Abstract
Background
Pancreatic cancer has been and still is associated with a very poor prognosis. This is due to a lack of major breakthroughs with respect to early diagnosis, prognostication, prediction, as well as novel, targeted therapies. The benefits of surgery and chemotherapy are evident, but the fact that only some 10% of all patients have early, localized disease highlights the unmet need for new early detection methods. An improved understanding of tumor biology and the development of molecular markers detectable both in the circulation and in cancer tissues may underlie the development of new tools for optimizing both diagnosis and treatment.
Material and methods
Review of the literature.
Results and conclusion
If we do not improve precision oncology for pancreatic ductal adenocarcinoma, the prognosis will still remain dismal and the” burden” on society will increase substantially.
Introduction
Pancreatic cancer is associated with a dismal prognosis. It is a fairly infrequent cancer, ranked number 9–14, and with an incidence of 10–15/100 000 inhabitants and year in the Western world. As the mortality regrettably remains high, the actual 5-year survival in pancreatic cancer is reported to be below 5% despite improvements in outcome following surgical resection [Citation1]. It is a disease of the elderly with a median age of 68 years at diagnosis and only 6.2% develop early-onset pancreatic cancer prior to the age of 50 years [Citation2]. This subgroup, though, has an even worse 5-year overall and cancer-specific survival and the need for more aggressive treatment, including surgery and chemotherapy. Data from European countries for the past decades confirm the lack of improvement that actually matters [Citation3]. The projection is that pancreatic cancer will become the second leading cause of cancer death by 2020–2030, which in many Western countries already is the case [Citation4]. Only some 15–20% of the total number of patients with pancreatic cancer are candidates for upfront pancreatic resection and it is evident that surgery is not enough for curing pancreatic cancer [Citation5–7].
Future trends
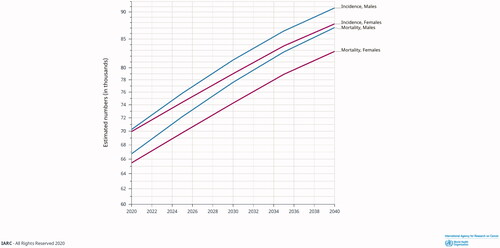
The highest incidence of pancreatic cancer is reported in Europe and the US (; [Citation8]). Both the incidence of and mortality from pancreatic cancer are expected to increase over the coming years, mainly due to the ageing population and lack of breakthroughs in early detection and treatment (; [Citation8]). Swedish data imply an exponential increase in societal costs for pancreatic cancer attributed to care-related costs and loss of production. Sweden has a population of about 10 million people. In 2009, the total yearly costs for managing pancreatic cancer, including loss of production, were in the range of 86–93 million EUR in Sweden [Citation9]. In 2018, the total costs in Sweden were calculated to be 125 million EUR and the prognosis for 2030 is in the range of 210–225 million EUR [Citation10].
Figure 1. Global incidence rates for pancreatic cancer in 2020. Source: GLOBOCAN 2020; https://gco.iarc.fr.
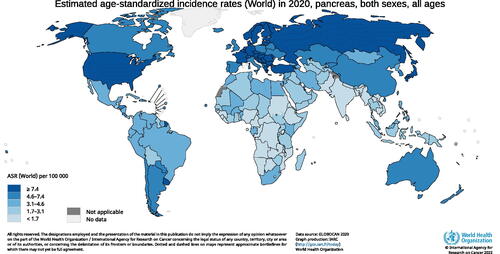
Figure 2. Projected incidence and mortality rates for pancreatic cancer from 2020 to 2040 in Europe. Source: GLOBOCAN 2020; https://gco.iarc.fr.
