Abstract
Background: The NS5A resistance-associated substitution (RAS) Y93H is found quite frequently (5–10%) at baseline in direct-acting antiviral agents (DAA) treatment-naïve genotype (GT) 3a patients when studied by the population-sequencing method (cut-off 20%). This RAS may impair HCV DAA treatment response, since it possesses a high fold in vitro resistance to daclatasvir (DCV) and velpatasvir (VEL) in GT 3. We investigated the effect of baseline Y93H in patients with GT 3a infection on treatment outcome, with or without resistance-based DAA-treatment during 2014–2017.
Patients/Methods: Treatment in the intervention group (n = 130) was tailored to baseline resistance-findings by population-sequencing method. Detection of baseline Y93H above 20% prompted a prolonged treatment duration of NS5A-inhibitor and sofosbuvir (SOF) and/or addition of ribavirin (RBV). Patients without baseline Y93H in the intervention group and all patients in the control group (n = 78) received recommended standard DAA-treatment.
Results: A higher sustained virologic response rate (SVR) in the intervention group was shown compared to the control group at 95.4% (124/130) and 88.5% (69/78), respectively (p = .06). All five patients with baseline Y93H in the intervention group achieved SVR with personalised treatment based on results from resistance testing; either with the addition of RBV or prolonged treatment duration (24w). In the control group, 2/4 patients with Y93H at baseline treated with ledipasvir/SOF/RBV or DCV/SOF without RBV, failed treatment.
Conclusion: The results from this real-life study are in accordance with the findings of the randomised controlled trials in 2015 and the EASL-guidelines of 2016, thus, baseline Y93H impacts on DCV and VEL treatment outcome.
Introduction
Hepatitis C virus (HCV) infection is considered the leading cause of liver cancer and liver transplantations in the Western world [Citation1]. Worldwide, an estimated 71 million people are living with viraemic HCV infection [Citation2]. In Sweden and Norway, about 0.4–0.5% of the population is infected with HCV, which approximately relates to 45,000 and 20,000 individuals, respectively [Citation3–5]. HCV is classified into seven genotypes (GT) and >100 subtypes [Citation6]. The most common GT in Sweden is 1a, followed by GT 3a [Citation7], while in Norway GT 3a is the most common, followed by 1a [Citation8].
Recently, HCV treatment has undergone a remarkable change and fixed-dose combinations of direct acting antivirals (DAAs) have replaced the traditional interferon (IFN)-based treatment. The DAAs can be classified into four classes, targeting three nonstructural proteins in HCV: NS3/4A protease inhibitors, NS5A inhibitors, and nucleoside and non-nucleoside inhibitors of the RNA-dependent NS5B polymerase [Citation9]. Using the latest approved drug combinations, a complete cure is possible with sustained virologic response (SVR) rates above 95%.The SVR rates have been somewhat lower for GT 3 compared to the other GTs [Citation10]. Almost all patients who fail treatment acquire resistance-associated substitutions (RASs), e.g., NS5A RAS that will persist for years [Citation9]. Even treatment-naïve patients could have RASs against currently approved DAAs, i.e., resistance at baseline [Citation10,Citation11]. Pre-existence of NS5A RASs with high fold resistance, together with other negative factors, such as high fibrosis stage, GT 3, or previous treatment with non-NS5A DAAs, could reduce the efficacy of DAAs [Citation10,Citation12].
RASs can be detected by population (Sanger) sequencing and next generation sequencing (NGS) methods. The Sanger method carries a 15–20% cut-off level for detecting RAS in the viral population, compared to 1% with NGS. However, the consensus is to recommend a cut-off level of 10–20% for detecting RASs within the HCV quasispecies, in order to be of clinical relevance [Citation10,Citation13,Citation14].
The NS5A RAS Y93H mutation/polymorphism is found quite often (5–10%) at baseline in DAA-treatment-naïve patients with GT 3 (with regard to subtype 3a) using the population sequencing method [Citation15]. This RAS possesses a high fold in vitro resistance of >2000 to daclatasvir (DCV) and >700 to velpatasvir (VEL) used for treatment of GT 3 infection [Citation16–18]. This was further revealed in clinical studies, the ALLY-3 study showed that 33% without liver cirrhosis and 75% with liver cirrhosis of the GT 3 patients with baseline RAS Y93H failed 12-week treatment with DCV plus sofosbuvir (SOF) [Citation19]. In the ASTRAL-3 study, which evaluated 12 weeks of VEL/SOF treatment, 4 out of 10 non-SVR patients had the Y93H mutation at baseline, and all non-SVR patients had Y93H at relapse [Citation20]. In another study, it was shown that baseline Y93H was associated with lower SVR rates for GT3, in particular for patients with cirrhosis [Citation21]. Furthermore, it has recently been demonstrated that addition of RBV with SOF/VEL was beneficial for treatment of GT3 with cirrhosis [Citation22].
