Abstract
Background and aim
Modern treatment strategies for inflammatory bowel disease (IBD) are postulated to change the natural disease course. Inception cohort studies are the gold standard for investigating such changes. We have initiated a new population-based inception cohort study; Inflammatory bowel disease in South Eastern Norway III (IBSEN III). In this article, we describe the study protocol and baseline characteristics of the cohort.
Methods
IBSEN III is an ongoing, population-based observational inception cohort study with prospective follow-up. Adult and pediatric patients with suspected IBD in the South-Eastern Health Region of Norway (catchment area of 2.95 million inhabitants in 2017), during the 3-year period from 2017 to 2019, were eligible for inclusion. Comprehensive clinical, biochemical, endoscopic, demographic, and patient-reported data were collected at the time of diagnosis and throughout standardized follow-up. For a portion of the patients, extensive biological material was biobanked.
Results
The study included 2168 patients, of whom 1779 were diagnosed with IBD (Crohn’s disease: 626, ulcerative colitis: 1082, IBD unclassified: 71). In 124 patients, there were subtle findings indicative of, but not diagnostic for, IBD. The remaining 265 patients were classified as symptomatic non-IBD controls.
Conclusion
We have included patients in a comprehensive population-based IBD cohort from a catchment population of 2.95 million, and a unique biobank with materials from newly diagnosed and treatment-naïve IBD patients and symptomatic non-IBD controls. We believe this cohort will add important knowledge about IBD in the years to come.
Introduction
In the early nineties, the Inflammatory Bowel disease in South Eastern Norway (IBSEN) study reported the highest incidence of inflammatory bowel disease (IBD) worldwide [Citation1]. The IBSEN cohort, which was considered to be a ‘gold standard’ of population-based inception cohorts at the time, has been followed for more than 20 years and contributed with important data about the natural disease course of IBD [Citation2–7]. However, the study included patients in the pre-biological era and new medications as well as more stringent treatment goals applied today are hypothesized to change the disease course [Citation8].
The current disease classification of Crohn’s disease (CD) and ulcerative colitis (UC) has also been challenged, e.g. as large genotype association studies have indicated that IBD is better classified as a continuum of diseases [Citation9]. New biomolecular mapping of IBD patients combined with comprehensive data on clinical disease course may unravel new disease classifications focusing on disease outcome rather than anatomy. Further, a main challenge in IBD care today is the lack of tools to predict disease course, which could aid individualized treatment and care [Citation10].
To investigate the possible impact of modern IBD management on disease course, study the rapid changes in epidemiology, and explore new and promising diagnostic and prognostic factors that can be the basis of future precision medicine in IBD, we have initiated a new population-based IBD cohort study; Inflammatory Bowel Disease in South-Eastern Norway III (IBSEN III). Within this cohort study we have included a control group of symptomatic patients without pathological findings. The study protocol and cohort’s baseline characteristics are presented here.
Methods
Design and study population
IBSEN III is a population-based observational inception cohort with prospective follow-up (Clinical Trials ID: NCT02727959). Newly diagnosed IBD patients and symptomatic non-IBD controls in the South-Eastern Health Region of Norway were included during the 3-year period from 2017 to 2019.The South-Eastern Health Region is the largest health region in Norway, constituting a catchment area of approximately 2.95 million inhabitants (56% of the Norwegian population) in 2017 (). Based on the latest IBD incidence data in Norway [Citation11] (assumed minimum incidence), and recently published incidence data from Sweden [Citation12] and Finland [Citation13] (assumed maximum incidence) we anticipated 531–876 eligible patients with CD and 1134–2196 eligible patients with UC. This would yield an estimated cohort of 1665–3072 newly diagnosed IBD patients.