Pancreatic cancer development
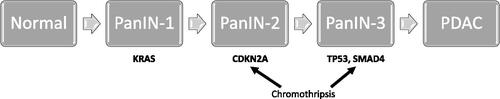
Most patients with pancreatic cancer are diagnosed in late, i.e. advanced, stages with locally advanced or more frequently metastatic disease with a poor prognosis. However, the patients that undergo surgical resection followed by adjuvant chemotherapy have a more favourable survival than those who do not undergo resection [Citation11]. Pathophysiologically, the malignant transformation to an invasive adenocarcinoma is a gradual and usually slow process with some initial driving mutations including KRAS, CDKN2A, TP53, and SMAD4 (). In most cases, it may take 10 years or more from the initial mutation until the invasive cancer lesion is established and another 5 years for the development of metastatic ability [Citation12,Citation13]. This means a potential window for diagnosis of a small lesion during the asymptomatic period when the lesion is still potentially curable. However, in a subset of cases, the genetic errors occur simultaneously, in a process known as chromothripsis [Citation14]. In fact, there is a comparably high rate (up to 30%) of pancreatic cancers that already with a tumor size ≤0.5 cm develop distant metastases [Citation15] and thus varying tumor biology may inflict on the general gradual tumor development.
Figure 3. Development model of pancreatic ductal adenocarcinoma (PDAC). The driver genes may be altered sequentially or simultaneously via chromothripsis. PanIN, pancreatic intraepithelial neoplasia.
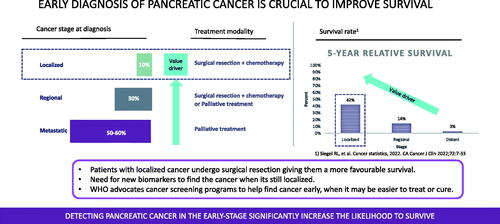
Overall, only 10% have a localized disease that allows for surgical resection which together with adjuvant chemotherapy renders a 5-year survival up to 42% [Citation16]. Some 30% have a regional disease where most patients are considered to have a non-resectable disease and receive palliative chemotherapy. In only a limited number in this group, upfront surgery or downstaging to resectable disease is possible. A majority (50–60%) of patients with pancreatic cancer have the metastatic disease already at diagnosis. They will receive palliative chemotherapy associated with a 5-year survival of a mere 3%. Increasing the number of localized tumors by early diagnosis would dramatically increase the overall 5-year survival rates ().
Epidemiology, genetics, and risk groups
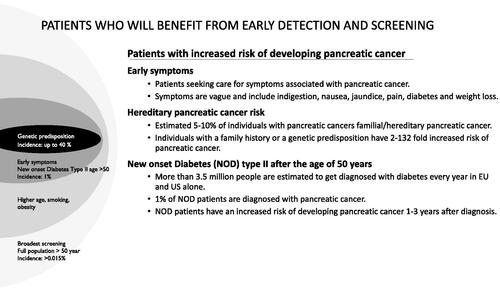
Between 5 to 10% of pancreatic ductal adenocarcinomas have been reported as hereditary forms. A number of genetic syndromes have been associated with increased pancreatic cancer risk, like Peutz Jeghers Syndrome, melanoma-pancreatic carcinoma syndrome (CDKN2A), Lynch Syndrome, and hereditary pancreatitis (PRSS1) [Citation17]. Clinical risk factors are relatively wide including smoking, obesity, diabetes and pancreatic cystic lesions (e.g. IPMN). Patients with new-onset diabetes mellitus type 2 above the age of 50 are an important risk group for pancreatic cancer with a risk ratio of up to 8, especially within the first 1–3 years after the diabetes diagnosis [Citation18]. It has generally been thought that inflammation as part of metabolic syndrome is associated with an increased risk of developing pancreatic cancer. However, a recent study indicated that high fasting glucose increases the risk of pancreatic cancer independent of metabolic syndrome [Citation19]. In summary, there are several patient groups that certainly would benefit from early detection and screening of pancreatic cancer, including hereditary and familial cases, new-onset diabetes mellitus type 2 (above the age of 50), and patients with early and vague symptoms ([Citation20,Citation21]; ).
