Abstract
Objectives
To understand current thinking and clinical decision-making in the treatment and management of patients with mild-to-moderate ulcerative colitis (UC).
Methods
This multinational, survey-based study was conducted in 2021. Two meetings were held, involving 11 IBD specialists, that used a series of questions and discussion to identify all factors possibly related to the management of UC. The importance of identified factors was assessed using an online questionnaire covering three scenarios – active disease, remission and patient empowerment. Each factor was scored on a scale of 0 (very-unimportant) to 100 (very-important) within each scenario, by a separate group of healthcare professionals working in IBD.
Results
A total of 157 individual factors were identified by the 11 IBD specialists and scored in the three scenarios by 56 respondents (52; 93% specialist gastroenterologists) from Europe and North America (25; 45%), South America (19; 34%) and the Middle East, Asia and Australia (12; 21%). For all scenarios, factors related to educating patients regarding UC and its treatment and understanding of patient goals ranked highest, ahead of clinical considerations regarding disease activity and treatment history. Setting realistic short-term treatment targets was a key consideration. 5-ASA optimisation and use of faecal calprotectin monitoring were core strategies across the three scenarios tested. Support for patients during longer-term management of their disease, starting from initial flare, was an important recurring theme.
Conclusion
The current management approach for mild-to-moderate UC was found to be guided primarily by the patient’s perspectives and goals, alongside assessment of their medical and disease history.
Introduction
Ulcerative colitis (UC) typically follows an episodic disease course with flares, characterised by diarrhoea, rectal bleeding and urgency, followed by periods of remission; although some patients suffer from persistent disease [Citation1–3]. The management approach is determined by the severity, extension and pattern of disease [Citation4,Citation5]. For mild-to-moderate disease, which represents most patients, 5-aminosalicylic acid (5-ASA) has been demonstrated to be effective for induction and maintenance of remission [Citation6–8] and is recommended as the first-line treatment [Citation4,Citation5]. Whilst symptomatic control remains a primary goal of treatment, it is now recognised that achieving sustained control of inflammation with mucosal healing, and possibly histological remission, should be prioritised [Citation2,Citation9–11]. This recognition has driven a shift towards a treat-to-target approach, which combines regular monitoring and therapy adjustment to achieve agreed treatment goals, based on symptom control, biomarker normalisation (e.g., C-reactive protein; faecal calprotectin), patient quality of life and endoscopic remission [Citation9,Citation11–13].
Achieving more ambitious therapy goals makes it vital for clinicians to optimise the use of 5-ASA therapy and promptly to identify patients who might benefit from escalating to more intensive treatment [Citation14,Citation15]. A survey from 10 years ago found that, while Spanish IBD specialists more often used 5-ASA optimisation than general gastroenterologists, this approach was often under-utilised when managing patients with mild-to-moderate UC [Citation16]. In order to understand current thinking and decision-making, detailed assessment of priorities in decision-making was undertaken with healthcare professionals experienced in managing patients with mild-to-moderate UC.
Methods
Study design
A survey-based study was conducted in two stages during 2021. The aim of part one was to identify all the factors potentially relating to the management of patients with UC, covering diagnosis, relapse, remission and patient involvement in their own care. This was accomplished by holding two online meetings (4 May and 1 June), with 11 locally practicing IBD specialists. The first meeting was attended by IBD specialists from the United Kingdom (S. P. L. T. – Chair), Australia (J. B.), Austria (A. M.), Belgium (E. L.), South Korea (J. H. C.) and the United Arab Emirates (S. A. A.); and the second meeting by attendees from the United Kingdom (S. P. L. T. – Chair), Canada (N. N.), Colombia (J. R. M.), Germany (A. D.), Poland (G. R.) and Portugal (F. M.). At each meeting, five questions designed to stimulate thoughts about the management of UC in different but complementary situations were presented (Supplementary File 1) and responses gathered in sequence. Participants were initially given 5–10 minutes to consider their responses to each question and then took turns to read out their answers in order to stimulate further thoughts from the other attendees. The aim was to capture an exhaustive list of responses – data saturation – on the current management of UC after having gone through all five questions. All responses were video recorded and documented. The results from each of the meetings were collated, compiled together into a list of distinct factors and used to construct a structured questionnaire, which constituted the second part of the study.
