Abstract
Background and aims
Therapy with two concomitant biologicals targeting different inflammatory pathways has emerged as a new therapy option for treatment refractory inflammatory bowel disease (IBD). Data on the efficacy and safety of dual biological therapy (DBT) are scarce and are investigated in this study.
Materials and methods
Data on all patients treated with a combination of two biologicals in four Finnish tertiary centres were collected and analysed. Remission was assessed by a physician on the basis of biomarkers, endoscopic evaluation and alleviation of symptoms.
Results
A total of 16 patients with 22 trials of DBT were included. Fifteen patients had Crohn’s disease. The most common combination of DBT was adalimumab (ADA) and ustekinumab (USTE; 36%) with median follow-up of nine months (range 2–31). Altogether seven (32%) patients were in remission at the end of follow-up and in two trials response to DBT was assessed to be partial with the relief of patient symptoms. In a total of four trials DBT reduced the need for corticosteroids. The majority of patients achieving a response to DBT were treated with the combination of ADA and USTE (56%). At the end of follow-up all nine (41%) patients responding to DBT continued treatment. Infection complications occurred in three patients (19%).
Conclusion
DBT is a promising alternative treatment for refractory IBD, and half of our patients benefitted from it. More data on the efficacy and safety of DBT are needed especially in long-term follow up.
Introduction
Up to 80% of patients with inflammatory bowel disease (IBD) experience relapse in the disease course at some point during long-term follow-up [Citation1,Citation2]. Biological therapies have a well-established role in the treatment of moderate to severe IBD. Treatment of IBD aims at clinical remission, mucosal healing, improved quality of life and reduction in the need for surgery [Citation3].
Despite evolving biological and small molecule therapies some patients remain refractory to treatment, and a significant portion lose response over time [Citation4]. In Crohn’s disease (CD), surgery is rarely curative and the disease recurs in many patients during follow-up [Citation5]. Most of the patients with treatment-refractory ulcerative colitis (UC) could become symptom-free after surgical treatment, but many patients are unwilling to undergo surgery. Extraintestinal manifestations (EIM) are seen in up to 50% of IBD patients [Citation6]. In some cases, EIM may be symptomatic despite a good response to IBD with ongoing treatment.
For the treatment of refractory IBD, combination therapy with two biologicals targeting different inflammatory pathways has emerged as a new treatment option [Citation7]. So far, data on the efficacy and safety of these combination therapies are scarce. However, some case reports and clinical case series on dual biological therapy (DBT) in IBD patients have reported promising results [Citation8–14].
The aim of this study was to evaluate the effectiveness and safety of DBT in IBD patients in a Finnish retrospective multicentre trial.
Patients and methods
Four Finnish hospitals participated in this retrospective multicentre study: Three university hospitals (Helsinki, Turku and Tampere) and one central hospital (Jyväskylä). Gastroenterologists identified DBT patients from electronic patient records. All patients receiving DBT for IBD between June 2015 and December 2020 were included.
Inclusion criteria were simultaneous use of two biological treatments (infliximab, adalimumab [ADA], golimumab, vedolizumab [VEDO] or ustekinumab [USTE]), age 16 years or over and follow-up for at least an induction period after the introduction of the second biological therapy. Data collected included demographic (gender, age, smoking status, prior medication and surgery), clinical (duration, extent and severity of the disease and laboratory results), and treatment data (clinical response, need for corticosteroids, immunomodulators or surgery during follow-up). The diagnosis of IBD was made on the basis of clinical history, symptoms, endoscopic and histological features. Disease location and behaviour were categorized by the Montreal classification [Citation15].
All trials of DBT were initiated based on physician’s clinical judgement of persistent disease activity assessed by clinical symptoms, laboratory tests and endoscopic or radiological findings. The documented infusion or injection of the second concomitant biological was considered as the start date of combination treatment and patients were followed up until the end of the observation period or until the enhancement of treatment with a different DBT trial. Data on clinical activity and laboratory results were collected at the onset of DBT and at 4, 12 and 18 months (±4 weeks) after initiation. Adverse events related to treatment were assessed throughout the duration of treatment.
