Abstract
Objectives
Patients with ulcerative colitis (UC) have shown an increased risk for colorectal cancer, hepatobiliary, hematologic, and skin cancers, but updated long-term data is needed. This study aimed to estimate the risk of cancer in patients with UC compared to the general Norwegian population, in a population-based cohort (the IBSEN study), 30 years after diagnosis; and to identify possible risk factors associated with cancer.
Methods
The IBSEN cohort prospectively included all incident patients between 1990 and 1993. Cancer incidence data were obtained from the Cancer Registry of Norway. The overall and cancer-specific hazard ratios (HR) were modelled using Cox regression. Standardized incidence ratios were estimated compared to the general population.
Results
In total, the cohort included 519 patients, and 83 cases were diagnosed with cancer. There was no statistically significant difference in the overall cancer risk (HR = 1.01, 95% CI: [0.79–1.29]) and colorectal cancer risk (HR = 1.37, 95% CI: [0.75–2.47]) between patients and controls. The incidence of biliary tract cancer was higher than expected (SIR = 9.84, 95%CI: [3.19–20.15]), especially when UC patients suffered from primary sclerosing cholangitis. Male UC patients were also more at risk of being diagnosed with hematologic malignancies (HR = 3.48, 95% CI: [1.55–7.82]). Being prescribed thiopurines was associated with a higher risk of cancer (HR = 2.03, 95% CI: [1.02–4.01]).
Conclusions
At 30 years after diagnosis, the risk of all cancer in patients with UC was not significantly increased compared with the general population. However, the risks of biliary tract cancer and hematologic cancers were increased, particularly in male patients.
Introduction
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD) that causes chronic inflammation of the large intestine. Patients with UC are prone to complications and have increased risks of several cancer types, including both intestinal and extraintestinal cancers [Citation1,Citation2]. This excess risk has primarily been described for colorectal cancer (CRC), hepatobiliary, hematologic, and skin cancers [Citation2,Citation3]. The extent of the risk of CRC is uncertain and has been diminishing in recent times in several Nordic studies [Citation4–7]. The increased risk of cancer for patients with UC has been linked to chronic inflammation, primary sclerosing cholangitis (PSC), and medical treatment, notably involving thiopurines (TP) [Citation8–10]. Therefore, updated estimates of cancer risk in longstanding disease are needed to evaluate surveillance guidelines, especially considering that the prevalence of UC in Norway is one of the highest in the world [Citation11,Citation12].
In this Norwegian population-based prospective cohort (the IBSEN study), 20 years after diagnosis, male patients with UC had an increased risk for all cancers and for CRC, compared to controls; in particular, male patients had an increased risk for CRC [Citation13]. This register-based study aimed to estimate the risk of cancer in patients with UC compared to matched controls, 30 years after diagnosis; furthermore, to assess the role of selected possible risk factors.
Materials and methods
Patient population
The Inflammatory Bowel South-Eastern Norway (IBSEN) study prospectively included all patients with newly diagnosed IBD residing in four counties in Southeast Norway from January 1, 1990, to December 31, 1993. The diagnosis was revised for up to 10 years after enrolment. All the patients were invited to participate in structured interviews, follow-up visits, and examinations including colonoscopy at one, five, 10, and 20 (±1) years after enrolment. Medical and surgical treatments were recorded until the last scheduled visit, at 20 (±1) years. In addition, colonoscopies were performed when clinically indicated. The design, methods, and procedures have previously been described in detail [Citation14–17]. Examination for PSC using magnetic resonance cholangiography was offered at 20 years of follow-up to 222 of 314 patients who consented [Citation18].
Registry and definitions
The cancer incidence and mortality data were obtained from the Cancer Registry of Norway (CRN). All medical doctors in Norway must report new cancer cases to the CRN. Data from the Cancer Registry were coded according to the International Classification of Disease 10th revision (ICD-10), International Classification of Diseases for Oncology 3rd Edition (ICD-O-3), and Surveillance, Epidemiology, and End Results (SEER) stage [Citation19]. Cancer diagnoses with low certainty were not included. CRC was defined by ICD-10 codes C18-C20 and C21.8, and biliary tract cancer was defined by codes C22.1 and C23–24.
The event was defined as the first occurrence of any cancer or of a specific cancer type from the date of enrolment until December 31, 2020, or the end of follow-up. Patients were censored for the risk of CRC when they underwent proctocolectomy. Prescription of TP (azathioprine) was defined by the presence of any prescription record at any time until the last scheduled visit, at 20 (±1) years.
