Abstract
Objectives
Faecal microbiota transfer (FMT) consists of the infusion of donor faecal material into the intestine of patients with the aim to restore a disturbed gut microbiota.
Methods
In this pilot study (NCT03275467), the effect of three repeated FMTs (day 0, two weeks, four weeks) was studied and followed up for six months in nine collagenous colitis (CC) patients, using two stool donors.
Results
Five patients had an active disease at the time of baseline sampling. The primary endpoint (remission at six weeks, defined as <3 stools whereof <1 watery stool per day) was achieved by two of these patients, and by one at eight weeks. Overall, in all nine patients, FMT did not result in a significant reduction of watery stools, assessed by daily diary. However, diarrhoea (assessed by gastrointestinal symptom rating scale) was significantly improved at four (p = .038) and eight weeks (p = .038), indigestion at eight (p = .045) and 12 weeks (p = .006), disease-related worries at four (p = .027) and eight weeks (p = .027), and quality of life at six months (p = .009). FMT resulted in an increased number of lamina propria lymphocytes, possibly indicating an initial mucosal immune activation. No serious adverse events, no systemic effects, and no changes in faecal calprotectin and psychological symptoms were observed.
Conclusions
FMT is able to improve symptoms in a yet undefined subset of CC patients. Further studies could help to characterise this subset and to understand if these results can be generalised to all microscopic colitis patients.
Introduction
Microscopic colitis (MC) is a chronic inflammatory disease mostly diagnosed in middle-aged or elderly women. Although MC does not lead to increased mortality, patients suffer from chronic watery diarrhoea, abdominal pain and weight loss, which strongly affects their quality of life [Citation1]. MC is primarily divided into the two entities collagenous colitis (CC) and lymphocytic colitis (LC). Both types are characterised by a macroscopically normal or almost normal mucosa; however, microscopically an increased number of lymphocytes can be observed. The colonic mucosa of CC patients also shows a thickened subepithelial collagen layer [Citation2,Citation3]. Currently, the most effective medication to treat MC is budesonide, a synthetic glucocorticoid. However, 80% of the patients relapse after ending the treatment and long-term use is associated with corticosteroids-associated side effects [Citation4].
The aetiology of MC is not well understood, but an aberrant immune response to unknown luminal agents in genetically predisposed individuals might be one of the underlying causes [Citation5]. The gut microbiota has been suggested to be such a potential luminal agent, and recent studies have shown that the faecal microbiota composition is altered in MC patients compared to healthy individuals [Citation6–8]. The emerging evidence of the gut microbiota being involved in the pathophysiology of MC as well as the need for alternative, non-pharmaceutical treatments suggest faecal microbiota transfer (FMT) as a new potential treatment option for MC. FMT consists of the introduction of faecal material from a healthy subject into the intestine of a patient with a disturbed gut microbiota, and has been shown to be a successful treatment in Clostridioides difficile infection (CDI) [Citation9]. The efficacy of FMT has also been studied in other diseases such as ulcerative colitis (UC) [Citation10–12], metabolic syndrome [Citation13,Citation14] and irritable bowel syndrome (IBS) [Citation15–17]. A recent casereport, in which one patient with active CC was treated with repeated FMTs, suggested that FMT could have a beneficial effect in CC [Citation18]. Additionally, a study investigating the outcome of FMT in patients with recurrent CDI and concurrent inflammatory bowel disease (IBD) or MC found that most of the patients with LC experienced normalised bowel habits after the CDI was cleared by FMT (12 out of 15 patients) [Citation19].
In this open-label pilot study, the effect of FMT on the number and consistency of bowel movements in ten patients with CC was assessed. Additionally, symptom scores, various blood markers and the colonic lymphocyte profile were assessed in order to address the possible mode of action of FMT.
Materials and methods
The study was conducted according to the principles of the Declaration of Helsinki and its revisions, and ethical approval was obtained from the Central Ethical Review Board of Uppsala, Sweden (registration number 2017/072). The trial was registered at ClinicalTrials.gov (NCT03275467) on September 7, 2017. The study was performed at Örebro University Hospital in Örebro, Sweden, from April 2018 to October 2019. Patients were recruited in the greater area of Örebro and asked to sign an informed consent before participation.
Study design
In this pilot study, nine CC patients were repeatedly treated with faecal microbiota material from two thoroughly screened, healthy donors. The first FMT was administered into the caecum by whole colonoscopy. During the colonoscopy, four biopsies each from the ascending colon and the right flexure were collected to confirm the CC diagnosis by histopathology according to clinical routines (subepithelial collagen layer ≥10 µm and lymphocyte infiltration). The second and third FMT were administered by enema, at two and at four weeks, respectively. At baseline and six weeks, the patients underwent an endoscopy without bowel cleansing during which biopsies were collected at a standard location (midsection of descending colon). At the same time points, blood samples were collected. Daily diaries, questionnaires and faecal samples were collected at every study visit. Patients were followed up at eight weeks, 12 weeks and six months, respectively. For an overview of the data and samples collected in the study, see . Patients were asked to keep their diet and medication stable during the course of the study.
Table 1. Data and sample collection throughout the course of the study.