Another NS5A RAS, A30K, is found at a similar level (5–10%) as a common baseline polymorphism in GT 3 patients. It’s in vitro resistance in GT 3a replicon assay towards DCV and VEL is in the fold ranges of 50 [Citation16,Citation17].
At the initiation of this study there were no available guidelines regarding baseline resistance testing before DAA treatment. The aim of this real-life study was to investigate the impact of Y93H at baseline on treatment outcome in GT 3 infected patients treated with DAAs, i.e., the intervention group was guided by baseline resistance testing and the control group was based on national guidelines without baseline resistance testing. The A30K RAS was also investigated, but only in the later phases as it was not considered as a clinically relevant RAS in 2014–2015. Known factors influencing treatment outcome were also evaluated.
Material and methods
Patients and treatment
Patients were consecutively included in this real-life, open label, nonrandomised Nordic multicenter study, consisting of an intervention group and a control group. The inclusion period for the intervention group was from 2 October 2014 to 5 December 2017 (when DCV/SOF or VEL/SOF were the recommended treatment regimes), and for the control group from April 2014 to 17 November 2016, Thus, the inclusion period was one year shorter for the control group in comparison to the intervention group and consequently no treatments with VEL/SOF (launched autumn 2016) in the control group. This was mainly due to the new EASL guidelines for baseline resistance testing, which became endorsed after 22 September 2016. Patients with chronic HCV GT 3a infection from Uppsala and Tromsø received treatment based on the results from resistance testing prior to treatment initiation (intervention group). The control group consisted of patients from Bodø, Falun, Stockholm and Örebro, who received treatment according to national guidelines [Citation23–26] without previous drug resistance testing.
In the intervention group (n = 130), treatment was adjusted to baseline resistance findings detected by the population sequencing method. Detection of baseline Y93H prompted either prolonged treatment duration (during 2014-16 DCV/SOF and during 2017 VEL/SOF) and/or addition of ribavirin (RBV) at the responsible MD’s discretion. At one occasion in 2016, 12 weeks SOF plus pegylated (PEG)-IFN with RBV was used. Patients without baseline Y93H in the intervention group and all patients in the control group (n = 78) received recommended standard DAA-treatment according to the National Boards. Note that A30K was not initially implemented in the intervention group as it was an unknown baseline RAS at the start of the study in 2014.
Samples for NS5A resistance analysis in the intervention group were analysed consecutively in routine diagnostics, while samples from the control group were analysed retrospectively.
Resistance analysis for emerging NS5A RAS was performed on all non-SVR patients at the time of relapse in the intervention group and retrospectively for the patients in the control group.
The inclusion criteria were: infection with HCV GT 3a; ≥18 years of age; informed consent; and treatment according to Swedish and Norwegian consensus recommendations. Patients previously treated with SOF plus ribavirin or other DAAs were excluded.
Liver elasticity (kPa) was measured with FibroScan® 502 (Echosens, France) (Swedish study sites) and FibroScan® 402 (Echosens, France; Norwegian study sites) by experienced nurses or doctors. For patients who had undergone a liver biopsy, the Metavir score was recorded [Citation27]. Presence of cirrhosis was determined by FibroScan >12.5 kPa [Citation28] or Metavir fibrosis score 4 in liver biopsy. The Child-Pugh score was estimated on the basis of available information from clinical examination, biochemical results and ultrasound. Patient data was extracted from the medical records by the responsible MD at each study site, anonymised and transferred to a joint database.
SVR was defined as undetectable HCV RNA 12 weeks after the end of treatment. Non-SVR was regarded as either viral breakthrough (a negative viral load nadir followed by a positive HCV RNA level during therapy), nonresponse (increase in HCV RNA levels after initial decrease during treatment) or viral relapse (nondetectable viral load at the end of treatment followed by an increase in HCV RNA-level beyond therapy).