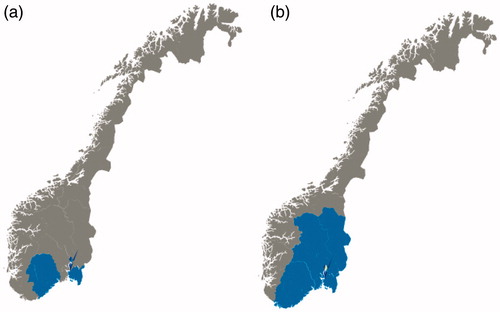
Figure 1. The geographical areas of inclusion of patients to the population-based inception cohort studies (a) IBSEN, recruited from the counties of Oslo, Østfold, Telemark and Aust-Agder during 1990–1994, and (b) IBSEN III, recruited from the counties of Agder (previously Aust-Agder and Vest-Agder), Innlandet (previously Oppland and Hedmark), Oslo, Vestfold and Telemark, and Viken (previously Buskerud, Akershus and Østfold), corresponding to the South-Eastern Health Region of Norway, during 2017–2019.
Setting
In Norway, there is free access to healthcare for all inhabitants. Patients with symptoms contact their general practitioner and are referred to the secondary health service when needed. All pediatric IBD patients and the large majority of adult IBD patients are diagnosed and receive their first treatment at public hospitals. There are a few private gastroenterology centers, e.g. eight in the South-Eastern Health Region in the inclusion period.
Inclusion process
All general practitioners and the private gastroenterology centers in the South-Eastern Health Region were informed of the ongoing study by letter and invited to refer all patients with symptoms and clinical findings suspicious of IBD to their local hospital. Individuals with suspected IBD based on referral letters were invited to participate in the study. Patients who fulfilled internationally accepted diagnostic criteria (Lennard-Jones criteria for adults [Citation14], revised Porto criteria for children [Citation15]) after initial diagnostic work-up were included as definite IBD cases. Patients with subtle findings indicative of IBD [on endoscopy or magnetic resonance imaging (MRI)], but who did not meet full diagnostic criteria, were also included and classified as ‘suspicion of small bowel IBD’ or ‘suspicion of large bowel IBD’. Patients with symptoms of IBD, but without endoscopic or histologic signs of inflammation, were classified as ‘symptomatic non-IBD controls’. These patients remained in the study as a control group. Exclusion criteria were other causes of acute or chronic bowel inflammation, i.e. infectious colitis, radiation colitis, diversion colitis, solitary rectal ulcer syndrome, graft versus host disease, diverticular colitis, medication associated colitis, ischemic colitis, microscopic colitis, and enema associated colitis.
Data collection and biobanking
The IBSEN III study design included two modules (). All hospitals performed Module 1, which included comprehensive clinical, endoscopic, demographic, and patient-reported data, as well as fecal samples for analyses of calprotectin and microbiota, before start of treatment. At the first ileocolonoscopy, biopsies were collected from six predetermined bowel segments (ileum, colon ascendens, transversum, descendens, sigmoideum and rectum) and additionally from the coecum in pediatric cases. In patients with diagnosed or suspected CD, systematic recording of early small bowel MRI was conducted. Analyses of routine blood samples and stool cultures were performed at the local laboratories as part of routine follow-up and the results were registered. The three largest hospitals in the region (Oslo University Hospital, Akershus University Hospital and Vestfold Hospital) also performed Module 2, which included extensive additional biobanking and additional PROMs in both incident IBD cases as well as symptomatic non-IBD controls. The collected data were based on reports from the patients, evaluations from the treating clinician, and information registered in the electronic patient records. All data were entered directly into an electronic clinical research form (CRF) system (Viedoc©, PCG Solutions AB, Uppsala, Sweden), including an internet-based system for patient-reported outcome measures (PROMs) (ViedocMe).
Table 1. Overview of data and biologic material collected in the IBSEN III study.
Prescheduled clinical follow-ups with the same systematic and comprehensive registration of data are planned at 1 and 5 years after diagnosis. After 3 and 6 months, and after 3 years, patient-reported data and fecal samples are collected (). The Standard Operation Procedures of IBSEN III (in Norwegian) are added as Supplemental Material.
