients.
Limitations of the present study were as follows: (1) this was a single center study; thus, selective bias was unavoidable. (2) The relatively small sample size (N = 78) was a major shortage of this study, which weakened the statistical power to some extent. Therefore, the findings of this study required verification by studies with a larger sample size in the future. (3) The study merely included adult Ph− ALL patients, while the prognostic value of CDC42 in other subtypes (such as adult Ph+ ALL) was uncertain. (4) This was a retrospective study; hence, confounding factors was hard to avoid.
In conclusion, increased CDC42 may link with pro-B ALL or early-T ALL, WBC elevation at diagnosis, unfavorable clinical response and worse survival in adult Ph− ALL patients, suggesting its potency as a prognostic biomarker for these patients. However, the findings require further validation in prospective studies with a larger sample size.
Author contributions
Qi Chen: conceptualization, formal analysis, project administration, writing – original draft, and writing – review and editing. Xiaoling Zhu: data curation, investigation, and writing – original draft. Di He: data curation, resources, and writing – original draft. Wanbao Ding: conceptualization, formal analysis, supervision, and writing – review and editing.
Ethical approval
The study was conducted with the consent of the Ethics Committee.
Consent form
Written informed consent was signed by the patient or his/her family.
Supplemental Material
Download TIFF Image (1.1 MB)Supplemental Material
Download MS Word (15 KB)Supplemental Material
Download MS Word (16 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yi M, Zhou L, Li A, et al. Global burden and trend of acute lymphoblastic leukemia from 1990 to 2017. Aging. 2020;12(22):22869–22891. doi: 10.18632/aging.103982.
- Chang JH, Poppe MM, Hua CH, et al. Acute lymphoblastic leukemia. Pediatr Blood Cancer. 2021;68(Suppl. 2):e28371.
- Davis K, Sheikh T, Aggarwal N. Emerging molecular subtypes and therapies in acute lymphoblastic leukemia. Semin Diagn Pathol. 2023;40(3):202–215. doi: 10.1053/j.semdp.2023.04.003.
- Iacobucci I, Mullighan CG. Genetic basis of acute lymphoblastic leukemia. J Clin Oncol. 2017;35(9):975–983. doi: 10.1200/JCO.2016.70.7836.
- Foà R, Chiaretti S. Philadelphia chromosome—positive acute lymphoblastic leukemia. N Engl J Med. 2022;386(25):2399–2411. doi: 10.1056/NEJMra2113347.
- Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(9):1079–1109. doi: 10.6004/jnccn.2021.0042.
- Rafei H, Kantarjian HM, Jabbour EJ. Recent advances in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60(11):2606–2621. doi: 10.1080/10428194.2019.1605071.
- DeAngelo DJ, Jabbour E, Advani A. Recent advances in managing acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book. 2020;40:330–342. doi: 10.1200/EDBK_280175.
- Locatelli F, Maschan A, Boissel N, et al. Pediatric patients with acute lymphoblastic leukemia treated with blinatumomab in a real-world setting: results from the NEUF study. Pediatr Blood Cancer. 2022;69(4):e29562.
- Boissel N, Chiaretti S, Papayannidis C, et al. Real-world use of blinatumomab in adult patients with B-cell acute lymphoblastic leukemia in clinical practice: results from the NEUF study. Blood Cancer J. 2023;13(1):2. doi: 10.1038/s41408-022-00766-7.
- Crosas-Molist E, Samain R, Kohlhammer L, et al. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102(1):455–510. doi: 10.1152/physrev.00045.2020.
- Maldonado MDM, Dharmawardhane S. Targeting Rac and Cdc42 GTPases in cancer. Cancer Res. 2018;78(12):3101–3111. doi: 10.1158/0008-5472.CAN-18-0619.
- Mizukawa B, O'Brien E, Moreira DC, et al. The cell polarity determinant CDC42 controls division symmetry to block leukemia cell differentiation. Blood. 2017;130(11):1336–1346. doi: 10.1182/blood-2016-12-758458.