Subjects
MC Patients
Patients with a previous CC diagnosis that reported to have an active disease, defined as more than three stools per day of which at least one watery (self-reported, assessed by interview two weeks before study start, stool diaries were not collected before final inclusion), between 18 and 70 years of age and with the willingness to stop budesonide treatment before the start of the trial, were included. CC diagnosis was confirmed later in the study by colonic biopsies taken during the colonoscopy for the first FMT. Reasons for exclusion were previous complicated gastrointestinal surgery, malignant disease except for non-melanoma skin cancer, dementia, severe depression, major psychiatric disorder or other incapacity for adequate cooperation, CDI or other current gastroenteritis, pregnancy or breastfeeding, severe endometriosis, antimicrobial treatment four weeks prior to first screening visit, antimicrobial prophylaxis, regular consumption of probiotics four weeks prior to first screening visit, recently diagnosed lactose intolerance (less than six months prior to first screening visit), recently diagnosed coeliac disease (less than six months prior to first screening visit), regular intake of non-steroidal anti-inflammatory drugs (NSAIDs), abuse of alcohol or drugs and any clinically significant disease/condition which in the investigator’s opinion could interfere with the results of the trial. Patients were asked to keep their diet stable during the study and completed a food frequency questionnaire at the beginning and the end of the study to monitor potential dietary changes.
Donors
Healthy subjects between 18 and 65 years of age were carefully screened before inclusion as potential donors. As butyrate-producing bacteria seem to be reduced in MC patients [Citation6,Citation7], we decided to include donors with a relatively high amount of butyrate-producing bacteria in their faecal samples. In short, these bacteria were quantified by quantitative real-time polymerase chain reaction (qPCR) detection of the butyryl-CoA CoA transferase gene, which encodes the last step of butyrate formation by gut bacteria [Citation16,Citation20]. Exclusion criteria are shown in . Donors were asked to sign an informed consent before participation. During the study, the donors regularly underwent blood and stool tests and were asked about their general health at every donation. The donors were asked to keep their diet stable over the course of the study. Both donors included were female and their age at inclusion was 28 and 31 years. Four CC patients received faecal material from donor A and five received faecal material from donor B (). Each individual patient received faecal material from only one donor.
Table 2. Exclusion criteria of FMT donors.
Table 3. Overview of patient characteristics and study outcomes throughout the study.
FMT procedure
The selected donors provided their faecal material at the study unit immediately after donation. Within two hours after donation, the faecal material was processed and frozen at −80 °C. The faecal material was covered with sterile saline (0.9% NaCl, 150 mL per 30 g) and carefully mixed manually in order to avoid oxygen exposure. Before freezing, sterile pharmaceutical grade glycerol was added to a final concentration of 10%. The frozen stool preparations were carefully thawed in a water bath at 37°C one hour before FMT. The first FMT consisted of one aliquot (30 g in 150 mL) administered into the caecum by colonoscopy performed under conscious sedation using midazolam and alfentanil after bowel cleansing. Two aliquots (in total 60 g in 300 mL) each were used for the second and third FMT and administered by enema without prior bowel cleansing. The recipients were asked to take 2 mg loperamide before and after the FMT, and an additional 2 mg if needed, in order to minimise the risk of losing the faecal material due to diarrhoea.
Adverse event assessment
Patients were asked to measure their body temperature and report adverse events for seven days after each FMT using a written form. Additionally, adverse events were reported to the investigators at each study visit.
Primary outcome
The primary endpoint of this study was defined as the proportion of MC patients in remission at six weeks. Remission was defined as less than three stools per day and a mean of less than one watery stool per day. The number and consistency of the bowel movements were assessed by a daily diary which is commonly used to diagnose and monitor MC [Citation4,Citation21]. The patients completed the daily diary approximately from one week before the first endoscopy until twelve weeks and for approximately two weeks before the six-month follow-up.
Secondary and exploratory outcomes
The secondary outcomes of this study were changes in number and form of bowel movements, changes in questionnaire scores as well as changes in lymphocyte infiltration, subepithelial collagen layer and immune cell composition of colonic biopsies. Exploratory outcome measures were changes in inflammation markers in faecal samples. To carefully monitor patients’ health, blood markers were assessed.
Questionnaires
Questionnaires assessing gastrointestinal symptoms and general health were completed by the patients before and after FMT. Gastrointestinal symptoms were assessed by the gastrointestinal symptom rating scale (GSRS). The GSRS includes 15 symptoms in five symptom clusters (diarrhoea, abdominal pain, constipation, indigestion, reflux) and uses a 7-point Likert scale in which ‘1’ represents no symptoms and ‘7’ very severe symptoms [Citation22–24]. The questionnaire is used for a variety of chronic gastrointestinal diseases and has been used for the assessment of MC previously [Citation25]. The hospital anxiety and depression scale (HADS) consists of 14 items divided into two subscales for anxiety (seven items) and depression (seven items). Patients rate each item on a four-point scale in which ‘0’ represents the most positive option and ‘3’ the most negative [Citation26]. The short health scale (SHS) is a simplified four-item questionnaire that addresses the four health dimensions symptom burden, social function, disease-related worry and general well-being. Patients rate each item on a six-point scale in which ‘1’ represents the most positive option and ‘6’ the most negative. No health-related quality of life questionnaire is specifically addressed to MC patients and validated in this patient group. The SHS questionnaire is well validated in IBD [Citation27–29] and also commonly used in MC [Citation21,Citation30]. The EQ-5D-5L is a measure of health status that provides descriptive measures of the five dimensions mobility, self-care, usual activities, pain/discomfort, anxiety/depression as well as the health status [Citation31]. In this study only the quantitative health status of the EQ-5D-5L was used for analysis which consists of a VAS scale and an index value with which the patients rate their current health status, with 100 being the best health status possible. The average of both these measurements was used for analysis.