The primary outcome was effectiveness defined as remission assessed by a physician on the basis of biomarkers, endoscopic evaluation and alleviation of symptoms after at least two months of DBT. In CD, clinical response was assessed using a modified Harvey–Bradshaw index (HBI, omitting abdominal palpation) and clinical remission was defined as HBI < 5. In UC, partial MAYO score <3 was cut the off value for clinical remission [Citation16]. Partial response was assessed by a physician based on overall response and alleviation of symptoms. Endoscopic remission was assessed by Mayo endoscopic score for UC, Rutgeerts score and simple endoscopic score for CD based on endoscopic characteristics (MAYO ≤ 1, i0-i1 and SES-CD < 3 as cut off values for endoscopic remission) [Citation17,Citation18]. Biochemical improvement was assessed by normalization in blood haemoglobin (≥117 g/L for females and ≥134 g/L for males) and plasma albumin levels (≥36 g/L) and reduction in C-reactive protein (CRP < 10 mg/L) and faecal calprotectin (FC) levels (FC cut-off value for remission ≤ 250 µg/g) [Citation19]. The secondary outcome was safety and any adverse or severe adverse event (SAE) detected and registered in electronic patient files during DBT.
Statistics
Frequencies and percentages were used as descriptive statistics for categorical variables. Continuous variables were summarized as median and range. Chi square test or Fisher’s exact test was used to analyse clinical characteristics and predictors in response to biological combination therapy. Changes in biomarkers (haemoglobin, albumin, CRP, faecal calprotectin) and clinical scores during follow-up were compared using the Wilcoxon test. Analyses were performed using the SPSS version 27 software.
Ethical considerations
The study was approved by the regional review board (R20590) and by the Finnish social and health data permit authority (THL 3866/14.02.00/2020). No informed consent was required under the Finnish regulations for registry-based studies without contact with study subjects.
Results
Altogether 16 patients receiving DBT were included. Baseline characteristics are shown in . Fifteen patients had Crohn’s disease (CD), all diagnosed before 40 years of age. The majority of CD patients had an ileocolonic disease (79%) with stricturing (60%) behaviour and perianal involvement (53%). Nine patients had undergone gut surgery prior to dual biological therapy (DBT). The only UC patient in this study had an endoscopically severe disease (defined as Mayo score 3) affecting the entire colon. All patients had failed three to five different treatments with biologicals prior to DBT. The first biological treatment was used for a median of nine (range 0–52) months prior to introducing the second biological.
Table 1. Baseline characteristics of 16 patients with IBD treated with biological combination therapy.
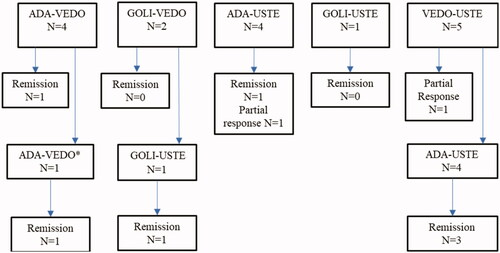
A total of 22 therapeutic DBT trials among 16 patients was implemented. The clinical characteristics of the different trials are shown in . The most common combination was ADA and USTE used in eight (36%) trials with median follow-up of nine months (range 1–31). The second concomitant biological had not previously been used for treatment in eight trials. Therapeutic drug monitoring was performed for the majority of patients during treatment. Three DBT trials were discontinued due to anti-drug antibody formation (adalimumab, golimumab, vedolizumab).
Table 2. Clinical characteristics of the 16 patients with IBD treated with 22 trials of biological combination therapy.
Clinical outcomes are presented in . Remission was achieved in a total of seven (32%) DBT trials. One of the patients achieved remission after surgery for occlusion during treatment and continued DBT. In two trials response to DBT was assessed to be partial with relief of symptoms. Of the nine patients achieving remission or partial response to DBT five were treated with a combination of ADA and USTE. One patient was treated with a combination of ADA and VEDO twice achieving remission at the second trial.
At baseline, ten patients underwent endoscopy, and the median SES-CD was 19 (range 10–32). The endoscopic Mayo score was 3 in the only UC patient’s endoscopy. After introduction of DBT endoscopy was performed in nine DBT trials within a median of four months (range 2–11). Only one patient using ADA-VEDO achieved endoscopic remission. A reduction in the SES-CD (from 19 to 5 points) occurred in another CD patient. The patient with UC failed to achieve any endoscopic response.
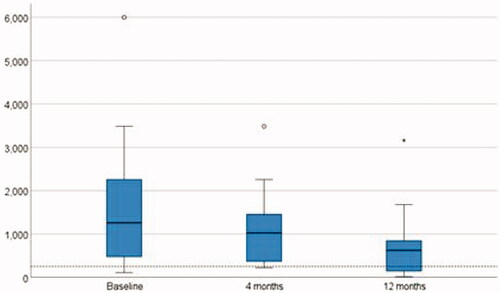
Changes in biomarkers (haemoglobin, albumin, CRP, FC) and clinical scores during follow-up are shown in . A significant reduction in median faecal calprotectin levels was seen from baseline to 12 months (p = .038) as seen in . For other biomarkers, no significant improvement was observed.