The primary objective was to study the treatment efficacy in the intervention group compared to the control group, with respect to the proportion of patients achieving SVR. Secondary objectives included to determine: (1) the proportion of patients with NS5A baseline Y93H and A30K RASs; (2) the proportion of patients with these baseline NS5A RASs experiencing viral breakthrough or relapse; and (3) the proportion of patients with baseline NS5A RASs not experiencing viral breakthrough or relapse.
Laboratory methods
Resistance testing of RASs (baseline and emerging) was performed at the Department of Clinical Microbiology at Akademiska Hospital, Uppsala and was performed on all available samples at baseline, i.e., on samples collected prior to treatment initiation and at treatment failure. Viral gene from patient samples was amplified by the Nested PCR method and then sequenced by the Sanger sequencing method (population sequencing). This pan-genotypic NS5A resistance analysis protocol has been published previously [Citation29]. In brief, RNA extraction from plasma samples was done using NucliSENS® easyMAG™ system (BioMérieux, Marcy-l'Étoile, France). cDNA was synthesised from RNA template with SuperScript™ III Reverse Transcriptase (Invitrogen™, Thermo Fisher, Waltham, MA, USA) using random hexamers. First round PCR and nested PCR were performed with in-house primers targeting parts of the NS5A-regions using the Taq PCR Master Mix (QIAgen, Hilden, Germany) [Citation29]. The amplicons were verified by agarose-gel electrophoresis. PCR-positive samples were purified using QIAquick® PCR Purification Kit (QIAgen, Hilden, Germany) before they were sent for sequencing.
The first two rounds of PCR amplification protocols preceding the sequencing step were revised on samples included in this study as of Q1 2017. Synthesis of cDNA and first round of PCR was done using Takara PrimeScript™ One Step RT-PCR Kit Ver.2 (Takara BIO Inc, Kusatsu, Shiga prefecture, Japan). Nested PCR was performed as previously [Citation29]. The nested PCR products were verified by e-Gel® 2% agarose electrophoresis (Invitrogen, ThermoFischer Scientific, Waltham, MA, USA). Samples were purified before sequencing using ExoSAP-IT™ (Applied Biosystems™, ThermoFischer Scientific, Waltham, MA, USA).
The purified products were, at the start of this study, sent to the Uppsala Genome Centre and as of Q3 2017 to EurofinsGenomics, Ebersberg, Germany for capillary electrophoresis (Sanger) sequencing, at both sites, on 3730xl DNA Analyser (Applied Biosystems™, ThermoFischer Scientific, Waltham, MA, USA) using the same primer pair used in the nested PCR.
Sequence analysis
Population-based sequencing generates a consensus sequence of the viral quasispecies with a sensitivity of approximately 20% for (minority) variants, recognised as mixed peaks in the electropherogram. The NS5A sequence results were aligned and analysed using SeqScape® Software v2.6 (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA) to generate consensus sequences. The NS5A sequence of HCV GT1a H77 strain (accession number: NC_004102.1) was used as a reference template to which the sample sequences were aligned. To simplify, we considered this reference GT 1a template suitable for GT 3 during the analysis with the Seqscape software as described in a previous reports [Citation7,Citation29,Citation30]. However, later on, the results were compared with GT 3a specific reference strain: D17763 (NZL1).
To detect relevant substitutions and evaluate their implications, the consensus sequences were submitted in the web-based mutation detection algorithm, Geno2Pheno [hcv] 0.92 (G2P) [Citation30]. Substitutions scored by G2P were further interpreted as clinically relevant RASs by relating scores with EASL guidelines 2016 and 2018, in addition to RASs reported to bear impact on DAA treatment outcome in vitro and/or in vivo in literatures [Citation10,Citation18,Citation31]. In this study, the NS5A RASs Y93H and A30K were defined as relevant for GT3 as reported in the literature [Citation14].
HCV-RNA quantification of the patient samples was performed at the Department of Clinical Microbiology at Uppsala Akademiska Hospital (2014–2017: COBAS® AmpliPrep/TaqMan® HCVQuantitative Test (Roche, Basel, Switzerland), v2.0 with a LOQ of 15 IU/mL and 2017 onwards Abbott m2000 HCV Viral Load Assay (Abbott Laboratories, Chicago, IL, USA) with a LOQ of 12 IU/mL. and at the Department of Microbiology, University Hospital of North Norway, Tromsø, Norway (ROCHE RT-PCR, Cobas Amplicor Hepatitis C Viral Polymerase Chain Reaction, Roche Molecular System Inc., Branchburg NJ, USA). All protocols used were performed according to the manufacturer’s instructions.