Clinical blood markers
Blood was collected by experienced nurses according to hospital routines at baseline and six weeks. The following blood markers were measured according to clinical routines: haemoglobin (Hb), erythrocyte mean corpuscular haemoglobin (Erc-MCH), erythrocyte count, leukocyte count, platelet count (all measured in whole blood), alanine transaminase (ALT), aspartate transaminase (AST), albumin, creatine, creatine kinase (CK) high sensitivity C-reactive protein (hsCRP), all measured in Li-hep plasma, and estimated glomerular filtration rate (eGFR).
Faecal calprotectin
Faecal samples were collected by the patients at home at every study visit (from baseline up to six months), immediately placed in their home freezer and delivered frozen to the study unit at their next visit using cool transport containers (Sarstedt, Germany). At the study unit samples were stored at −80°C until analysis. Before analysis, faecal samples were transferred to CALEX® Cap tubes (Bühlmann Laboratories AG, Switzerland). Analysis was performed on an Advia 1800 instrument (Siemens Healthcare, Sweden) with Bühlmann fCAL® turbo reagents according to the manufacturer’s instructions. Method for analysis was a particle enhanced turbidimetric immuno assay (PETIA), according to clinical routines.
Histopathologic examination of colonic biopsies
Biopsies were collected at baseline and six weeks from an uncleansed descending colon (midsection) and paraffin-embedded for pathological examination according to clinical routines. Colonic biopsies were used to assess collagen layer thickness (hematoxylin and eosin staining) and intraepithelial lymphocyte infiltration (antibodies used: mouse anti-human CD8, clone C8/144B, and rabbit anti-human CD3, polyclonal, both from Agilent/Dako, USA).
Flow cytometry analysis of colon mucosal lymphocytes
Biopsies were collected at baseline and six weeks from an uncleansed descending colon (midsection) and immediately transferred to PBS on ice. Colonic biopsies were used to isolate intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) in order to characterise surface and intracellular markers by flow cytometry as previously described [Citation32,Citation33]. For a detailed method description, see Supplementary Material.
Statistical analysis
Daily diary data
The number and consistency of stools was assessed by daily diary. In order to perform a conservative analysis, on days on which it was unclear if the patient had no stools or missed to report the number, this was interpreted as ‘0’ before the first FMT and as missing data after the first FMT. If the patient could not decide if the consistency was watery or non-watery, it was interpreted as ‘0.5’ for both consistencies. For analysis, the average number of total stools, watery stools and non-watery stools between the study visits was calculated. Data collected at the days of study visits and bowel cleansing were excluded. Normal distribution of the data sets was tested with the Shapiro-Wilk normality test, and statistical significance was calculated with the non-parametric Friedman test with Dunn’s multiple comparison. All time points were compared to baseline. The average of the data collected two weeks before FMT and until the first FMT was used as the baseline value. One patient was lost to follow-up after attending the six-week visit. Two patients resumed their budesonide treatment after attending the eight-week visit; these values of patients using budesonide were excluded. The missing or excluded values were imputed with the respective mean baseline values, adopting a conservative approach.
Results are presented as median and interquartile range. In this study, daily diary data showed that five patients had an active disease at baseline while four were in remission. We present their results as two separate entities; however, sample size was not sufficient to perform separate statistical analysis.
Questionnaire and faecal calprotectin data
All data was compared to baseline using the non-parametric Friedman test and post hoc Dunn’s multiple comparison test. Missing or excluded values were imputed with the respective mean baseline values as described for the daily diary data, and tests were performed in the same way, adopting a conservative approach.
Blood markers, histopathologic assessment of colonic biopsies and flow cytometry data
If normally distributed (Shapiro-Wilk normality test), the paired t-test was performed to test statistical significance, otherwise the Wilcoxon matched-pairs signed rank test was used.
Results
Patient characteristics
Ten patients were included in the study (). All patients were female and between 44 and 70 years of age. If using budesonide before the start of the study, this was discontinued at least 11 days before the baseline visit. In nine of the ten patients, the CC diagnosis was confirmed by histopathology of the biopsies collected from the ascending colon and right flexure during the whole colonoscopy for the first FMT. In one patient, an earlier histology-based CC diagnosis could not be confirmed, although the inclusion criteria (based on symptoms assessed by interview) were fulfilled. The results of this patient were excluded from the analyses. Three of the nine remaining patients had co-morbid autoimmune or chronic inflammatory disease. shows the concomitant medications of the patients which were kept stable until after the 12-week visit. In addition, four patients occasionally took loperamide and one patient occasionally took simeticone (an anti-foaming agent) during the course of the study. Three patients were current smokers, five patients had smoked in the past and one patient had never smoked. One patient was lost to follow-up after the six-week visit for unknown reasons. Two patients started with budesonide between eight and twelve weeks, both as they were not content with the effect of the FMT treatment. No dietary changes were reported.