Table 3. Changes in biochemical markers, clinical and endoscopic scores during follow-up.
In ten (45%) trials DBT patients were also receiving corticosteroids (Cs) at the onset of the second biological and in three (14%) Cs therapy was initiated during DBT. Of all the trials with concomitant Cs at baseline DBT reduced the need for Cs in four (40%).
Two out of three trials with active perianal involvement at the onset of DBT reported clinical response in perianal fistulas. A total of six CD patients were operated on during follow-up of whom five underwent abdominal surgery and one had treatment for a perianal abscess. At the end of follow-up nine patients continued with DBT. One patient switched to ADA-VEDO after a failure with ADA-USTE at the end of follow-up.
Infection complications (erysipelas [ADA-VEDO], recurring clostridium difficile [VEDO-USTE], perianal abscess [VEDO-USTE], and conjunctivitis [ADA-VEDO]) occurred in three patients (19%). All reported infections were treated with antimicrobials with no need to discontinue DBT. No severe adverse events were reported.
Clinical characteristics and predictors of response to treatment are shown in . Of the nine patients achieving partial response or remission five were male, four had extraintestinal manifestations and six had perianal disease. Due to the small size of the series none of the predictors were significant when evaluating response to DBT.
Table 4. Clinical characteristics and predictors to response to biological combination therapy in 22 trials.
Discussion
In this series only one-third of patients on dual biological therapy (DBT) achieved remission. In two trials treatment resulted in symptom relief without significant improvement in biochemical markers. Only one of the trials with endoscopic assessment achieved full mucosal healing. In four trials DBT led to a reduction in corticosteroid (Cs) use and one third of all patients needed surgery during follow-up.
In a study from Norway all 10 patients treated with DBT, a combination of tumour necrosis factor (TNF) inhibitor and VEDO were in clinical remission at the end of median seven months of follow-up [Citation10]. A recent Italian study reported clinical response in all 16 patients treated with DBT and in 75% of the cases treatment led to a reduction in Cs within seven months of follow-up [Citation12]. Similar results were reported in a paediatric series with 75% of patients achieving corticosteroid-free remission within six months of follow-up in combination therapy with biologicals or biologicals and small molecules [Citation20]. There are also other studies reporting clinical responses of up to 70% for DBT [Citation21,Citation22].
In our study, DBT resulted in remission less often than in the Norwegian and Italian case series. More than half of the DBTs showed no efficacy. Results in our study resemble those reported into our very best of knowledge in the largest DBT cohort reported by Yang and co-workers. That study included 22 patients with 24 DBT trials and resulted in clinical and endoscopic remission in 41% and 26% of patients, respectively [Citation11]. Similar results were obtained in a study of paediatric patients where only half of the eight patients responded to treatment with DBT [Citation13]. These results suggest that although using DBT may increase the effectiveness of treatment compared to one agent alone, many patients still fail to respond to these combinations.
Earlier studies have shown a significant reduction in CRP and erythrocyte sedimentation rates as well as an increase in haemoglobin and albumin levels during treatment [Citation12,Citation20,Citation23]. In our study only FC level decreased significantly during the combination therapy. Although FC levels improved during DBT, endoscopic remission was rarely achieved.
A previous randomized controlled trial of CD patients treated with a combination of TNF-inhibitor infliximab and natalizumab reported no increase in infections compared to treatment with TNF-inhibitor alone [Citation24]. The studies mentioned before reported no severe adverse events (SAE) [Citation8,Citation10,Citation12–14]. However, doubts about the safety of DBT have been raised, at least in rheumatoid patients [Citation7]. A meta-analysis by Ahmed and co-workers reported 288 trials of DBT showing respective pooled rates of adverse and SAE of 31% and 6.5%. When comparing that series to our own, in this study the patients were young and free from severe comorbidity. In our study infections occurred in three (19%) patients. Although none of the adverse events lead to discontinuation of treatment, erysipelas and recurrent clostridium difficile infection should be assessed as significant adverse events. In earlier studies clostridium difficile infection was one of the most common complications during DBT [Citation11,Citation13,Citation23]. It should be noted that adverse events such as severe infections are likely to develop in the long run and therefore more information on the safety of DBT is needed, especially in long-term follow-up.
Enhancing pharmacotherapy by combining different agents is an attractive idea. The tricky part is to find the right combination for the right patient as shown in our study, where five patients achieved remission with another trial of DBT after failing with the first combination. For now, with the lack of international guidelines, the choice of therapeutic combination basis on evaluation of individuals prior medical response and experimenting. In our series the combination of ADA and USTE proved to be the most effective option. To optimize the treatment, we still need specific biomarkers to help choose the right biological targeting the right inflammatory pathway in selected patients.