Statistics
The null hypothesis of this study was that the SVR12 rate was equal in the intervention and control groups. The basic statistical computing was done in Microsoft® Excel® 2013 (Microsoft Office professional plus 2013, Microsoft Corporation) and in Statistical Package for Societal Sciences, version 24 (IBM Corp., Armonk, NY, USA). The Chi-square-test was used to test the differences between groups (or Fishers exact test if expected cell count was small). A two-tailed p value <.05 was considered significant.
Ethics
The protocol of this multicentre study was in compliance with the Helsinki Declaration. The regional committee of medical research ethics, Committee in Uppsala (Dnr: 2013/185, Dnr: 2013/185/1 and Dnr: 2013/185/2) and the Data Protection Official at The University Hospital of Northern Norway (Nr. 0574) approved this study. All participants received written information and the opportunity to withdraw from this study.
Results
Patient baseline characteristics
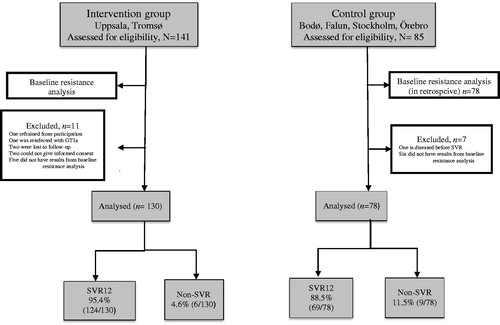
In total, 226 patients with HCV GT3, 141 in the intervention group and 85 in the control group were assessed to be eligible for the study. Eleven patients from the intervention group were omitted from further analyses: one refrained from participation, one was re-infected with GT 1a, two were lost to follow-up, two could not give informed consent, and baseline resistance results could not be obtained from five of the patients. In the control group, seven patients were omitted; one deceased before SVR and six had no results from baseline resistance analysis. Thus, week 12 follow-up data were obtained from 208 patients; 130 in the intervention group and 78 in the control group ().
Figure 1. Flowchart of patients included in the study. Baseline resistance testing in the control group was performed retrospectively.
Detailed demographic and baseline clinical characteristics are described in . Most patients were men, 73.1% (95/130) and 66.7% (52/78) in the intervention and control groups, respectively, and the majority was treatment-naïve to previous PEG-IFN/RBV regimen, 76.9% (100/130) and 59.0% (46/78) in the intervention and control groups, respectively. Amongst the patients who were PEG-IFN/RBV treatment-experienced, 77.4% (24/31) in the control group had response-relapse compared to 60.7% (17/28) in the intervention group. The median age at start of treatment was 55 and 52 years in the intervention and control groups, respectively. The distribution of patients with cirrhosis was 37.7% (49/130) and 61.5% (48/78) in the intervention and control groups, respectively. Median viral load at start of treatment was similar in both groups.
Table 1. Genotype 3 patient baseline characteristics.
Most cirrhotic patients in both groups were Child-Pugh class A (data not shown).
describes treatment characteristics. The distribution and variety of treatment regimens were higher in the intervention group since patients were included in this group also during 2017 when more DAAs were available. While the great majority of the patients in the control group were administered DCV/SOF (93.6%, 73/78), the remaining were administered ledipasvir (LED) plus SOF. Among the patients in the intervention group, 47.7% (62/130) were treated with DCV/SOF, 10.0% (13/130) were treated with LED/SOF, and furthermore, only patients in the intervention group were treated with VEL/SOF (41.5%, 54/130). Of the patients on VEL/SOF-treatment, 29.6% (16/54) also had RBV included in the treatment regime (data not shown). Most of the patients in both groups received treatment for 12 weeks, but it was more common in the intervention group compared to the control group, 76.9% (100/130) and 53.8% (42/78), respectively (p < .001). Simultaneous prolonged treatment of 24 weeks and addition of RBV was administered to 32.1% (25/78) in the control group compared to only 3.8% (5/130) in the intervention group (data not shown).
Table 2. Treatment characteristics of the GT3 patients.