Table 4. Concomitant medications (until 12 weeks).
Adverse events
No serious adverse events were observed after the FMTs. shows the mild adverse events that were reported by the patients up to seven days after each FMT. The patients’ body temperature did not exceed 37.8°C in the first seven days after each FMT. One of the patients experienced an MC flare-up, which was presumably triggered by an upper respiratory tract infection, as this patient experienced flare-ups after upper respiratory tract infections several times in the past, although an association to the FMT cannot be ruled out. Abdominal discomfort and flare-ups could be related to the FMT but could also be the course of the original disease.
Table 5. Adverse events after FMT.
Number of stools
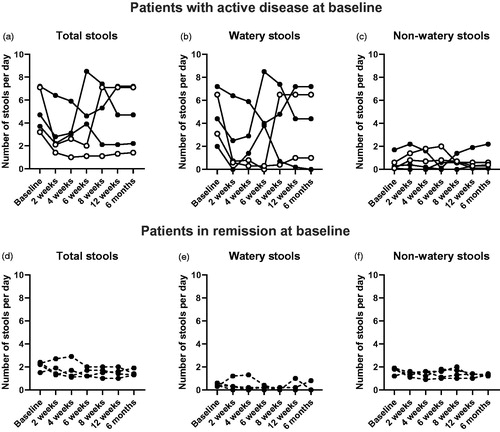
The primary outcome of this study was defined as remission (<3 stools per day of which <1 watery stool) at six weeks. Six out of nine patients were in remission at six weeks, but only five out of nine patients had an active disease (>3 total stools of which at least one watery) according to the daily diary collected at the baseline visit (). Two of these patients that had an active disease at baseline (A and E) achieved remission at six weeks and were defined as clinical responders at six weeks. Patient A received the faecal material from donor A, patient E received faecal material from donor B (). A third patient (D) who had an active disease at baseline was a responder at eight and twelve weeks and six months and received faecal material from donor B.
Overall, in all nine patients, number of stools at the primary endpoint of six weeks was: Total stools: median 2.0, interquartile range (IQR) 1.2 to 4.3 (baseline: 3.2, IQR 2.3 to 5.9); watery stools: 0.3, IQR 0.1 to 3.9 (baseline: 2.0, IQR 0.4 to 5.5); non-watery stools: 1.0, IQR 0.4 to 1.7 (baseline: 1.2, IQR 0.2 to 1.8).
Adopting a conservative analysis approach (missing or excluded values were imputed with the respective mean baseline values), no statistically significant differences were observed in the number of stools (total, watery and non-watery) at any time point compared to baseline ().
Figure 1. Number of stools after FMT. a–c Number of stools per day of patients with active disease at baseline. d–f Number of stools per day of patients in remission at baseline. These patients were in remission also after FMT. The missing/excluded values of some patients during the later time points were replaced with the individual baseline values when performing the statistical tests. No statistically significant differences were found. Open symbols show clinical responders, dashed lines show patients who were already in remission at baseline.
General health and symptom questionnaires
The effect of FMT on gastrointestinal and psychological symptoms as well as general health and quality of life was evaluated by self-assessed questionnaires.
Gastrointestinal symptom rating scale (GSRS)
The total GSRS score was significantly reduced at eight weeks (median 2.1, IQR 1.2 to 3.7, p = .020) and 12 weeks (1.8, IQR 1.1 to 3.4, p = .014) compared to baseline (3.2, IQR 2.7 to 3.8) when adopting a conservative analysis approach ().
Figure 2. Gastrointestinal symptom rating scale scores before and after FMT. * indicates p < .05, ** indicates p < .01 compared to baseline when adopting a conservative approach. The missing/excluded values of some patients during the later time points were replaced with the individual baseline values when performing the statistical tests. Open symbols show clinical responders, dashed lines show patients who were already in remission at baseline.
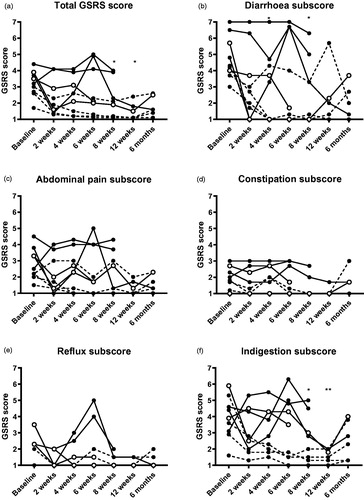
The patients rated significantly lower on the diarrhoea subscore at four weeks (3.3, IQR 1.0 to 4.5, p = .038) and eight weeks (3.3, IQR 1.0 to 4.5, p = .038) compared to before the FMT (4.3, IQR 3.9 to 6.1). The ratings for the indigestion subscore decreased significantly at eight weeks (2.8, IQR 1.4 to 4.2, p = .045) and twelve weeks (2.0, IQR 1.4 to 3.5, p = .006) compared to baseline (3.9, IQR 3.0 to 5.0). The constipation, the abdominal pain and the reflux subscores did not differ significantly over the course of the study.