An increased need for biomarkers in blood and pancreatic cancer tissue
Novel biomarkers in blood and cancer tissue need to be developed and validated. By earlier detection of precursor lesions and small asymptomatic early cancers, a dramatic improvement in the 5-year survival could be expected. There is a need for diagnosis, in general, to differentiate ductal adenocarcinomas in the pancreas from non-neoplastic pancreatic lesions. The serum tumor marker CA 19-9 was discovered more than 40 years ago [Citation22,Citation23]. CA 19-9 is not cancer nor pancreatic cancer-specific and the sensitivity to detecting local disease is not sufficient enough. Moreover, since CA 19-9 is a sialylated Lewisa blood group antigen, those 5–10% of the Caucasian population who are Lewis negative cannot express CA 19-9 at all [Citation24]. Thereby, there is a high demand for new biomarkers that can detect early pancreatic cancer with adequate sensitivity and specificity. Furthermore, we need prognostic markers for the stratification of pancreatic cancer patients, complementary to the traditional TNM stage. Biomarkers are also needed for monitoring patients and selecting responders to treatment, avoiding unnecessary, ineffective treatment, adverse events, and costs.
Pancreatic cancer surgery and the effects of centralization
Pancreatoduodenectomy (Whipple’s procedure) has undergone a very positive development concerning postoperative morbidity and mortality. A key element seems to be centralization of pancreatic surgery and high volumes both for an institution and the individual surgeon. It has repeatedly been shown that centralization of pancreatic cancer surgery results in an increased resection rate, increased number of radical (R0) resections and increased disease-free and overall survival rates. Furthermore, increased volumes have enabled more complex surgery and substantially improved the possibilities for research and development [Citation25–27].
Centralization of pancreatic surgery to high-volume centers has significantly improved outcomes in terms of the operation time, bleeding amounts, need for transfusions, reoperations, length of hospital stay and postoperative mortality [Citation28,Citation29]. The introduction of fast-track programs (enhanced recovery) has further improved outcomes with fewer gastric retentions, shorter hospital stays and less costs, without affecting the patient’s quality of life [Citation30]. The centralization of pancreatic surgery has increased the demand and value of multi-disciplinary conferences, improving regional/national and interdisciplinary collaboration.
It is to state that complications after pancreatoduodenectomy are expensive and double hospital care costs. Despite centralization, postoperative pancreatic fistula grades B and C still remain in the range of 8–12% increasing hospital stay and costs by 1.5 times [Citation31].
In line with the general trend toward minimally invasive surgery, laparoscopic surgery has also to some extent been introduced in pancreatic resections. This has been performed mostly for distal pancreatic resections but also for pancreaticoduodenectomy. Results show that laparoscopic as compared to open pancreatoduodenectomy has similar operative outcomes (morbidity and mortality) but seems associated with a shortening in-hospital stay and blood loss, but with a longer operative time [Citation32,Citation33]. Further development using robot-assisted pancreaticoduodenectomy confirms advantages such as operative time, blood loss and quicker recovery without affecting postoperative complications [Citation34].
When reviewing reported prognostic factors in resectable pancreatic cancer, it is to note that tumor size matters, as does lymph node positivity, especially with a lymph node ratio >0.3. Tumor grade, vascular invasion and perineural invasion are also important prognostic factors, as is the surgical resection margin status (R0 vs R1) [Citation35]. However, the prognostic significance of resection margin status may be less pronounced if we enter an era of neoadjuvant treatment [Citation36].
Why is pancreatic cancer so difficult to treat?
Pancreatic ductal adenocarcinoma is genetically complex within a mean of 63 genetic mutations from the initiating driving upstream mutations [Citation37]. It is characterized by a large, hypovascular stromal compartment, representing 85–90% of the tumor, that contributes to chemoresistance. Surgery still remains the fundament for a potential cure together with chemotherapy, administered as adjuvant therapy or potentially in a neoadjuvant setting also in upfront resectable patients. What we need is early diagnosis, prediction of outcome, and individualized precision tumor treatment to improve outcomes. Through future biomarker studies in serum and pancreatic cancer tissue, this type of optimized stratification may be provided, stratifying and selecting different types of treatment in a precision oncology fashion.