Baseline RASs
The prevalence of baseline Y93H RAS was similar in both the intervention and control groups; 3.8% (5/130) and 5.1% (4/78), respectively, and baseline A30K prevalence was 3.8% (5/130) and 2.6% (2/78), respectively. The prevalence of baseline Y93H in terms of country/cohort was also similar with 4.9% (6/122) and 3.5% (3/86) in the Swedish and Norwegian cohorts, respectively (). In contrast, only one baseline A30K was found in the Swedish cohort as all the other A30K was found in the Norwegian patients, 0.8% (1/122) and 7.0% (6/86), respectively. Detailed description of clinical characteristics of the patients harbouring baseline RASs A30K and Y93H are summarised in .
Table 3. Clinical and treatment characteristics in patients with baseline A30K and Y93H RASs.
Of the five patients with baseline A30K in the intervention group, one was detected in a patient who subsequently relapsed. In the control group, prevalence of A30K was lower but found in one of the two patients who relapsed.
All five patients with baseline Y93H in the intervention group achieved SVR with treatment based on results from the resistance testing. These patients were treated with either 24 weeks DCV/SOF or LED/SOF treatment without RBV, 12 weeks VEL/SOF with RBV, or 12 weeks SOF plus PEG-IFN with RBV.
One patient with A30K in the intervention group did not have the treatment adjusted since the A30K was not considered a clinically relevant NS5A RAS at the time of start of treatment in 2014 (and during 2015). This patient was treated 12 weeks with DCV/SOF but subsequently failed and relapsed.
In the control group, 50.0% (2/4) of the patients with Y93H at baseline failed treatment and relapsed. These two patients were treated with LED/SOF and RBV for 16 weeks and DCV/SOF without RBV for 24 weeks, respectively ( and ).
Table 4. Clinical characteristics, baseline, and emerging NS5A RASs in the non-SVR patients.
In total, 15 patients failed to achieve SVR12, four of them had baseline RAS (three in the control group; one in the intervention group). The main reason for non-SVR was viral relapse, however, in two patients viral breakthrough ensued, and one patient was nonresponding. In three of these patients, one A30K and two Y93H were detected at baseline in the control group. One A30K was detected in the intervention group ().
SVR12 rates
SVR12 rates were consistently higher in the intervention group compared to the control group (). The most distinct differences between the intervention and control groups are: (1) an overall SVR rate of 95.4% (124/130) and 88.5% (69/78), respectively (p = .06); (2) a SVR rate in patients with baseline Y93H of 100% (5/5) and 50% (2/4), respectively (p = .07); and (3) a SVR rate in 12-week treatment of 98% (98/100) and 90.2% (38/42), respectively (p = .04).
Figure 2. Sustained virologic response rates (SVR) in the intervention and control groups. Treatment period for the intervention group was from 2 October 2014 to 5 December 2017, and for the control group from 10 April 2014 to 17 November 2016. SVR rates in the intervention group (blue bars) and the control group (orange bars).
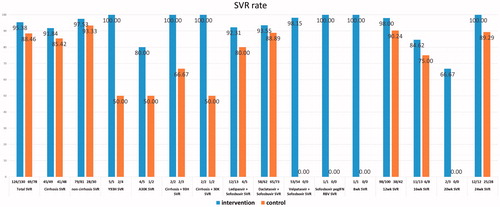
Of the patients in the intervention group who received a 12-week treatment and relapsed, one was treatment-naïve and noncirrhotic but harboured baseline A30K, and another had cirrhosis but did not have any baseline RAS but had failed previous treatment history with PEG-IFN/RBV. Neither had RBV added to their regimens with DCV plus SOF. In the control group, 4/42 failed 12-week treatment regimens. All were male and had previous treatment history with PEG-IFN/RBV; three had relapsed and one was nonresponding. Three were cirrhotic and had 12 weeks of DCV plus SOF and RBV. One was noncirrhotic and did not receive RBV. Subsequent analyses for presence of baseline RAS showed that one of them had A30K at baseline ().
Discussion
This Nordic multicenter study from April 2014 to December 2017 was performed when data on optimal regimens were emerging and guidelines were rapidly changing. For GT 3 treatment, the EASL guidelines recommended DCV plus SOF during 2014–2015 and a change to VEL/SOF in 2016–2017. Thereby, this study was conducted prior to recently approved (in Sweden January 2018 and in Norway February 2018) medications [glecaprevir (GLE)/pibrentasvir (PIB) and VEL/SOF plus voxilaprevir (VOX)]. Thus, these regimens have in a global perspective greatly improved the SVR rates, even for GT 3 in presence of NS5A RASs. This is mainly due to the inclusion of GLE or VOX, which are effective NS3 protease inhibitors against GT 3.