Statistical analysis
All Norwegian citizens are assigned a unique digital identification number which allows for the linking of data between different registries. Each patient in the IBSEN cohort was age and sex-matched with five controls residing in the same geographical area at the time of diagnosis, randomly drawn from the Norwegian Population Register.
Categorical data are presented as counts and percentages. Categorical data were compared using the chi-squared test. The cumulative incidence of the first cancer occurrence was plotted using the Kaplan–Meier method adjusted for competing risks, and overall cumulative mortality using the Kaplan–Meier method.
Cancer hazard ratios (HR) for patients compared to controls were modelled using the Cox proportional hazard model stratified by matched case–controls sets. The regression models were adjusted for age and sex when the analyses were restricted to patients only.
When estimating the rates of rare cancer types, standardized incidence ratios (SIR) were calculated as the ratio between the observed and expected numbers. Expected incident cancer cases were derived from data openly available from the Cancer Registry of Norway for the corresponding time, regions, sex, and age groups [Citation20]. The expected numbers for ‘intrahepatic bile duct cholangiocarcinoma’ (ICD-10 C22.1) or ‘overlapping cancer of the anus and rectum’ (ICD-10 C21.8) were not separately available from this data source and therefore account for the SIRs of liver cancer and anal cancer, respectively.
Confidence intervals (CI) for the SIRs were calculated using the Wilson and Hilferty approximation. P-values less than 0.05 were considered statistically significant. All analyses were considered exploratory; no correction for multiple testing was done. All analyses were performed using R statistical software version 4.2.1 and the survival package version 3.3.
Ethical considerations
The Regional Committee for Medical and Health Research Ethics (2010/1540 and 2019/1256) and the Oslo University Hospital Data Protection Officer approved this register-based study. Reporting and interpretation of data from the Cancer Registry of Norway are the sole responsibilities of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should it be inferred.
Results
The cohort included 519 patients with UC, followed by a total of 12,473 person-years, and 371 patients were still alive at the end of the study. Nine patients were lost to follow-up because of emigration. Thirty-four patients underwent proctocolectomy.
Overall, 83 patients were diagnosed with cancer, accounting for a total of 99 cancer diagnoses ( and ). Patients with UC did not have a higher overall risk of cancer (HR = 1.01, 95% CI: [0.79–1.29]) than their matched controls, regardless of sex ().
Figure 1. Cumulative incidence for cancer and mortality, stratified by sex, from the time of diagnosis. (a) Cumulative incidence of cancer and of death in women. (b) Cumulative incidence of cancer and of death in men.
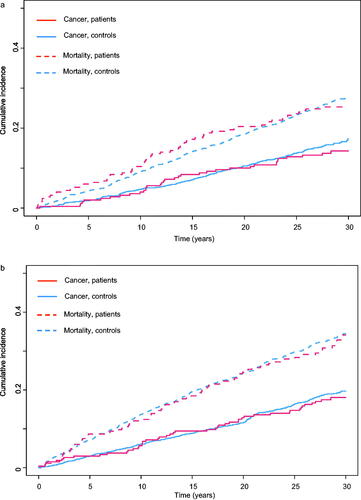
Table 1. Demographic and clinical characteristics of the IBSEN study population.
Table 2. Hazard ratios (HR) of the most frequent cancer types in patients compared to controls.
Colorectal cancer
The hazard rates for CRC were slightly increased in patients with UC compared to controls, although not statistically significant, neither in male nor female patients (HR = 1.37, 95% CI: [0.75-2.47]) (). There were three cases of colorectal cancer located in the intestinal remnant. The extent of inflammation at diagnosis did not change the HR for CRC (Left-sided colitis HR = 1.30, 95% CI: [0.26–6.44]; Pancolitis HR =2.46, 95% CI: [0.85–7.10]; Proctitis as reference). CRC was significantly more often detected at a localized stage in patients than in controls and less often at a distant metastatic stage (p = 0.029) (). The 5-year survival rate for CRC was similar between patients and controls (49.2%, 95% CI: [29.7–81.6%] and 47.1%, 95% CI: [30.3–57.7%], respectively).
Table 3. Distribution of colorectal cancer (CRC) SEER stages in patients and controls.
Biliary tract cancer
The incidence of biliary tract cancer was significantly higher in patients with UC than expected (SIR = 9.84, 95%CI: [3.19–20.15]), with five extrahepatic biliary tract cancer (). PSC was a considerable risk factor for developing biliary tract cancers in patients with UC: out of five biliary tract cancer cases, three had been diagnosed with PSC ().
Table 4. Number of observed incident cancer diagnoses and standardized incidence ratios (SIR) of the common cancer types in patients.