Hospital anxiety and depression score (HADS)
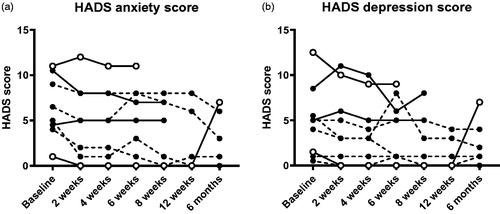
No statistically significant differences were observed in the HADS anxiety or depression score at any time point compared to baseline ().
Figure 3. Hospital anxiety and depression scale scores before and after FMT. No statistically significant differences were found. The missing/excluded values of some patients during the later time points were replaced with the individual baseline values when performing the statistical tests. Open symbols show the clinical responders, dashed lines show patients who were already in remission at baseline.
Short health scale (SHS)
The SHS showed that patients had significantly less disease-related worries at four weeks (median 2.0, IQR 1.0 to 2.5, p = .027) and eight weeks (1.0, IQR 1.0 to 2.5, p = .027) compared to baseline (2.5, IQR 2.3 to 3.8) (). The SHS subscores ‘symptom burden’, ‘social function’ and ‘general well-being’ were not significantly affected by FMT.
Table 6. Short health scale questionnaire before and after FMT.
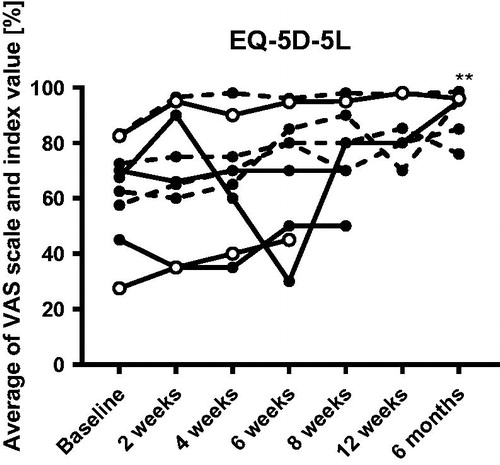
EQ-5D-5L
The patient’s quality of life, assessed by EQ-5D-5L VAS scale and index value, was significantly improved at six months (85.0, IQR 57.5 to 95.5, p = .009) compared to baseline (67.5, IQR 51.3 to 77.5) ().
Figure 4. EQ-5D-5L scores before and after FMT. ** indicates p < .01 compared to baseline when adopting a conservative approach. The missing/excluded values of some patients during the later time points were replaced with the individual baseline values when performing the statistical tests. Open symbols show the clinical responders, dashed lines show patients who were already in remission at baseline.
Systemic effect of FMT
In order to carefully monitor the patients’ health, several blood markers were assessed. No statistically significant changes were found between baseline and six weeks in the blood markers (Supplementary Table 1). The values for haemoglobin, erythrocyte count, leukocyte count, platelet count and erythrocyte mean corpuscular haemoglobin of two patients were missing at baseline due to technical reasons. Additionally, the value for high sensitivity C-reactive protein was missing for one patient at six weeks.
Effect of FMT on faecal calprotectin
To investigate potential modes of action, the effect of FMT on gastrointestinal inflammation was determined based on faecal (F) calprotectin levels. No statistically significant differences were found in F-calprotectin concentrations at any time point compared to baseline (Supplementary Table 2). Median F-calprotectin concentrations were in general quite stable, while individual F-calprotectin concentrations showed variations during the course of the study.
Effect of FMT on collagen layer thickness and intraepithelial lymphocyte infiltration
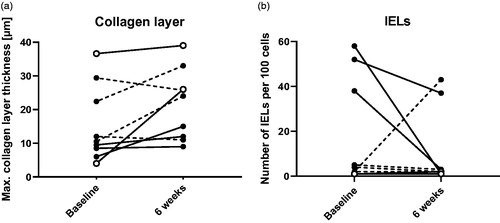
Microscopic characteristics of CC are a thickened subepithelial collagen layer and lymphocyte infiltration. The effect of FMT on collagen layer and intraepithelial lymphocytes (IELs) was assessed in distal colonic biopsies (descending colon, midsection) by immunohistochemistry. The collagen layer was slightly thicker at six weeks (median 24.0 µm, IQR 11.5 to 29.5 µm, p = .054) compared to baseline (10.5 µm, IQR 7.3 to 25.9 µm) (). No statistically significant changes were found between baseline and six weeks in IEL numbers ().