- Wurzer H, Filali L, Hoffmann C, et al. Intrinsic resistance of chronic lymphocytic leukemia cells to NK cell-mediated lysis can be overcome in vitro by pharmacological inhibition of Cdc42-induced actin cytoskeleton remodeling. Front Immunol. 2021;12:619069. (doi: 10.3389/fimmu.2021.619069.
- Yan J, Wan D. Dysregulation of circulating CDC42 and its correlation with demographic characteristics, comorbidities, tumor features, chemotherapeutic regimen and survival profile in non-small-cell lung cancer patients. J Clin Lab Anal. 2022;36(2):e24140. doi: 10.1002/jcla.24140.
- Gao S, Xue J, Wu X, et al. The relation of blood cell division control protein 42 level with disease risk, comorbidity, tumor features/markers, and prognosis in colorectal cancer patients. J Clin Lab Anal. 2022;36(7):e24572.
- Yang D, Zhang Y, Cheng Y, et al. High expression of cell division cycle 42 promotes pancreatic cancer growth and predicts poor outcome of pancreatic cancer patients. Dig Dis Sci. 2017;62(4):958–967. doi: 10.1007/s10620-017-4451-z.
- Ramakrishnan A, Datta I, Panja S, et al. Tissue-specific biological aging predicts progression in prostate cancer and acute myeloid leukemia. Front Oncol. 2023;13:1222168. doi: 10.3389/fonc.2023.1222168.
- Wang W, Zou H, Chen N, et al. Knockdown of CLAUDIN-6 inhibited apoptosis and induced proliferation of bovine cumulus cells. Int J Mol Sci. 2022;23(21):13222.
- Hematology Oncology Committee, Chinese Anti- Cancer Association; Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of acute lymphoblastic leukemia (2016). Zhonghua Xue Ye Xue Za Zhi. 2016;37(10):837–845.
- Wang J, Rao Q, Wang M, et al. Overexpression of Rac1 in leukemia patients and its role in leukemia cell migration and growth. Biochem Biophys Res Commun. 2009;386(4):769–774. doi: 10.1016/j.bbrc.2009.06.125.
- Jain N, Lamb AV, O'Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127(15):1863–1869. doi: 10.1182/blood-2015-08-661702.
- Roberts KG, Mullighan CG. The biology of B-Progenitor acute lymphoblastic leukemia. Cold Spring Harb Perspect Med. 2020;10(7):a034835. doi: 10.1101/cshperspect.a034835.
- Petrides PE, Dittmann KH. How do normal and leukemic white blood cells egress from the bone marrow? Morphological facts and biochemical riddles. Blut. 1990;61(1):3–13. doi: 10.1007/BF01739426.
- Wu A, Jiang X. p21-Activated kinases as promising therapeutic targets in hematological malignancies. Leukemia. 2022;36(2):315–326. doi: 10.1038/s41375-021-01451-7.
- Yao D, Li C, Rajoka MSR, et al. P21-activated kinase 1: emerging biological functions and potential therapeutic targets in cancer. Theranostics. 2020;10(21):9741–9766. doi: 10.7150/thno.46913.
- Hou Y, Zi J, Ge Z. High expression of RhoF predicts worse overall survival: a potential therapeutic target for non-M3 acute myeloid leukemia. J Cancer. 2021;12(18):5530–5542. doi: 10.7150/jca.52648.
- Iwasaki T, Katsumi A, Kiyoi H, et al. Prognostic implication and biological roles of RhoH in acute myeloid leukaemia. Eur J Haematol. 2008;81(6):454–460. doi: 10.1111/j.1600-0609.2008.01132.x.
- Mulloy JC, Cancelas JA, Filippi MD, et al. Rho GTPases in hematopoiesis and hemopathies. Blood. 2010;115(5):936–947. doi: 10.1182/blood-2009-09-198127.
- Del Principe MI, Maurillo L, Buccisano F, et al. Central nervous system involvement in adult acute lymphoblastic leukemia: diagnostic tools, prophylaxis, and therapy. Mediterr J Hematol Infect Dis. 2014;6(1):e2014075. doi: 10.4084/MJHID.2014.075.