The remit of the second-stage questionnaire was objectively to assess the importance and contribution of each of the factors identified during the online meetings, by a wide range of healthcare professionals from different countries, when considering three defined scenarios:
When your patient presents with active mild-to-moderate UC.
When your patient achieves remission following a mild-to-moderate UC flare.
Self-management and empowerment of patients with mild-to-moderate UC (Supplementary File 2).
Each factor was scored on an end-anchored analogue scale from zero (very unimportant) to 100 (very important) within that particular scenario. The option to score any factor as ‘not relevant’ was also provided. The order of the individual factors was randomised for scoring within each scenario in order to minimise (unintended) rationalisation of responses.
The questionnaire was available in English on a secure online website (open 27 August–5 October), a link to which was distributed by the authors to healthcare professionals (HCPs) working in IBD within their respective countries. Demographic details including country of practice, position (job title), years’ experience in gastroenterology, time spent managing patients with IBD and medicines prescribed for mild-to-moderate UC were also captured as optional fields on the questionnaire. A target of 50 completed questionnaires was set, with previous experience showing that 25 was the minimum number required to yield statistical significance for differences between two analogue scale points [Citation17].
Analysis of questionnaire
Questionnaire responses were analysed to address three key questions:
What are the common factors influencing decisions regarding management of mild-to-moderate UC across the three scenarios being explored?
How does decision-making differ between the three scenarios?
How do the different factors fit together in a decision network?
Question 1 was addressed by principal component analysis. Varimax rotation was performed to enhance the separation of the three scenarios. Mean scores with SDs were calculated univariately for each factor overall and on a per scenario basis. Question 2 was addressed using linear discriminant function analysis (DFA) which defined components (mathematical functions) that created maximum discrimination between the scenarios. These components were centred in decision domains (groups of factors strongly related to one scenario but not the others) related to each scenario. The strength of the relationship between a factor and its scenario was indicated by a loading score; the further from zero the score was, the more closely related it was to the scenario at the corresponding pole of the DFA (for the purposes of the analysis the poles were assigned positive and negative, but this has no bearing on interpretation of results). Question 3 was addressed using a form of hierarchical cluster analysis, in which individual items with mean scores, which were one SD above 50 (for positive associations) or below 50 (for negative associations) within any treatment scenario, were assembled and stacked hierarchically according to these scenario associations and their decreasing mean scores within these associations. These hierarchical stacks (in the form of a dendrogram) were then used to construct a hypothetical decision environment based on the association (or lack thereof) of individual factors to each scenario. This provides insight into which factors were consistently important in decision-making versus those that were only important (or not important) in a particular circumstance/scenario. To aid interpretation, factors were grouped by general topic; there was no selection of factors by the authors.
All questionnaires were analysed after replacing any missing data. For all analyses, missing data were handled using the individual item mean for each treatment scenario. Factors identified as ‘not relevant’ were fulfilled with a value of 50, which set these factors as neutral during subsequent analysis. The data from an intercept on an end-anchored analogue line are, by definition, parametric, as the values are continuous and on a linear scale. All analyses were carried out using SPSS for Windows 15.0 (IBM Corporation, New York, NY) and Excel 365 (Microsoft Corporation, Washington, DC).
Results
Stage 1 – online meetings
From the two meetings, a total of 157 individual factors were identified as potentially relating to the management of patients with UC (Supplementary File 2). The factors included broad areas, such as patient history and disease progression, use of medications, disease monitoring, therapeutic goals, communication with other healthcare professionals, and patient interaction and communication.
Stage 2 – questionnaire
A total of 56 questionnaires were completed before the survey was closed. Feedback and analytics indicated that the questionnaire typically took 45‒60 minutes to complete. The dispersion of questionnaire data was calculated as mean 68.9 and SD 68.8, indicating a good spread of responses on the end anchored scale.