Pancreatic cancer staging and resectability decision
Pancreatic cancer is staged according to the Amerian Joint Committee on Cancer (AJCC), currently in its 8th edition [Citation38]. Subclassification of lymph node metastases has been introduced in the recent edition. For accurate detection of lymph node metastases, a minimum of 12 lymph nodes should be examined [Citation39].
Endoscopic ultrasound (EUS) is increasingly used for pancreatic cancer staging (lymph nodes, vascular invasion, etc) and also allows for diagnostic confirmation with fine needle aspiration (EUS- FNA) [Citation40,Citation41]. EUS has also become an acknowledged tool for pancreatic cancer screening in high-risk individuals as an alternative to magnetic resonance imaging (MRI) [Citation42].
Clinically, pancreatic tumors are classified as non-metastatic or metastatic. The non-metastatic disease includes resectable, borderline resectable, and locally advanced pancreatic cancer [Citation43]. Borderline resectable pancreatic tumors may potentially be removed by surgery, but there is a high risk that a radical resection cannot be achieved. Borderline tumors often have vascular involvement with a need for vascular resections [Citation43]. According to the criteria created by the National Comprehensive Cancer Network (NCCN) borderline resectable disease is defined as tumors having less than 180° vascular involvement [Citation44]. By adding biological factors that raise suspicion of metastases (like highly increased CA 19-9 levels or confirmed regional lymph node metastases) together with conditional factors taking the patient’s performance status and co-morbidity into account, an international consensus definition of borderline resectable pancreatic cancer has been provided [Citation45].
Systemic therapy in pancreatic cancer
Systemic chemotherapy, administered as neoadjuvant, adjuvant or palliative, has its place in the management of pancreatic cancer, in some cases combined with radiotherapy. Neoadjuvant chemotherapy has proven beneficial in borderline resectable tumors. In selected patient series, neoadjuvant treatment turned up to 60–90% resectable by this” downstaging” approach [Citation46,Citation47]. Neoadjuvant therapy may induce tumor regression and treatment of micro-metastatic disease make radical (R0) resections more likely. Of specific value is that neoadjuvant therapy seems to offer longer survival than upfront surgery for poorly differentiated and higher-stage borderline pancreatic cancers [Citation48]. Also in unresectable, and more locally advanced diseases, up to 25% of patients can undergo an R0 resection after neoadjuvant chemotherapy [Citation49]. The most commonly used treatment regimes are FOLFIRINOX and the combination of gemcitabine with nab-Paclitaxel. One argument for neoadjuvant chemotherapy is identifying patients with biologically more aggressive diseases in whom progression is seen during neoadjuvant treatment, thus avoiding unnecessary pancreatic surgery [Citation50]. When interpreting the effect of neoadjuvant therapy it is to remember that computed tomography scanning is unable to truly differentiate between tumor tissue and the fibrosis that actually occurs following neoadjuvant chemoradiotherapy [Citation51]. A powerful tool, though, is the CA 19-9 response during preoperative chemotherapy for patient selection and prognosis. Actually, combining preoperative CRP and CA 19-9 has proven to be a useful diagnostic-prognostic score [Citation52]. In borderline resectable patients, changes in these parameters during neoadjuvant treatment were highly predictive; CRP remaining below the cut-off value and CA 19-9 decreasing >90% during preoperative treatment predicted a favourable postoperative outcome [Citation53].