When we started this real-life study, the knowledge on outcome of baseline NS5A RAS in GT 3 treatment was very limited. However, we suspected that the clinically most relevant RAS in GT 3 should be Y93H, which according to in vitro data from the literature confers a high level of resistance to DCV and VEL, where the fold-change values in resistance compared to GT 3a wild type replicon are 2100 and 700 fold, respectively [Citation16,Citation17]. In the ALLY-3 and the ASTRAL-3 clinical studies, it was later shown that the Y93H in GT 3 was associated with lower SVR rates to treatment with DCV/SOF and VEL/SOF, especially in cirrhosis patients with baseline Y93H [Citation19,Citation20]. Furthermore, the natural prevalence of Y93H is as high as 5–10% in GT 3 patients with no prior exposure to NS5A treatment [Citation10]. Thereby, since September 2016 it is stated in the EASL guidelines that physicians who have easy access to reliable resistance tests can use baseline testing of Y93H in GT 3 patients to guide their decisions prior to treatment with VEL/SOF [Citation14,Citation32]. In case of baseline Y93H findings, these guidelines recommend the addition of RBV and/or extended treatment duration, which were actually the same recommendations (during 2014–2017) for retreatment of GT 3 patients with previous NS5A DAA-failure. In the recent report from the surveillance system against Antivirals in Norway (RAVN), resistance testing of Y93H at baseline is recommended in patients with GT 3 and cirrhosis [Citation33].
It was, therefore, relevant for the study group, as early as 2014, to evaluate treatment outcome based on baseline analysis of Y93H. As displayed in , all five patients with baseline Y93H in the intervention group achieved SVR with personalised resistance-based treatment. In the control group, 2/4 patients with Y93H at baseline, one treated for 16 weeks with LED/SOF and RBV and the other 24 weeks with DCV/SOF, failed treatment. Thereby, Y93H appeared to have a negative impact on treatment outcome (p = .073). However, because of a lower prevalence of baseline Y93H than expected and the limited sample sizes this could not be statistically determined.
In our study, an overall higher SVR was shown in the intervention group compared to the control group with values at 95.4% (124/130) and 88.5% (69/78), respectively (p = .06). However, there could be several confounding factors for the results in the intervention group compared to the control group. As shown in , some known negative predictors for SVR outcome were found in the control group compared to the intervention group in terms of the proportion of treatment naive patients (59.0% versus 76.9%), and more patients with cirrhosis (61.5% versus 37.7%). However, negative factors were also found in the intervention group, which had a higher rate of male patients; 73.1% compared to 66.7% in the control group, and a lower proportion of patients receiving 24 weeks of treatment; 9.2% compared to 35.9% in the control group. In addition, the newer treatment regimen VEL/SOF was used in the intervention group (41.5%) and not at all in the control group. However, it has been shown in a recent large real-life study of 2824 GT 3 patients that the chance of obtaining SVR was the same irrespective of whether a patient received DCV/SOF or VEL/SOF [Citation34]. It should also be noted that a few patients in our study, thirteen in the intervention group and five in the control group, were prescribed LED/SOF. This was done in 2014 and early 2015, before DCV was available in Norway. At this time, it was still not known that LED is less potent against GT 3 than DCV and VEL [Citation14]. Despite the many dissimilarities between the intervention and control groups, still higher SVR rates for the intervention groups than for controls were found for all different parameters displayed in .
Interestingly, even when data are adjusted for inclusion-period, the specified data from both groups are confined to treatment start between 2014 and 2016, SVR-rates in all examined parameters still show a tendency to be higher in the intervention group than in the control group (Supporting Information, Figure S1). Correspondingly, all other parameters (i.e. age, sex, previous treatment experience, prevalence of baseline Y93H and A30K, proportion of cirrhotic patients, proportion of prolonged treatment duration and the addition of RBV) included in this study remain very similar in both groups even after adjustment of time period. It should be mentioned that ten patients in the intervention group received VEL/SOF, compared to none in the control group during the matched period (data not shown).