The large majority (52; 93%) of respondents were specialists in gastroenterology/IBD, with over half (33; 59%) having over 10 years’ experience working in the area (Supplementary File 3). Approximately two-thirds of respondents (38; 68%) spent at least 25% of their clinic time managing patients with IBD, with most (47; 89%) practicing in an Academic centre. All respondents (100%) had prescribed oral 5-ASA to their patients with mild-to-moderate UC, 93% topical 5-ASA, 72% budesonide MMX, 40% other topical steroids, 55% systemic steroids and 59% biologics. Nine countries were represented in the survey, with 25 (45%) questionnaires received from Europe and North America, 19 (34%) from South America and 12 (21%) from the Middle East, Asia and Australia.
Common factors influencing decision-making across the three scenarios
A number of common factors were identified that contributed most strongly to clinicians’ considerations and decision-making in their patients with mild-to-moderate UC across all three scenarios ( and Supplementary File 4). Factors related to educating patients regarding UC and its treatment and engaging with patients to understand their previous experience and goals were ranked highest, ahead of more clinical considerations regarding disease activity, response to treatment (both current and historic) and recognition of the role of 5-ASA treatment. This was corroborated in the univariate analyses, wherein 9/10 most highly scored factors were associated with patient education, engagement and consideration of their goals and priorities (mean scores 79.9‒82.1; SD 19.8‒23.4; ).
Table 1. Top 25 factors contributing most strongly to clinical decision-making across all three scenarios in multivariate analysis.a
Table 2. Top 10 factors contributing most strongly to clinical decision-making across all three scenarios in univariate analysis.
Differences in decision-making between the three scenarios
Clinical decision-making related to active disease was found to have several distinct differences to that associated with remission and patient empowerment, which were much more closely aligned ( and Supplementary File 5). The decision domain relating to active disease was focussed on avoiding unnecessary escalation of treatment by not targeting (at least initially) overly ambitious goals and fully assessing the patient to ascertain underlying remission status, disease severity and potential exacerbating factors; whereas, the other two scenarios showed a strong focus on the optimisation of long-term therapy in line with disease characteristics alongside consideration of the challenges of ensuring compliance in the maintenance setting.
Table 3. Top 10 factors contributing most strongly to differences in clinical decision-making between active disease and the other two scenarios in multivariate analysis.a
Separation of the ‘empowerment and self-management’ and ‘remission following flare' scenarios was driven by a greater focus on supporting the patient in identifying and attaining realistic treatment goals via education and communication in the former scenario ( and Supplementary File 5). This compared to a strong focus on the role of oral 5-ASA dose escalation alongside the use of faecal calprotectin monitoring and consideration of disease and treatment history to inform treatment decisions.
Table 4. Top 10 factors contributing most strongly to differences in clinical decision-making between self-management and empowerment of patients and remission in multivariate analysis.a
In univariate analysis, the most important factors for managing active disease reflected key themes associated with assessment of disease activity and severity, optimisation of 5-ASA therapy and engaging with the patient to support adherence and the success of maintenance following control of the disease flare (Supplementary File 6). For patients in remission, the focus on education expands to include a more long-term perspective that encompasses helping them manage and maintain their quality of life, understand their therapy options and have a clear idea of when to re-engage with healthcare. Clinical factors considered were related to ensuring that a patient continues to receive appropriate therapy during the maintenance period. The factors associated with the self-management scenario align with patient education and helping them to understand their therapeutic options.
Overall decision network
Factors associated with patient education, communication and support for their long-term involvement in the management of their disease, as well as information on frequency of relapse and disease extent, were identified as overarching considerations across all three scenarios ( and Supplementary File 7). Clinical thinking then coalesced around two groups of factors, all of which were relevant to active disease, whilst highlighting some divergence in thinking for the maintenance and patient empowerment scenarios. For patients with active disease and those in remission, these considerations were related to assessment of remission, treatment history and preparing the patient for ongoing monitoring. For active disease and patient empowerment, treatment history and disease assessment were also important, but patient-related factors, such as promoting involvement in their care and adherence, were key considerations. In addition, the concept of optimisation of 5-ASA therapy was a prominent part of clinical thinking.
Figure 1. Overall decision environment in mild-to-moderate UC*. *Hierarchical cluster analysis. The full list of factors associated with each level of the analysis are available in Supplementary File 7. GP: general practitioner.