Definitions
Disease phenotype, location, and extent were evaluated according to the Montreal classification [Citation16] at time of diagnosis, and re-evaluated at each clinical follow-up. Change in phenotype at 1-year follow-up was defined by the appearance of newly affected segments or complications (stenosis or fistula) after the initial diagnostic work-up. Disease relapse was defined as symptoms of IBD that led to changes in the treatment. Numbers of relapses were registered and ‘chronic symptoms without remission’ during the observed periods were also registered. Local complications were defined as stenosis, fistula and/or abscess. Number of hospital admissions and total number of admitted days due to IBD were registered. Surgeries were registered as numbers of events, type of surgery (appendectomy, colectomy, segmental resection of colon, small bowel resection, perianal drainage of abscess, perianal fistula surgery), and for colectomy, its indication (acute severe colitis, refractory colitis, dysplasia/cancer). IBD-relevant medication (including elemental diet) was registered with the relevant drug and number of months during which the drug was used. The reason for stopping a biological drug was registered as ‘development of antibodies’, ‘side effects’, ‘non-response without antibodies’, or ‘other’. Curves visually illustrating disease course were also adapted from the IBSEN study [Citation2].
Registries
The unique personal identification number of Norwegian citizens provides the opportunity to link the clinical cohort to several nationwide health registries and population-based health studies. Linkage to the Norwegian Patient Registry will enable evaluation of completeness of the cohort and increase data quality. Linkage to other health registries will also be performed in substudies of IBSEN III investigating topics such as mortality, cancer risk, and socioeconomic impact ().
Table 2. Overview of National registries and databases to be linked with the IBSEN III study.
Statistics
Continuous data are presented as median with ranges, categorical data as counts and percentages. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (Armonk, NY).
Ethical considerations
The IBSEN III study was approved by the South-East Regional Committee for Medical and Health Research Ethics (Ref 2015/946-3). All patients signed an informed consent form before participating in the study. For patients younger than 18 years, parents/legal guardians signed the informed consent form. Patients 16 or 17 years of age co-signed together with their parents/legal guardians.
Results
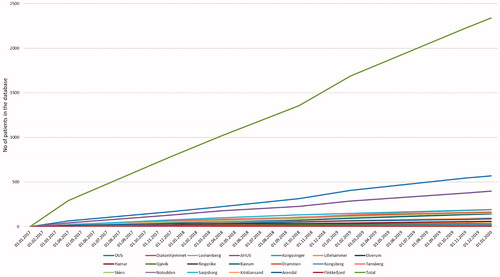
A total of 2168 patients were included in the study. A flow chart of included patients is presented in . A total of 1779 patients were diagnosed with IBD: 626 patients with CD, 1082 patients with UC and 71 patients with IBD unclassified. In 124 patients, there were subtle findings indicative of, but not diagnostic for, IBD: 76 suspicious of small bowel IBD and 48 suspicious of large bowel IBD. The remaining 265 patients were classified as symptomatic non-IBD controls. The inclusion rate was stable at approximately 63 patients per month (). Only one of the hospitals (catchment area of approximately 83 000 in 2017, 2.8% of the South-Eastern Health Region) failed to include any patients.
Figure 2. Flow chart of patients included in the study. Diagnosis of inflammatory bowel disease was set based on Lennard-Jones criteria for adults [Citation14] and Porto criteria for children [Citation15].
![Figure 2. Flow chart of patients included in the study. Diagnosis of inflammatory bowel disease was set based on Lennard-Jones criteria for adults [Citation14] and Porto criteria for children [Citation15].](/cms/asset/eccb34de-3105-47c0-93ea-93f4357484d8/igas_a_1922746_f0002_c.jpg)
Figure 3. Number of included patients during the study period, from each center and total inclusion rate.
During the study period, 373 patients with newly diagnosed or suspected IBD did not consent (i.e. were not asked or declined) to inclusion in the IBSEN III study. Due to ethical regulations, we were not allowed to collect any further information on this group of patients.
Baseline characteristics of the patients included in the cohort are shown in . In , we present an overview of biological material collected from baseline in the IBSEN III biobank subgroup (Module 2), including the number of fecal samples collected from the entire cohort at baseline.