Neoadjuvant therapy in upfront resectable pancreatic cancer
The role of neoadjuvant therapy in patients with upfront resectable pancreatic cancer is not fully elucidated and should presently only be investigated in randomized clinical trials [Citation54]. Several studies are ongoing or under completion addressing neoadjuvant chemotherapy before surgery versus upfront surgery in resectable pancreatic cancer [Citation55]. Neoadjuvant chemotherapy has many potential benefits, including a higher proportion of patients receiving chemotherapy, early treatment of micrometastatic disease, as well as downstaging of tumors (). Up to 40% may have disease progression during neoadjuvant treatment due to an aggressive tumor and these patients are not subjected to futile surgery [Citation56]. However, reduced risk of progression has been described during neoadjuvant therapy for example in the PREOPANC-1 [Citation57], PACT-15 [Citation58], and Prep-02/JSAP-05 trials [Citation59,Citation60], although distant metastases may occur in up to 15% indicating aggressive tumor biology [Citation58]. This is to be compared with the fairly frequent development of early distant metastases (at similar rates) following upfront surgical resection. It is also to notice that some 30% of patients with upfront surgery never obtain adjuvant chemotherapy due to complications after resection or early disease recurrence and progression [Citation61,Citation62]. There are indications of the overall effectiveness of neoadjuvant therapy in resectable pancreatic cancer with fewer positive lymph nodes and increased survival [Citation58–60,Citation63]. As mentioned, early metastatic and aggressive tumor potential may be defined during neoadjuvant treatment [Citation14] and circulating tumor cells and associated occult metastatic disease is frequent and the associated survival is poor [Citation64].
Table 1. Potential advantages and disadvantages with neoadjuvant chemotherapy in resectable pancreatic cancer.
Adjuvant therapy in pancreatic cancer
The ESPAC trials have shown a survival advantage of adjuvant chemotherapy after pancreatic resection. Initially, 5-FU was used but was later replaced by gemcitabine which proved to be as effective, but with fewer side effects, and adjuvant gemcitabine together with capecitabine was superior to gemcitabine alone (ESPAC-4) [Citation65–68]. The CONKO-001 trial also confirmed the use of gemcitabine in the adjuvant setting [Citation69]. Recent studies have demonstrated that modified FOLFIRINOX significantly increases survival as compared to gemcitabine, though the incidence of toxic effects is higher. The median overall survival in the FOLFIRINOX study was 54.4 months compared to 35.0 months among the gemcitabine group [Citation70]. When the combination of gemcitabine and nab-Paclitaxel was compared to gemcitabine alone the combined Gem-Nab treatment improved overall survival to 40.5 months compared to 36.2 months for gemcitabine alone [Citation71]. A FOLFIRINOX regime was approximately 1.7 times more costly per month as compared to the gemcitabine/nab-Paclitaxel treatment, but was considered cost-effective due to improved outcome [Citation72].
Palliative chemotherapy in pancreatic cancer
Initial studies demonstrated that gemcitabine increases the median survival from 3–4 to 5.5–7 months, and since then most regimes have been gemcitabine-based. FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, fluorouracil) compared with gemcitabine alone significantly increased survival in metastatic pancreatic cancer from 6.8 to 11.1 months. Toxicity problems reported, though, were concerning [Citation73]. Another improvement was that the combination of gemcitabine and nab-Paclitaxel increased survival in metastatic pancreatic cancer from 6.7 to 8.5 months when compared to gemcitabine alone [Citation74].
Radiotherapy in pancreatic cancer
Radiotherapy may be used as part of a neoadjuvant radiochemotherapy protocol, seemingly less in Europe with a shift towards chemotherapy rather than radiochemotherapy [Citation75]. In resectable pancreatic cancer, there are indications that the use of intraoperative radiation therapy (IORT) may improve locoregional control and overall survival [Citation76] and IORT in borderline resectable and locally advanced pancreatic ductal adenocarcinoma subjected to surgical resection appeared to be associated with improved survival, though more supportive evidence seems to be needed [Citation77]. Following the outcome of the ESPAC-1 study where no survival benefit could be seen for adjuvant chemoradiotherapy [Citation65] continued studies have used various chemotherapy combinations in the adjuvant setting. Stereotactic radiotherapy in locally advanced pancreatic cancer may have a role in achieving pain relief [Citation78].