Local effect of FMT on the immune system
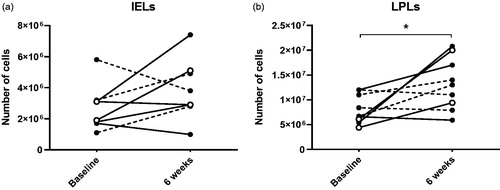
The effect of FMT on colon mucosal T cells was assessed by flow cytometry after isolation of intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs). Isolation of IELs from one patient’s colonic biopsies failed due to technical reasons. Cells of ten colonic biopsies were manually counted using a Bürker counting chamber under the microscope after isolation. No significant differences were found between IEL cell numbers at baseline and six weeks (). LPL cell counts were significantly increased at six weeks (baseline: 6.7 × 106, IQR 5.8 × 106 to 1.2 × 107; six weeks: 1.3 × 107, IQR 8.7 × 106 to 1.9 × 107, p = .034, ). Next, the cells were characterised using flow cytometry. No significant differences in surface markers were found between baseline and six weeks in neither the IELs (Supplementary Figure 1) nor the LPLs (Supplementary Figure 2). The surface and intracellular markers of the clinical responders did not show a distinctive pattern.
Figure 6. Cell counts after isolation of intraepithelial lymphocytes (IELs) (a) and lamina propria lymphocytes (LPLs) (b) from colonic biopsies at baseline and six weeks. * indicates p < .05. Open symbols show the clinical responders, dashed lines show patients who were already in remission at baseline.
Discussion
Exchanging the gut microbiota by FMT is a potential treatment for disorders with a suggested microbial dysbiosis. This is the first pilot study describing the effect of FMT on CC in multiple patients. Repeated FMTs resulted in clinical remission and reduced number of watery stools in a subset of patients, while it had no effect in others. No serious adverse events were observed, and blood markers showed no substantial systemic effects of FMT. However, one patient experienced a flare-up during the study and although an association to the FMT cannot be ruled out, it could also be related to the original MC disease. Faecal calprotectin was not affected. The number of LPLs in colon biopsies was significantly increased after repeated FMTs, indicating a locally occurring immune response. However, flow cytometry analysis did not reveal that FMT resulted in more active or proliferating lamina propria T cells, and IELs were not affected.
This pilot study primarily aimed at investigating the effect of repeated FMTs on symptoms of patients with CC. Overall, no significant decrease in diarrhoea was observed at six weeks, both according to the daily diary and the GSRS; however, GSRS diarrhoea symptom scores decreased significantly at four and eight weeks. A limitation of this study was the low number of patients with active disease at baseline, which made it difficult to assess improvements due to FMT. Four of the patients that were in remission at six weeks did not have an active disease at baseline (based on number and consistency of stools assessed by daily diary), even though they had reported to have more than three stools a day at the screening visit and had previously been diagnosed with CC. This could be due to the fluctuating character of the disease, with flare-up and remission periods succeeding each other. Other reasons could be the unpredictable disease state after ending budesonide treatment, reporting bias, or a placebo effect due to the patients’ anticipation of receiving a potential beneficial treatment.
Out of the five patients that had an active disease at baseline, two were in remission at six weeks. They were defined as responders based on their number of stools at baseline (>3 total stools of which at least 1 watery) and six weeks (<3 total stools of which <1 watery). One of the responders was lost to follow-up after the six-week visit due to unknown reasons, while the other one was still in remission at six months. One additional patient with an active disease at baseline achieved remission at eight weeks, which was maintained until the six-month visit.
FMT treatment led to clinically relevant symptom improvement in a subset of CC patients, especially in the responders. In our study, for all nine patients, GSRS ratings improved from mild or moderate discomfort to minor discomfort whenever significant, showing a clinically relevant improvement. In the future, an identification of the patients who benefit the most from FMT could provide a lead to personalised treatment modalities.
Even though it is still unknown what defines a successful donor, possible donor dependence in FMT studies has been reported previously [Citation10,Citation11,Citation34,Citation35]. In our study, two patients resumed budesonide treatment between eight and 12 weeks as they were not satisfied with the effect of the FMT. These two patients received faecal material from donor A (). Also, the responder that was lost to follow-up after six weeks received faecal material from the same donor (donor A). The other two responders (one at six weeks and one at eight weeks) received faecal material from donor B. This could indicate that the effectiveness of FMT might differ depending on the donor, even though our sample size was too small to draw definite conclusions (two donors for four or five patients each). Future analysis of the microbiome of this patient cohort could give more information about donor-recipient compatibility.
In the present study, three repeated FMTs were administered with two weeks in between. The first FMT was administered into the caecum by whole colonoscopy. The second and third FMT were administered by enema. The enemas did not require prior bowel cleansing, avoiding removal of the new microbiota that potentially colonised after the first FMT. We have chosen to administer the FMTs via the lower gastrointestinal route as this has been shown to be more efficient than the upper gastrointestinal route in UC [Citation36].
Even though there is no evidence that repeated FMTs increase efficacy, it is commonly used in UC. In addition, optimal time points for repeated FMTs are still unknown. In UC, Moayyedi et al. used weekly FMTs for six weeks [Citation10], while Paramsothy et al. used repeated FMTs several times per week for eight weeks [Citation11] and Rossen et al. applied two FMTs with three weeks in between [Citation12]. As a compromise, in this pilot study, we decided to apply a two-week interval for administration of the FMTs. Additionally, repeated FMTs have been shown to be effective for one CC patient in a recent case report [Citation18].
To assess the effect of FMT on the microscopic characteristics of CC, distal colonic biopsies from the midsection of the descending colon were collected at baseline and six weeks. These could be obtained without prior bowel cleansing, which would have affected the newly introduced microbiota. Surprisingly, an increase, albeit non-significant, in collagen layer thickness was found in these biopsies at six weeks, also in the responders.