Table 3. Baseline characteristics of patients included in the IBSEN III cohort study.
Table 4. Overview of collected biobank material at baseline.
Discussion
We present a new population-based inception cohort of IBD patients, the IBSEN III cohort. This large-scale, unselected and treatment-naïve study population is optimal for studies of IBD disease course in the biological era as well as prognostic studies.
In the nineties, the original IBSEN study was considered to represent the ‘gold standard’ of population-based cohorts. Since then many large-scale, well-characterized cohorts have been developed, all with their strengths and limitations [Citation17–20]. The IBSEN III study has many advantages compared to other IBD cohorts. Norway has a stable, traceable population combined with a public health care system. This allows for inclusion of a large cohort of newly diagnosed and treatment naïve patients from a well-defined geographical area. The population-based design of the IBSEN III study, including both adult and pediatric cases, increases the potential generalizability of the study results. Based on previous studies of incidence in similar Nordic populations and countries, we estimated 531–876 eligible patients with CD and 1134–2196 eligible patients with UC, resulting in a predicted cohort of 1665–3072 after three years’ inclusion. The cohort size of 1779 newly diagnosed IBD patients, and additionally the 373 patients, who did not consent to inclusion, indicates that the cohort size is within the lower range of this estimate. To assess the completeness of the cohort, we will link data from the Norwegian Patient Registry and compare the number of incident patients in this registry within the corresponding geographical area and inclusion years, with the number of included patients. Validation of correct diagnoses in the Norwegian Patient Registry has been performed with other diagnoses [Citation21], but not within IBD. However, a Swedish study found high validity of IBD diagnoses within the Swedish Patient Registry [Citation22].
The first IBSEN study generated important data on IBD’s natural disease course [Citation2,Citation3], prognostic factors for disease course [Citation4–6], and risk of cancer and mortality [Citation7]. However, the IBSEN study included patients diagnosed in the pre-biological era (early nineties) and the introduction of biologics is postulated to change the disease course of IBD. The IBSEN III cohort is recruited from the same geographical area and followed with the same study design and corresponding or improved methods as the first IBSEN study. This will generate comparable data from both cohorts in general, and will also allow for analyses of the possible disease-modifying effects of biologics and immunomodulators on disease course.
The era of multi-omics proposes a future treatment strategy based on diagnostic, prognostic and predictive models [Citation23]. The IBSEN III cohort is thoroughly characterized, both clinically and biochemically. We expect that results from further biomolecular mapping of the cohort can identify diagnostic markers and possibly a new biomolecular-based disease classification, as well as predictors of disease outcome.
We have included a symptomatic non-IBD control group in the present study. We will also continue to follow patients with possible IBD but with subtle findings that did not meet the diagnostic criteria. Prospective follow-up including re-evaluation of the diagnosis will provide the opportunity to study diagnostic and prognostic markers in these patients.
Limitations
To include all newly diagnosed IBD patients in the region was challenging and selection bias cannot be excluded. We are aware of almost 400 patients that were considered for inclusion but not invited or declined inclusion. The final diagnosis of these patients is not known to us. The sensitivity of the inclusion will be evaluated by comparison of IBD cases in the Norwegian Patient Registry as described above. Also, the study was designed with ‘other causes of acute or chronic bowel inflammation’ as an exclusion criterion, and we only kept the symptomatic patients without any findings in our control group. As one of our aims is to increase diagnostic precision, this might turn out to be a limitation in further studies.
The study is investigator-initiated, was designed with limited resources, and relies on the efforts of many healthcare workers in 19 hospitals. Implementation had to be adapted to local procedures. Three-month and six-month follow-ups are therefore not performed as clinical visits. The patients received text messages that reminded them to answer questionnaires and deliver fecal samples. This approach may have led to reduced response rates at the 3- and 6-month follow-ups.
Due to differences in disease severity at onset, some patients were unable to deliver fecal samples, and in some cases other biological material as well, before start of treatment. Therefore, not all of the biobanked material is collected from treatment-naïve patients. Still, the quantity of biological material from purely treatment-naïve patients is substantial and will generate important future study results.