Precision oncology
It seems quite evident that stratification of patients down to group or even individual level is essential and should be done already during diagnostic work-up before the decision/choice of a treatment regimen. Improved information from biomarker studies on pancreatic cancer tissue and blood may render more precise prognostic and predictive information helping the clinicians avoid ineffective treatment, side effects, and unnecessary costs [Citation6,Citation79].
Increased knowledge about the genomic landscape in pancreatic ductal adenocarcinoma can potentially provide future implications for management and a more personalized treatment selection [Citation80]. In recent years, new molecular subtypes of pancreatic cancer based on gene expression profiles and outcome data have been presented. According to the Collisson classification [Citation81], there are three molecular subtypes: classical, quasi-mesenchymal and exocrine-like. The Moffitt classification [Citation82] separated the stroma from pancreatic tumors and identified two stroma subtypes (“normal” and “activated”) and two tumor-specific subtypes (“classical” and basal-like). Bailey et al. [Citation83] proposed four subtypes; squamous, pancreatic progenitor, immunogenic and aberrantly differentiated endocrine exocrine (ADEX). Altogether, these molecular classifications may provide better stratification and subtyping. For example, the immunogenic type may provide possibilities for immunotherapy using immune check inhibition, and dendritic cell vaccination [Citation84]. Immunotherapy, though, up to now has not provided convincing evidence of effect in pancreatic cancer and probably has to be administered to selected subgroups of patients, combined with agents sensitizing the tumor microenvironment by reprogramming tumor vasculature and immune compartment and optimize drug delivery and targeting [Citation85].
The search for novel treatments includes targeting the crosstalk between tumor cells and tumor microenvironment, stroma targeting and macrophage targeting, among others [Citation85]. Of interest is inhibition of PARP1 (poly(ADP-ribose) polymerase, PARP being a key sensor of DNA damage and BRCA1/BRCA2 deficiency [Citation41,Citation86].
The clinical and molecular subclassification of pancreatic cancer needs to be complemented with adequate preclinical and translational tumor models. The use of patient-derived organoids or PDX models generated from patient tumor tissue may provide information on the biology of the tumor and also predictive information on expected drug response based on high throughput drug screening. It may very well be that driving mutations and crucial signaling pathways can be made targetable (drugable) and facilitate therapeutic decision-making.
Conclusion
The number of cases and deaths due to pancreatic cancer is increasing globally. This is mainly attributed to an ageing population and a lack of improvement in early diagnosis and treatment. Preventive strategies should focus on modifiable risk factors. Novel types of biomarker tests need to be developed and applied to high-risk populations to help detect early curable lesions. Centralization of pancreatic surgery has helped reduce perioperative mortality and long-term survival benefit has been achieved as a result of recent developments in chemotherapy. Although adjuvant chemotherapy remains the gold standard for resected patients, encouraging data on the benefits of neoadjuvant treatments are emerging from randomized trials. Future molecular subtyping approaches and individualized treatment options will most definitely help improve systemic treatment for pancreatic cancer, which will be beneficial both for patients with localized tumors, as well as for those with advanced disease.
Disclosure statement
The authors report no conflict of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10(1):16425.
- Ansari D, Althini C, Ohlsson H, et al. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg. 2019;404(5):565–571.
- Pancreatic Cancer Across Europe: Taking a united stand 2018. Published by United European Gastroenterology.
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921.
- Ansari D, Andersson R. Pancreatic ductal adenocarcinoma: is a cure possible? Future Oncol. 2017;13(10):863–865.
- Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. WJG. 2015;21(11):3157–3165.
- Kommalapati A, Tella SH, Goyal G, et al. Contemporary management of localized resectable pancreatic cancer. Cancers. 2018;10(1):24.
- GLOBOCAN 2020. https://gco.iarc.fr.
- Tingstedt B, Andersson E, Flink A, et al. Pancreatic cancer, healthcare cost, and loss of productivity: a register-based approach. World J Surg. 2011;35(10):2298–2305.