Even if the role of a thickened subepithelial collagen layer in CC is not completely understood, it has been suggested to contribute to diarrhoea symptoms by, for example, reducing the absorptive capacity of the epithelium [Citation37]. However, this did not seem to be the case in our patient group. In addition, the pattern of CC can vary throughout the large intestine, and the collagen layer thickness in distal parts of the colon is probably not representative for the more proximal parts [Citation2].
We hypothesise that the increase in the number of LPLs indicates that the host mucosa reacted to the introduction of a new microbial ecosystem with a stimulation of the local immune response. This is in line with a recent study in IBS patients, where FMT resulted in local activation of immune-related gene sets of the host mucosa in addition to symptom improvement [Citation16,Citation38].
Although four patients occasionally used loperamide during the course of the study, we do not believe that this influenced the results. Primary and secondary outcome parameters were assessed over longer periods of time (e.g., questionnaires assessed effects over several weeks) rather than at single time points, whereas loperamide intake occurred only on single days and its effect is short-term only [Citation39].
One patient experienced a flare-up of their MC during the course of the study which could be related to the FMT, but could also originate from their MC disease. This flare-up was close to the time of sampling at six weeks where this patient had a slightly thickened collagen layer. However, the thickened collagen layer does not need to be a sign of the flare-up, as also the responders to the FMT treatment had an increased collagen layer at six weeks. For this patient also numbers of IELs and LPLs and F-calprotectin concentrations were altered at some of the time points investigated. However, especially the F-calprotectin concentrations varied rather a lot for most of the patients during the course of the study. Flare-ups have been reported previously for FMT in UC [Citation40,Citation41].
To assess possible modes of action of FMT on MC, flow cytometry analysis was applied to assess the effect of FMT on mucosal immune cell composition in detail. A recently published case report, showing that repeated FMTs resulted in remission of CC in one female patient, also investigated the immunomodulatory effect of FMT by flow cytometry analysis of colonic IELs and LPLs [Citation18]. After the second FMT, a decreased proportion of intraepithelial cytotoxic T cells (CD3+CD8+), decreased proportions of lamina propria activated/memory T-helper (CD4+CD45RO+) and cytotoxic T cells (CD8+CD45RO+), as well as increased lamina propria regulatory T cells (CD4+FoxP3+) were found. Most of these cells were not notably changed after repeated FMTs in our study, however, lamina propria regulatory T cells (CD4+FoxP3+) were also slightly increased in seven of our patients, including the responders, but this did not reach significance [Citation32]. Overall, no large impact of FMT on the mucosal T cell composition was found using flow cytometry analysis. It could be that the effect of FMT is mediated by other immune cells, or that a different mode of action plays a role, such as improved intestinal barrier function [Citation37].
In conclusion, this is the first pilot study to show that repeated FMT is able to improve symptoms in a subgroup of CC patients. Response rates were similar to recent studies investigating FMT in UC (36% of UC patients with active disease obtained clinical remission according to a recent meta-analysis [Citation42]). FMT was shown to be safe in CC patients and did not result in systemic inflammation or other major adverse events, although one patient experienced a flare-up during the course of the study which could be related to the FMT but also to the original disease. FMT resulted in a significantly increased number of LPLs which could give possible insights into the mode of action by initial activation of the local immune system preceeding clinical improvement. Limitations of this pilot study were a low number of patients with active disease at baseline and a rather short follow-up period. Nevertheless, this study provides important information for the design of future clinical trials in MC. Stricter inclusion based on, for example, daily registration of stool patterns, could help to include more patients with active disease. Future trials should of course include a control group. This is especially important in FMT studies, as bowel cleansing and re-infusion of the patient’s own microbiota were shown to affect symptoms as well as the gut microbiota in both UC and IBS [Citation12,Citation16]. In addition, the placebo effect in MC patients is around 15–30% [Citation4,Citation43]. Also, the role of different routes of administration, such as via the upper or lower gastrointestinal tract, should be investigated. It is also of interest to assess whether patients with LC, the other subtype of MC, could benefit from FMT. In addition, future studies, including analysis of patients’ and donors’ gut microbiome, are needed to further investigate possible modes of action of FMT and identify MC patients who are most likely to benefit from FMT, further developing personalised FMT treatments.
Author contributions
SH, JR, JB, EHH, RJB, and JK designed the study; JB and JK recruited patients; SH, JR, JK and RJB collected the data; SH, JR, AKK, GV and JK analysed data and performed statistical analysis; SH, JR and JK drafted the manuscript; SH, JR, JB, AKK, GV, EHH, RJB, and JK interpreted the data and critically revised the manuscript. SH and JR contributed equally. All authors have reviewed and approved the final version of the manuscript.
Supplemental Material
Download MS Word (328.7 KB)Acknowledgements
The authors want to thank Ida Svanerud, research nurse at the Department of Gastroenterology at University Hospital Örebro, for her help with performing the study and collecting samples and Seta Kurt for her help with the flow cytometry.