The NS5A RAS A30K exists as a polymorphism in GT 3 in the same prevalence range 5–10% as Y93H. This RAS in GT 3a replicon assay confers resistance levels of 44 fold to DCV and 50 fold to VEL [Citation16,Citation17]. Even though these resistance levels are lower than for Y93H, it could be a negative factor in patients who are difficult to treat, e.g., with severe cirrhosis. Combination of HCV RASs can often confer greater level of phenotypic resistance, however, we did not detect the combination of A30K and Y93H, neither at baseline or at treatment failure, which is in accordance with a recent report [Citation35]. At the beginning of our study during 2014–2015, the RAS A30K was not considered a clinically relevant NS5A RAS but during 2016 the intervention group had its regimen tailored to this baseline RAS. It could be noted that a clinical study of the recently approved GLE/PIB treatment suggests a negative effect of baseline A30K. In treatment-experienced (PEG-IFN or SOF) GT 3 patients without cirrhosis who received 12 weeks of GLE/PIB treatment, significant lower SVR rate (25%) was observed for those patients with A30K at baseline, compared to those without A30K at baseline (SVR 96%) [Citation36].
The current Swedish and Norwegian recommendations for first-line treatment of treatment-naive GT 3 patients is, due to cost-effectiveness considerations, still VEL/SOF [Citation26,Citation37]. Thereby, baseline resistance testing in treatment of GT 3 may also, in addition to optimising responses and economic consequences, lead to less RBV use and shorter durations than dosing purely based on disease characteristics such as treatment experiences or cirrhosis.
Conclusion
In this real-life study conducted in Q2 2014 to Q4 2017, we found a low prevalence of baseline Y93H RAS in HCV genotype 3 in Sweden and Norway. Even though a trend was observed for Y93H being a negative predictor for DCV/SOF or VEL/SOF treatment outcome (p = .07), it could not be statistically determined, probably due to the small sample sizes. However, the findings are in line with the randomised controlled trials (ALLY-3 and ASTRAL-3) and the EASL-guidelines of 2016 and 2018. This could, therefore, have positive implications for the latest approved regimes, GLE/PIB and VEL/SOF/VOX, combinations that are known to be more potent against Y93H. However, these regimes often are more expensive than VEL/SOF and mainly considered as retreatment options. Since the resistance analysis cost per individual, even today, is 20–50 fold lower than the cost of current DAA-treatment, selection of cost-effective treatment combinations/duration should still be of importance, both in a perspective of evidence-based healthcare delivery, resistance-surveillance, and for the individual patient to avoid relapse with uncertain retreatment options.
Johan Lennerstrand has received a research grant from Medivir, honorary for lectures from AbbVie and Gilead, and travel grants from BMS.
Soo Aleman has received honorary for lectures and expert groups from AbbVie, BMS, Gilead and MSD, and research grants from AbbVie and Gilead.
Ann-Sofi Duberg has received honorary for lectures and expert groups from AbbVie, BMS, Gilead and MSD.
Tore Gutteberg has received honorary for lectures and expert groups from AbbVie, BMS, Gilead and MSD.
Anders Lannergård has received honorary from Gilead, MSD and Abbvie.
Supplemental Material
Download PDF (226.9 KB)Supplemental Material
Download MS Word (25.5 KB)Acknowledgements
The authors would like to thank Anders Bergqvist, Christina Öhrmalm and Kåre Bondeson for their help with the resistance analysis.
Additional information
Funding
References
- Seeff LB. The history of the “natural history” of hepatitis C (1968–2009). Liver Int. 2009;29:89–99.
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2: 161–176.
- Büsch K, Waldenström J, Lagging M, et al. Prevalence and comorbidities of chronic hepatitis C: a nationwide population-based register study in Sweden. Scand J Gastroenterol. 2017;52:61–68.
- Dalgard O, Jeansson S, Skaug K, et al. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand J Gastroenterol. 2003;38:864–870.
- Duberg A-S, Blach S, Falconer K, et al. The future disease burden of hepatitis C virus infection in Sweden and the impact of different treatment strategies. Scand J Gastroenterol. 2015;50:233–244.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327.
- Palanisamy N, Danielsson A, Kokkula C, et al. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res. 2013;99:12–17.
- Alberti A, Lacoin L, Morais E, et al. Literature review of the distribution of hepatitis C virus genotypes across Europe. J Med Virol. 2016;88:2157–2169.
- Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504.
- Wyles DL. Resistance to DAAs: when to look and when it matters. Curr HIV/AIDS Rep. 2017;14:229–237.