- Draus T, Ansari D, Wikstrom F, et al. Projected economic burden of pancreatic cancer in Sweden in 2030. Acta Oncol. 2021;60(7):866–871.
- Pappalardo A, Giunta EF, Tirino G, et al. Adjuvant treatment in pancreatic cancer: shaping the future of the curative setting. Front Oncol. 2021;11:695627.
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909.
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117.
- Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature. 2016;538(7625):378–382.
- Ansari D, Bauden M, Bergstrom S, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg. 2017;104(5):600–607.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA A Cancer J Clinicians. 2022;72(1):7–33.
- Danielsson K, Ansari D, Andersson R. Personalizing pancreatic cancer medicine: what are the challenges? Per Med. 2013;10(1):45–59.
- Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129(2):504–511.
- Jacobson S, Dahlqvist P, Johansson M, et al. Hyperglycemia as a risk factor in pancreatic cancer: a nested case-control study using prediagnostic blood glucose levels. Pancreatology. 2021;21(6):1112–1118.
- Greer JB, Whitcomb DC, Brand RE. Genetic predisposition to pancreatic cancer: a brief review. Am J Gastroenterol. 2007;102(11):2564–2569.
- Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4(11):1366–1372.
- Koprowski H, Herlyn M, Steplewski Z, et al. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212(4490):53–55.
- Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5(6):957–971.
- Scara S, Bottoni P, Scatena R. CA 19-9: Biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:247–260.
- Ahola R, Siiki A, Vasama K, et al. Effect of centralization on long-term survival after resection of pancreatic ductal adenocarcinoma. Br J Surg. 2017;104(11):1532–1538.
- Delitto D, Black BS, Cunningham HB, et al. Standardization of surgical care in a high-volume center improves survival in resected pancreatic head cancer. Am J Surg. 2016;212(2):195–201 e1.
- Seppanen H, Juuti A, Mustonen H, et al. The results of pancreatic resections and long-term survival for pancreatic ductal adenocarcinoma: a single-institution experience. Scand J Surg. 2017;106(1):54–61.
- Ansari D, Williamsson C, Tingstedt B, et al. Pancreaticoduodenectomy–the transition from a low- to a high-volume center. Scand J Gastroenterol. 2014;49(4):481–484.
- Yoshioka R, Yasunaga H, Hasegawa K, et al. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg. 2014;101(5):523–529.
- Williamsson C, Karlsson N, Sturesson C, et al. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg. 2015;102(9):1133–1141.
- Williamsson C, Ansari D, Andersson R, et al. Postoperative pancreatic fistula-impact on outcome, hospital cost and effects of centralization. HPB. 2017;19(5):436–442.
- Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020;271(1):54–66.
- Mazzola M, Giani A, Crippa J, et al. Totally laparoscopic versus open pancreaticoduodenectomy: a propensity score matching analysis of short-term outcomes. Eur J Surg Oncol. 2021;47(3):674–680.
- Shi Y, Jin J, Qiu W, et al. Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg. 2020;155(5):389–394.
- Akerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol. 2016;22(28):6424–6433.
- Schmocker RK, Delitto D, Wright MJ, et al. Impact of margin status on survival in patients with pancreatic ductal adenocarcinoma receiving neoadjuvant chemotherapy. J Am Coll Surg. 2021;232(4):405–413.
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806.
- Kaka S, Pawlic TM, PJ A, et al. Exocrine pancreas. Pancreatic adenocarcinoma. In: Amin MB, editor. AJCC cancer staging manual. 8th ed. New York: Springer-Verlag; 2016.
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15(1):165–174.
- Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc. 2002;55(2):232–237.
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020.
- Aslanian HR, Lee JH, Canto MI. AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology. 2020;159(1):358–362.
- Evans DB, George B, Tsai S. Non-metastatic pancreatic cancer: resectable, borderline resectable, and locally advanced-definitions of increasing importance for the optimal delivery of multimodality therapy. Ann Surg Oncol. 2015;22(11):3409–3413.