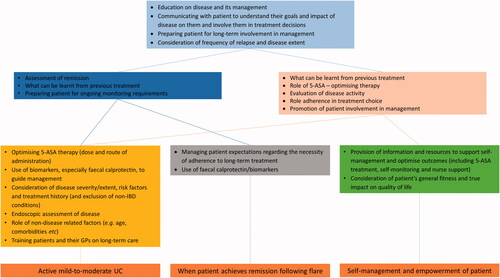
The decision network next moved onto the factors more directly related to each scenario. When considering active disease, clinical factors broadened to include consideration of non-IBD related conditions and co-morbidities together with a more detailed clinical picture of the patient’s UC including risk factors. Optimisation of 5-ASA by dose and route of administration and use of biomarkers, such as faecal calprotectin, were primary therapy considerations. The training of patients and their primary care physicians on long-term care of UC was seen as a key component of management at this stage even before achievement of remission. The factors specifically associated with maintenance of remission were more tightly concentrated around managing patients’ expectations, particularly the importance of adherence to therapy in the longer term and the use of faecal calprotectin for monitoring. Specific considerations for patient self-management and empowerment included the provision of education, information and resources about UC as well as their general health including access to nurse-led support.
Discussion
Our study has provided novel insights into clinical decision-making in the management of mild-to-moderate UC. Across the three defined scenarios – active disease, remission and patient empowerment – it was found that factors related to patient education regarding disease and treatment options and establishing/accounting for patient goals and perspectives were pre-eminent considerations when deciding upon treatment and management. Whilst this might seem surprising at first, it probably reflects the paucity of disease-specific biomarkers in UC compared to diseases such as diabetes with HbA1c [Citation18,Citation19], for example, and, therefore, the need for a more holistic approach to management. It also highlights the interest in faecal calprotectin as a biomarker and why this has had an increasingly prominent role in the management of both active UC and remission, as shown in this survey and in clinical practice [Citation20,Citation21].
The key clinical considerations identified to guide treatment and management were, as might be expected, multifactorial and included disease characteristics (severity, extent, etc.) and risk factors, along with response to past and current treatment. Importantly, the highest-ranking factor when considering active disease was not to consider histologic remission as the primary treatment goal. This aligns with the recent STRIDE II consensus [Citation13] which positions histologic healing as a supplementary long-term treatment target that is secondary to the achievement of symptomatic response and remission, followed by endoscopic healing accompanied by normalised function and quality of life. Whilst treatment goals in UC are undoubtedly becoming more ambitious, this survey highlights how clinicians are still aligning and agreeing treatment goals with patients that are achievable in the first instance.
To achieve and maintain remission, there was a strong focus on optimising 5-ASA therapy before escalating to other therapies. Interestingly, 59% of respondents had reported prescribing biologics in mild-to-moderate UC, so such strong support for 5-ASA optimisation indicates a shifting attitude towards maximising use of initial therapy, in the context of more ambitious long-term therapy goals. Optimisation included using oral and topical therapy (‘top and tail’) as well as higher doses in both induction and maintenance therapy. This thinking aligns with evidence from a meta-analysis that high dose (≥3.3 g/day) and combined oral/topical 5-ASA were significantly superior to standard dose 5-ASA (1.7‒3.2 g/day) and low dose 5-ASA (≤1.6 g/day) for inducing remission and preventing relapse [Citation22]. An important factor supporting the optimisation strategy was the use of faecal calprotectin to guide maintenance dosing of 5-ASA. Patient-led management of 5-ASA dose was also identified as a key contributor to patient empowerment. Optimisation of 5-ASA therapy can include switching patients to once daily from divided doses, which have been shown to be equally effective [Citation6,Citation23]. Reducing the dosing frequency has been associated with improved adherence [Citation4,Citation24], with adherence to therapy shown to be another salient theme in decision-making.