Disclosure statement
RB received personal fee from Microbiome Expert Board, Ferring A/S outside the submitted work. All other authors have no conflicts of interest to declare.
Additional information
Funding
References
- Munch A, Aust D, Bohr J, et al.; European Microscopic Colitis Group (EMCG). Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012;6:932–945.
- Bohr J, Wickbom A, Hegedus A, et al. Diagnosis and management of microscopic colitis: current perspectives. Clin Exp Gastroenterol. 2014;7:273–284.
- Miehlke S, Guagnozzi D, Zabana Y, et al. European guidelines on microscopic colitis: United European Gastroenterology (UEG) and European Microscopic Colitis Group (EMCG) statements and recommendations. United European Gastroenterol J. 2020. DOI:10.1177/2050640620951905
- Munch A, Bohr J, Miehlke S, et al. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut. 2016;65:47–56.
- Jarnerot G, Tysk C, Bohr J, et al. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449–455.
- Fischer H, Holst E, Karlsson F, et al. Altered microbiota in microscopic colitis. Gut. 2015;64:1185–1186.
- Carstens A, Dicksved J, Nelson R, et al. The gut microbiota in collagenous colitis shares characteristics with inflammatory bowel disease-associated dysbiosis. Clin Transl Gastroenterol. 2019;10:e00065.
- Rindom Krogsgaard L, Kristian Munck L, Bytzer P, et al. An altered composition of the microbiome in microscopic colitis is driven towards the composition in healthy controls by treatment with budesonide. Scand J Gastroenterol. 2019;54:446–452.
- Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493.
- Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109e6.
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228.
- Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118 e4.
- Kootte RS, Levin E, Salojarvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26:611–619e6.
- Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916 e7.
- Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107–2115.
- Holster S, Lindqvist CM, Repsilber D, et al. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin Transl Gastroenterol. 2019;10:e00034.
- Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24.
- Gunaltay S, Rademacher L, Hultgren Hornquist E, et al. Clinical and immunologic effects of faecal microbiota transplantation in a patient with collagenous colitis. World J Gastroenterol. 2017;23:1319–1324.
- Khoruts A, Rank KM, Newman KM, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Gastroenterol Hepatol. 2016;14:1433–1438.
- Louis P, Flint HJ. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol. 2007;73:2009–2012.
- Hjortswang H, Tysk C, Bohr J, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis. 2009;15:1875–1881.
- Svedlund J, Sjodin I, Dotevall G. GSRS-a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134.
- Dimenas E, Glise H, Hallerback B, et al. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30:1046–1052.
- Dimenas E, Glise H, Hallerback B, et al. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–687.
- Roth B, Bengtsson M, Ohlsson B. Diarrhoea is not the only symptom that needs to be treated in patients with microscopic colitis. Eur J Intern Med. 2013;24:573–578.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370.
- Hjortswang H, Jarnerot G, Curman B, et al. The Short Health Scale: a valid measure of subjective health in ulcerative colitis. Scand J Gastroenterol. 2006;41:1196–1203.
- Stjernman H, Granno C, Jarnerot G, et al. Short health scale: a valid, reliable, and responsive instrument for subjective health assessment in Crohn’s disease. Inflamm Bowel Dis. 2008;14:47–52.
- Ludvigsson JF, Myrelid P. Årsrapport SWIBREG: SWIBREG Swedish Inflammatory Bowel Disease Registry; 2015.
- Nyhlin N, Wickbom A, Montgomery SM, et al. Long-term prognosis of clinical symptoms and health-related quality of life in microscopic colitis: a case-control study. Aliment Pharmacol Ther. 2014;39:963–972.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736.
- Kumawat AK, Strid H, Elgbratt K, et al. Microscopic colitis patients have increased proportions of Ki67(+) proliferating and CD45RO(+) active/memory CD8(+) and CD4(+)8(+) mucosal T cells. J Crohns Colitis. 2013;7:694–705.
- Sundin J, Rangel I, Kumawat AK, et al. Aberrant mucosal lymphocyte number and subsets in the colon of post-infectious irritable bowel syndrome patients. Scand J Gastroenterol. 2014;49:1068–1075.
- Wilson BC, Vatanen T, Cutfield WS, et al. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019;9:2.
- König J, Brummer RJ. Faecal microbiota transplantation in IBS — new evidence for success? Nat Rev Gastroenterol Hepatol. 2020;17:199–200.
- Cao Y, Zhang B, Wu Y, et al. The value of fecal microbiota transplantation in the treatment of ulcerative colitis patients: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5480961.
- Burgel N, Bojarski C, Mankertz J, et al. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443.
- Holster S, Hooiveld GJ, Repsilber D, et al. Allogenic faecal microbiota transfer induces immune-related gene sets in the colon mucosa of patients with irritable bowel syndrome. Biomolecules. 2019;9:586.
- Killinger JM, Weintraub HS, Fuller BL. Human pharmacokinetics and comparative bioavailability of loperamide hydrochloride. J Clin Pharmacol. 1979;19:211–218.
- Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164.
- Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019;13:1311–1317.
- Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11:1180–1199.
- Stewart MJ, Seow CH, Storr MA. Prednisolone and budesonide for short- and long-term treatment of microscopic colitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2011;9:881–890.