- Tempero MA. New year, new look!. J Natl Compr Canc Netw. 2019;17(1):1–605.
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270(2):340–347.
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18(3):619–627.
- Nurmi A, Mustonen H, Parviainen H, et al. Neoadjuvant therapy offers longer survival than upfront surgery for poorly differentiated and higher stage pancreatic cancer. Acta Oncol. 2018;57(6):799–806.
- Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20(31):10740–10751.
- Michelakos T, Pergolini I, Castillo CF, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733–740.
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457–463.
- Nurmi A, Mustonen H, Stenman U, et al. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci Rep. 2021;11(1):781.
- Nurmi AM, Mustonen H, Haglund C, et al. Changes in CRP and CA19-9 during preoperative oncological therapy predict postoperative survival in pancreatic ductal adenocarcinoma. Oncology. 2021;99(11):686–698.
- Blair AB, Sorber R, Rozich NS, et al. A qualitative review of neoadjuvant chemotherapy in resectable pancreatic adenocarcinoma. Pancreas. 2019;48(8):973–984.
- Labori KJ, Lassen K, Hoem D, et al. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (norwegian pancreatic cancer trial – 1 (NorPACT-1)) – study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017;17(1):94.
- Maggino L, Malleo G, Marchegiani G, et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 2019;154(10):932–942.
- Eijck CHJV, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: long-term results of the multicenter randomized phase III PREOPANC trial. J Clin Oncol. 2021;39(15_suppl):4016–4016.
- Reni M, Balzano G, Zanon S, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):413–423.
- Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (prep-02/JSAP05). Jpn J Clin Oncol. 2019;49(2):190–194.
- Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (prep-02/JSAP-05). J Clin Oncol. 2019;37(4_suppl):189–189.
- Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–945.
- Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma – a population-based cohort study. Acta Oncol. 2016;55(3):265–277.
- Artinyan A, Anaya DA, McKenzie S, et al. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117(10):2044–2049.
- Court CM, Ankeny JS, Sho S, et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann Surg Oncol. 2018;25(4):1000–1008.
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–1585.
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–156.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024.
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–1081.
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481.
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406.
- Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15_suppl):4000–4000.
- Kharat AA, Nelson R, Au T, et al. Cost-effectiveness analysis of FOLFIRINOX vs gemcitabine with nab-paclitaxel as adjuvant treatment for resected pancreatic cancer in the United States based on PRODIGE-24 and APACT trials. J Manag Care Spec Pharm. 2021;27(10):1367–1375.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348.
- Jin L, Shi N, Ruan S, et al. The role of intraoperative radiation therapy in resectable pancreatic cancer: a systematic review and meta-analysis. Radiat Oncol. 2020;15(1):76.
- Harrison JM, Wo JY, Ferrone CR, et al. Intraoperative Radiation Therapy (IORT) for borderline resectable and locally advanced Pancreatic Ductal Adenocarcinoma (BR/LA PDAC) in the era of modern neoadjuvant treatment: short-term and long-term outcomes. Ann Surg Oncol. 2020;27(5):1400–1406.
- Buwenge M, Macchia G, Arcelli A, et al. Stereotactic radiotherapy of pancreatic cancer: a systematic review on pain relief. JPR. 2018;ume 11:2169–2178.
- Ansari D, Tingstedt B, Andersson R. Pancreatic cancer – cost for overtreatment with gemcitabine. Acta Oncol. 2013;52(6):1146–1151.
- Dreyer SB, Chang DK, Bailey P, et al. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23(7):1638–1646.
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503.
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178.
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52.
- Johansson H, Andersson R, Bauden M, et al. Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol. 2016;22(43):9457–9476.
- Andersson R, Pereira CF, Bauden M, et al. Is immunotherapy the holy grail for pancreatic cancer? Immunotherapy. 2019;11(17):1435–1438.
- Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–1447.