Preparing patients for the demands of a chronic disease and long-term therapy alongside provision of education and support was found to be an important feature of management throughout all scenarios. This patient-centric approach to management is encouraging when considering reports that patients often feel under-educated regarding their disease and treatment options [Citation25]; that patient quality of life is often under-discussed in IBD [Citation26] and that good patient-HCP communication is a key determinant of good care [Citation27]. Indeed, active patient involvement in decision-making has been recognised to be of high value in the management of mild-to-moderate UC [Citation28], with data demonstrating that management approaches that directly engage with the patient can improve adherence and treatment outcomes [Citation29,Citation30]. The increasing use of e-health and remote monitoring systems, particularly since COVID [Citation31], provide important engagement and feedback to patients and can help their sense of control and empowerment. Nurse-led support was another important requirement raised in the survey. IBD nurses are often the first point of contact for education, advice and support for patients and their value is perhaps best demonstrated in a recent single centre study which reported a halving of hospital admissions (28 versus 56; p = 0.002) in the year following appointment of an IBD nurse [Citation32].
When considering the limitations of this survey, it should be recognised that respondents to the questionnaire were mostly consultants/specialists (93%), practising at Academic centres (89%), with over half (59%) having over 10 years’ experience working in gastroenterology. Hence, the results reflect the decision-making and thinking of experienced clinicians working at large centres and might not necessarily reflect the situation with more general gastroenterologists working in smaller IBD services. The scope of the survey in terms of geography (covering Europe, North and South America, the Middle East, Asia and Australia) does, however, suggest a conformity in management practices amongst the respondents. Another limitation was that the questionnaire was available only in English, so some subtle differences in interpretation of the factors might have occurred, although the authors felt that this introduced less bias than those potentially introduced by multiple translations. Finally, as with any survey-based study, those that were willing to complete a long (≥45 min) questionnaire might not reflect the general population of practitioners. The use of two meetings to generate a list of factors and then to score them for importance, rather than to conceive a fixed number of questions, as well as randomisation of the 157 factors within the three scenarios helped mitigate any tendency to rationalise responses, which is known to introduce bias in surveys [Citation33].
The current management approach for mild-to-moderate UC adopted by experienced clinicians was found to be guided by the patient’s perspectives and goals, as well as assessment of their medical and disease history. Optimisation of 5-ASA was considered a central tenet of this management approach as was providing patients with long-term support. It is hoped that the detailed exposition of how experienced IBD clinicians make their clinical decisions provided herein will support and guide general gastroenterologists and community doctors operating outside specialist services to optimise their practice when managing patients with mild-to-moderate UC. To further support this process, these insights should also be used to help inform future educational initiatives and further define best practice as we move towards the era of personalised medicine in IBD.
Supplemental Material
Download PDF (1.7 MB)Disclosure statement
A. D. has received fees for participation in clinical trials, review activities, such as data monitoring boards, statistical analysis, end point committees from Falk, Abbvie, Janssen, Gilead and Pfizer; consultancy fees from Abbvie, MSD, Ferring, Roche/Genentech, Takeda, Vifor, Pharmacosmos, Boehringer-Ingelheim, Gilead, Galapagos, Falk, Janssen, Pfizer, Sandoz/Hexal, BMS/Celgene, Tillotts, Amgen and Fresenius Kabi; payment from lectures including service on speakers bureaus from Falk Foundation, Ferring, MSD, Abbvie, Vifor, Janssen, Pfizer, Tillotts, Takeda, Gilead/Galapagos; payment for development of educational presentations from Tillotts and Ferring.