The IBSEN III study has also been affected by the COVID-19 pandemic. In the period from March to June 2020, most of the scheduled 1-year follow-up visits had to be postponed or cancelled. We find it likely that the most severe cases were prioritized for clinical follow-up during this period, thus this might have introduced bias. Luckily, this was only relevant for a minor group of patients included in 2019, and as of autumn 2020 1-year follow-ups seem to be running normally again.
Conclusion
With limited resources, we have built a comprehensive population-based cohort from a catchment population of 2.95 million, and a unique biobank with materials from newly diagnosed and treatment-naïve IBD patients and symptomatic non-IBD controls. This cohort will increase knowledge about IBD in the years to come.
Author contributions
Study conception: MLH. Study design: MLH, VAK, RO, GP, GHH, SB, PR. Collection of data: All authors. Statistical analyses, interpretation of data and draft of the manuscript: VAK, MLH. Critical revision of the manuscript for intellectual content: All authors. Approval of the final manuscript: All authors.
Supplemental Material
Download PDF (467.9 KB)Acknowledgements
The authors thank all the study nurses and local study personnel who have contributed to the inclusion of patients in the IBSEN III study. We also thank the Clinical Trial Unit, Division of Medicine, Akershus University Hospital, and Unger-Vetlesen Institute, Department of Medicine, Lovisenberg Diaconal Hospital, for their contributions to the collection and handling of the biobank material.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Moum B, Vatn MH, Ekbom A, et al. Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996;31(4):362–366.
- Solberg IC, Lygren I, Jahnsen J, IBSEN Study Group, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44(4):431–440.
- Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438.
- Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133(2):412–422.
- Solberg IC, Cvancarova M, Vatn MH, et al. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn's Disease (the IBSEN Study). Inflamm Bowel Dis. 2014; 20:60–68.
- Kristensen VA, Cvancarova M, Hoivik ML, et al. Serological antibodies and surgery in a population-based inception cohort of Crohn's disease patients - the IBSEN study. Scand J Gastroenterol. 2020;55(4):436–441.
- Hovde O, Kempski-Monstad I, Smastuen MC, et al. Mortality and causes of death in Crohn's disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63(5):771–775.
- Colombel JF, D'Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–266.
- Cleynen I, Boucher G, Jostins L, International Inflammatory Bowel Disease Genetics Consortium, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387(10014):156–167.
- Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017;38(2):127–142.
- Moum B, Ekbom A, Vatn MH, et al. Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in south eastern Norway. Gut. 1997;40(3):328–332.
- Sjoberg D, Holmstrom T, Larsson M, et al. Incidence and clinical course of Crohn's disease during the first year - results from the IBD Cohort of the Uppsala Region (ICURE) of Sweden 2005-2009. Journal of Crohn's & Colitis. 2014;8(3):215–222.
- Jussila A, Virta LJ, Kautiainen H, et al. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: a nationwide register study in Finland. Inflamm Bowel Dis. 2012;18(3):555–561.
- Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.
- Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795–806.
- Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753.
- Chaparro M, Barreiro-de Acosta M, Benítez JM, EpidemIBD study group of GETECCU, et al. EpidemIBD: rationale and design of a large-scale epidemiological study of inflammatory bowel disease in Spain. Therap Adv Gastroenterol. 2019;12:1756284819847034.
- Pittet V, Juillerat P, Mottet C, Swiss IBD Cohort Study Group, et al. Cohort profile: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol. 2009;38(4):922–931.
- Burisch J, Cukovic-Cavka S, Kaimakliotis I, et al. Construction and validation of a web-based epidemiological database for inflammatory bowel diseases in Europe an EpiCom study. Journal of Crohn's & Colitis. 2011;5(4):342–349.
- Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149(10):916–924.
- Benjaminsen E, Myhr KM, Grytten N, et al. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
- Jakobsson GL, Sternegård E, Olén O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–221.
- Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–361.