The limitations of this series are due to the retrospective nature and to relying on computerized data. However, our study includes the majority of IBD patients treated with DBT in Finland within the last five years and can be considered a nation-wide study of the use of DBT in IBD. The number of patients and duration of follow-up in this series are comparable with those in previously published studies. This series is a Finnish multi-centre study providing new information on the efficacy of DBT in patients with IBD when published data on the treatment is still very limited. However, at least two prospective trials (Clinical Trials.gov Identifier: NCT02764762 and NCT03662542) are currently underway to assess the effectiveness and safety of DBT and may provide more insights into the treatment.
In conclusion, DBT may provide an alternative treatment in refractory IBD, but not for all. More data on the efficacy and safety of DBT are needed, especially in long-term follow.
Disclosure statement
H. E. reports receiving a research grant from the Emil Aaltonen foundation and congress and travel fees from Abbvie, Takeda and Pfizer. S. K. and H. H. have nothing to declare. J. K. has received speaker or consultation fees from Pfizer, Oriola, Tevapharm, Boehringer-Ingelheim and Viatris. I. K. reports receiving speaker fees from Tillots and congress and travel fees from Abbvie, Janssen and Vitris. P. O. advisory board Gilead, consultation fee Tillotts, congress and travel fees from Abbvie, Ferring, Pfizer. A. J. has received speaker or consultant fees from Abbvie, Pfizer, Orion Pharma and Takeda. T. S. has received speaker or consultant fees from Bristol-Myers Squibb, Jansen-Cilag, Pfizer and Takeda in addition to research grants from Janssen-Cilag and Takeda. T. I. advisory Board Janssen, Celltrion, Takeda and speaker’s fee Tillotts, Takeda, Janssen. The authors alone are responsible for the content and writing of the paper.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Henriksen M, Jahnsen J, Lygren I, et al.; IBSEN Study Group. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12(7):543–550.
- Solberg IC, Lygren I, Jahnsen J, et al.; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol. 2009;44(4):431–440.
- Turner D, Ricciuto A, Lewis A, et al.; International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583.
- Hazel K, O'Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020; 11:2040622319899297.
- Ananthakrishnan AN. Surgery for Crohn’s disease: look harder, act faster. Lancet. 2015;385(9976):1370–1371.
- Harbord M, Annese V, Vavricka SR, et al.; European Crohn’s and Colitis Organisation. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239–254.
- Hirten RP, Iacucci M, Shah S, et al. Combining biologics in inflammatory bowel disease and other immune mediated inflammatory disorders. Clin Gastroenterol Hepatol. 2018;16(9):1374–1384.
- Mao EJ, Lewin S, Terdiman JP, et al. Safety of dual biological therapy in Crohn's disease: a case series of vedolizumab in combination with other biologics. BMJ Open Gastroenterol. 2018;5(1):e000243.
- Liu EY, Loomes DE. Ustekinumab and vedolizumab dual biologic therapy in the treatment of Crohn’s disease. Case Rep Med. 2017;2017:5264216.
- Buer LCT, Høivik ML, Warren DJ, et al. Combining anti-TNF-α and vedolizumab in the treatment of inflammatory bowel disease: a case series. Inflamm Bowel Dis. 2018;24(5):997–1004.
- Yang E, Panaccione N, Whitmire N, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;51(11):1031–1038.
- Privitera G, Onali S, Pugliese D, et al. Dual targeted therapy: a possible option for the management of refractory inflammatory bowel disease. J Crohns Colitis. 2021;15(2):335–339.
- Olbjørn C, Rove JB, Jahnsen J. Combination of biological agents in moderate to severe pediatric inflammatory bowel disease: a case series and review of the literature. Paediatr Drugs. 2020;22(4):409–416.
- Roblin X, Paul S, Ben-Horin S. Co-treatment with golimumab and vedolizumab to treat severe UC and associated spondyloarthropathy. J Crohns Colitis. 2018;12(3):379–380.
- Gomollón F, Dignass A, Annese V, et al.; ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25.
- Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;315(8167):514.
- Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6(10):965–990.
- Magro F, Gionchetti P, Eliakim R, et al.; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670.
- D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–2224.
- Dolinger MT, Spencer EA, Lai J, et al. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(8):1210–1214.
- Kwapisz L, Raffals LE, Bruining DH, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19(3):616–617.
- Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(3):e361–e379.
- Glassner K, Oglat A, Duran A, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21(5):264–271.
- Sands BE, Kozarek R, Spainhour J, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13(1):2–11.