S. P. L. T. has received Grants/Research Support from: AbbVie, Buhlmann, Celgene, IOIBD, Janssen, Lilly, Pfizer, Takeda, UCB, Vifor, and Norman Collisson Foundation; Consulting Fees from: Abacus; AbbVie; Actial; ai4gi; Alcimed; Allergan; Amgen; Aptel; Arena; Asahi; Aspen; Astellas; Atlantic; AstraZeneca; Barco; Biocare; Biogen; BLPharma; Boehringer Ingelheim; BMS; Buhlmann; Calcico; Celgene; Cellerix; Cerimon; ChemoCentryx; Chiesi; CisBio; ComCast; Coronado; Cosmo; Ducentis; Dynavax; Elan; Enterome; Equillium; Falk; Ferring; FPRT Bio; Galapagos; Genentech/Roche; Genzyme; Gilead; Glenmark; Grunenthal; GSK; GW Pharmaceuticals; Immunocore; Immunometabolism; Indigo; Janssen; Lexicon; Lilly; Medarex; Medtrix; Merck; Merrimack; Millenium; Neovacs; Novartis; Novo Nordisk; NPS-Nycomed; Ocera; Optima; Origin; Otsuka; Palau; Pentax; Pfizer; Pharmaventure; Phillips; P&G; Pronota; Proximagen; Resolute; Robarts; Sandoz; Santarus; Satisfai; Sensyne; Shire; SigmoidPharma; Sorriso; Souffinez; Syndermix; Synthon; Takeda; Theravance; Tigenix; Tillotts; Topivert; Trino Therapeutics with Wellcome Trust; TxCell; UCB Pharma; Vertex; VHsquared; Vifor; Warner Chilcott and Zeria; Speaker Fees from: AbbVie, Amgen, Biogen, Falk; Ferring, Janssen, Pfizer, Shire, Takeda, UCB; ST holds no stocks or share options. K. P. is an employee of Ferring Pharmaceuticals. S. A. A. has participated in advisory board for Ferring. J. B. has received honoraria, research grants or consulting fees from Abbvie, Janssen, Takeda, Pfizer, Ferring, Bristol Myers Squibb, Gilead, Tillott’s, Sandoz, Chiesi, Celltrion, Microba and Antara. J. H. C. has received personal fees from Celltrion Inc., Eisai Korea, Ferring Korea, IQVIA, Ferring, Janssen Korea, Shire Korea, and Takeda Korea. J. F. is an employee of Violicom Medical Limited that has received funding from Ferring for work on various projects. E. L. has received research grants from Janssen, Pfizer, and Takeda; educational grants from AbbVie, Janssen, MSD, and Takeda; speaker fees from AbbVie, Falk, Ferring, Hospira, Janssen, MSD, Pfizer, and Takeda; participated in advisory boards for AbbVie, Celgene, Ferring, Hospira, Janssen, MSD, Pfizer, Takeda, Galapagos, Gilead, Arena, Elli Lilly; consultant for AbbVie. F. M. has served as speaker and received honoraria from Abbvie, Biogen, Bristol-Myers Squibb, Falk, Ferring, Hospira, Janssen, Laboratórios Vitoria, Pfizer, Lilly, Merck Sharp & Dohme, Sandoz, Takeda, UCB and Vifor. J. R. M. has received sponsorship as speaker from AbbVie, Biopas, Biotoscana, Farma, Ferring, Janssen, and Takeda. A. R. M. is receiving research support from AbbVie and Takeda under the framework of the Christian Doppler Research Society; has received further consultation fees and/or speaker honoraria from AbbVie, Merck Sharp & Dohme, Takeda, Janssen-Cilag, Amgen, Sandoz, Nestlé, Ferring, Falk, and Pfizer. N. N. has received grants, advisory board fees, or speakers bureau honoraria from Janssen, Abbvie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. G. R. has received grants/research support or speakers fee from Abbvie, AlfaSigma, Astellas, Ferring, Janssen, Pfizer, Pharmabest, Recordatti, Sanprobi, Sandoz, Vitama and Takeda.
Data availability statement
All data supporting the findings of this study are available upon reasonable request to the corresponding author.
Additional information
Funding
References
- Solberg IC, Lygren I, Jahnsen J, IBSEN Study Group, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population based inception cohort (IBSEN study). Scand J Gastroenterol. 2009;44(4):431–440.
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413.
- Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343–356.
- Harbord M, Eliakim R, Bettenworth D, European Crohn’s and Colitis Organisation [ECCO], et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784.
- Ko CW, Singh S, Feuerstein JD, American Gastroenterological Association Institute Clinical Guidelines Committee, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748–764.
- Murray A, Nguyen TM, Parker CE, et al. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;8(8):CD000543.
- Murray A, Nguyen TM, Parker CE, et al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;8(8):CD000544.
- Paridaens K, Fullarton JR, Travis SPL. Efficacy and safety of oral pentasa (prolonged-release mesalazine) in mild-to-moderate ulcerative colitis: a systematic review and meta-analysis. Curr Med Res Opin. 2021;37(11):1891–1900.