- Kileng H, Kjellin M, Akaberi D, et al. Personalized treatment of hepatitis C genotype 1a in Norway and Sweden 2014–2016: a study of treatment outcome in patients with or without resistance-based DAA-therapy. Scand J Gastroenterol. 2018;53:1347–1353.
- Lontok E, Harrington P, Howe A, et al. Hepatitis C virus drug resistance–associated substitutions: state of the art summary. Hepatology. 2015;62:1623–1632.
- Pawlotsky J-M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151:70–86.
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153–194.
- Bergfors A, Leenheer D, Bergqvist A, et al. Analysis of hepatitis C NS5A resistance associated polymorphisms using ultra deep single molecule real time (SMRT) sequencing. Antiviral Res. 2016;126:81–89.
- Hernandez D, Zhou N, Ueland J, et al. Natural prevalence of NS5A polymorphisms in subjects infected with hepatitis C virus genotype 3 and their effects on the antiviral activity of NS5A inhibitors. J Clin Virol. 2013;57:13–18.
- Lawitz EJ, Dvory-Sobol H, Doehle BP, et al. Clinical resistance to velpatasvir (GS-5816), a novel pan-genotypic inhibitor of the hepatitis C virus NS5A protein. Antimicrob Agents Chemother. 2016;AAC:00763–00716.
- Palanisamy N, Kalaghatgi P, Akaberi D, et al. Worldwide prevalence of baseline resistance-associated polymorphisms and resistance mutations in HCV against current direct-acting antivirals. Antivir Ther. 2018;23:485–493.
- Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135.
- Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617.
- Leroy V, Angus P, Bronowicki J-P, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY‐3+). Hepatology. 2016;63:1430–1441.
- Esteban R, Pineda JA, Calleja JL, et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology. 2018;155:1120–1127.e4.
- The Norwegian Medical Association. Faglig veileder for utredning og behandling av hepatitt C. 2014 [cited 2018 Jan 8]. Available from: http://legeforeningen.no/PageFiles/246436/Veileder%20sept%202014.pdf
- The Norwegian Medical Association. Faglig veileder for utredning og behandling av hepatitt C. 2015 [cited 2015; National guidelines]. Available from: http://gastroenterologen.no/filer/Veileder-Revisjon-mars-2015.pdf
- Sykehusinnkjøp HF. LIS recommondations for HCV treatment. Oslo (Norway): Sykehusinnkjøp HF; 2016.
- RAV Referensgruppen för AntiViral terapi. Antiviral treatment of hepatitis C. 2017 [cited 2017]. Available from: https://www.sls.se/rav/rekommendationer/hepatit-c-virus/
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20.
- Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350.
- Lindström I, Kjellin M, Palanisamy N, et al. Prevalence of polymorphisms with significant resistance to NS5A inhibitors in treatment-naive patients with hepatitis C virus genotypes 1a and 3a in Sweden. Infect Dis. 2015;47:555–562.
- Kalaghatgi P, Sikorski AM, Knops E, et al. Geno2pheno [HCV]–a web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS One. 2016;11:e0155869.
- Sorbo MC, Cento V, Di Maio VC, et al. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: update 2018. Drug Resist Updat. 2018;37:17–39.
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
- Norwegian Institute of Public Health. Usage of Antivirals and the Occurence of Antiviral resitance in Norway in 2017. 2018 [cited 2019 Jan 23]. Available from: https://www.fhi.no/en/publ/2018/usage-of-antivirals-and-the-occurrence-of-antiviral-resistance-in-norway-20/
- Belperio PS, Shahoumian TA, Loomis TP, et al. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70:15–23.
- Howe A, Cento V, Garcia F, et al. A real world resistance profile of virologic failures collected from an international collaboration (SHARED). In: Hepatology. Hoboken (NJ): Wiley; 2018.
- Krishnan P, Pilot-Matias T, Schnell G, et al. Pooled resistance analysis in HCV genotype 1-6 infected patients treated with glecaprevir/pibrentasvir in phase 2 and 3 clinical trials. Antimicrob Agents Chemother. 2018;62:e01249–18.
- Sykehusinnkjøp HF. LIS recommondations for HCV treatment. 2019 [cited 2019 Feb 14]. Available from: https://sykehusinnkjop.no/Documents/Legemidler/Avtaler%20og%20anbefalinger/2019/Uten%20priser%20Hepatitt%20C%20anbefalinger%202019%20og%202020.pdf