- Sandborn WJ, Feagan BG, Hanauer SB, et al. The guide to guidelines in ulcerative colitis: interpretation and appropriate use in clinical practice. Gastroenterol Hepatol (NY). 2021;17(4 Suppl 4):3–13.
- Ungaro R, Colombel J-F, Lissoos T, et al. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114(6):874–883.
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338.
- Colombel J-F, D'haens G, Lee W-J, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–266.
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583.
- Solitano V, D’Amico F, Fiorino G, et al. Key strategies to optimize outcomes in mild-to-moderate ulcerative colitis. JCM. 2020;9(9):2905.
- Nguyen NH, Fumery M, Dulai PS, et al. Comparative efficacy and tolerability of pharmacological agents for management of mild to moderate ulcerative colitis: a systematic review and network meta-analyses. Lancet Gastroenterol Hepatol. 2018;3(11):742–753.
- Gisbert JP, Gomollón F, Hinojosa J, et al. Adherence of gastroenterologists to European Crohn's and Colitis Organisation consensus on ulcerative colitis: a real-life survey in Spain. J Crohns Colitis. 2010;4(5):567–574.
- Carbonell-Estrany X, Dall’Agnola A, Fullarton JR, et al. Interaction between healthcare professionals and parents is a key determinant of parental distress during childhood hospitalisation for respiratory syncytial virus infection (European RSV Outcomes Study [EROS]). Acta Paediatr. 2018;107(5):854–860.
- Wu C-Y, Yang H-Y, Luo S-F, et al. From rheumatoid factor to anti-citrullinated protein antibodies and anti-carbamylated protein antibodies for diagnosis and prognosis prediction in patients with rheumatoid arthritis. IJMS. 2021;22(2):686.
- Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol. 2019;72(1):12–19.
- Louis E. Fecal calprotectin: towards a standardized use for inflammatory bowel disease management in routine practice. J Crohns Colitis. 2015;9(1):1–3.
- Honig G, Heller C, Hurtado-Lorenzo A. Defining the path forward for biomarkers to address unmet needs in inflammatory bowel diseases. Inflamm Bowel Dis. 2020;26(10):1451–1462.
- Barberio B, Segal JP, Quraishi MN, et al. Efficacy of oral, topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and network meta-analysis. J Crohns Colitis. 2021;15(7):1184–1196.
- Flourié B, Hagège H, Tucat G, MOTUS Study Investigators, et al. Randomised clinical trial: once- vs. twice-daily prolonged-release mesalazine for active ulcerative colitis. Aliment Pharmacol Ther. 2013;37(8):767–775.
- Dignass AU, Bokemeyer B, Adamek H, et al. Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(7):762–769.
- Rubin DT, Sninsky C, Siegmund E, et al. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflam Bowel Dis. 2021;27(12):1942–1953.
- Mitchell R, Kremer A, Westwood N, et al. Talking about life and IBD: a paradigm for improving patient-physician communication. J Crohns Colitis. 2009;3(1):1–3.
- Irving P, Burisch J, Driscoll R, et al. IBD2020 global forum: results of an international patient survey on quality of care. Intest Res. 2018;16(4):537–545.
- Danese S, Banerjee R, Cummings JRF, et al. Consensus recommendations for patient-centered therapy in mild-to-moderate ulcerative colitis: the i support therapy-access to rapid treatment (iSTART) approach. Intest Res. 2018;16(4):522–528.
- Pedersen N, Thielsen P, Martinsen L, et al. EHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20(12):2276–2285.
- Moshkovska T, Stone MA, Smith RM, et al. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17(9):1874–1881.
- Lees CW, Regueiro M, Mahadevan U. Innovation in inflammatory bowel disease care during the COVID-19 pandemic: results of a global telemedicine survey by the international organization for the study of inflammatory bowel disease. Gastroenterology. 2020;159(3):805–808.e1.
- Martinez-Vinson C, Le S, Blachier A, et al. Effects of introduction of an inflammatory bowel disease nurse position on healthcare use. BMJ Open. 2020;10(5):e036929.
- Furnham A. Response bias, social desirability and dissimulation. Pers Individ Dif. 1986;7(3):385–400.