Table 5. Number of incident biliary tract cancers in patients with ulcerative colitis (UC), patients with primary sclerosing cholangitis (PSC), and in controls.
Hematologic cancers
The risk for hematologic cancer was increased in male patients with UC, compared to controls (HR = 3.48, 95% CI: [1.55–7.82]) (). Two occurrences of lymphoma in the intestinal region contributed to this increase, whereas only one was observed in the control group. The SIRs for breast and thyroid cancers were not significantly increased. In female patients with UC, there were fewer cases of cancers of the reproductive and intrathoracic organs than expected ().
Patients who had been prescribed TP were then at a higher risk of subsequent cancer than patients who did not use TP (HR = 2.03, 95% CI: [1.02–4.01]). Although the hazard ratio was increased, being prescribed TP was not significantly associated with a higher risk of ensuing hematologic cancer in patients (HR = 2.94, 95% CI: [0.58–14.90]) or non-melanoma skin cancer (HR = 6.23, 95% CI: [0.54–71.4]).
Discussion
Overall, patients with UC in the IBSEN cohort did not show a higher risk of cancer than the general Norwegian population, despite the increased incidence of biliary tract cancer and hematologic cancers. The increased overall cancer risk in male patients of the IBSEN cohort, 20 years after diagnosis, has not been replicated in our extended follow-up [Citation13]. In a nationwide Danish cohort with 30 years of follow-up, the SIR of gastrointestinal malignancies has been declining with time [Citation21]. The IBSEN cohort may have the same trend, which could suggest that the current treatment strategies restrain the UC-related risk of cancer.
The increased hazard rate for colorectal cancer was not statistically significant, independently of the extent of intestinal inflammation at diagnosis. This observation differs from the results of recent larger studies from the Nordic countries, which have found higher incidence ratios of CRC than in the present study [Citation4–6]. These studies have reported that the excess risk of CRC may be highest shortly after diagnosis, partly due to detection bias, and appears to have decreased with time [Citation5,Citation6]. Norway has a high and still increasing national incidence of CRC, which may explain the absence of a significant difference in the present study [Citation22].
CRC 5-year survival rate was not different compared with controls, and accordingly, in a previous study, we did not observe increased mortality caused by all gastrointestinal cancers together or CRC specifically [Citation23]. CRC was diagnosed at an earlier stage than that in the controls, which may be attributable to the awareness of the risk of CRC and the regular examinations undergone by patients with UC, including polypectomy when indicated [Citation5,Citation24,Citation25]. In the first 20 years of the IBSEN study, patients with UC underwent endoscopies every 6.5 years on average. Early detection probably contributed to a more favourable survival prognosis [Citation24,Citation26]. CRC survival in Nordic countries has generally improved throughout the IBSEN study [Citation27].
Together, these findings indicate that the excess risk for CRC was not as elevated as previously estimated and that good adherence to endoscopic surveillance and current medical management probably reduces the risk of this severe complication [Citation28].
The hazard ratio for biliary tract cancer was considerably increased, which is particularly serious since the survival of biliary tract cancer is poor [Citation29]. PSC-UC was strongly associated with this risk in our study. The precise mechanism of patients with UC developing PSC is unknown. PSC was diagnosed in 4.8% of the patients in the IBSEN study. This prevalence is close to the national estimate and these of the other Nordic countries, where the SIRs for biliary tract cancer are similarly very high as a consequence of PSC [Citation4,Citation30,Citation31]. These findings support stringent surveillance strategies for the detection and follow-up of patients with PSC–UC [Citation29].
PSC is also a known risk factor for CRC [Citation2,Citation32]. However, the moderate size of the IBSEN cohort precluded estimating this risk. The incidence of hematologic cancer was increased in male patients. Fourteen cases during the study period (1.1 per 1000 person-years) represent a substantial absolute risk. There were two lymphomas in the intestinal region, which is a known risk for patients with IBD [Citation33]. Local inflammation itself may be a risk factor for the occurrence of lymphoma and hematologic malignancies, together with the long-term use of TP, inhibitors of TNF-α, and Epstein–Barr virus [Citation10,Citation34]. In our study, we did not find any significant association between the use of TP and hematologic cancer or non-melanoma skin cancer, which several reviews have reported [Citation35–38]. However, our data revealed that the use of TP was associated with a higher risk of all cancers. The benefit/risk ratio for every patient prescribed TP should be assessed and relevant prevention measures implemented.
The SIR for thyroid cancer was not significantly increased compared to the general population, differing from what has been shown in other cohorts [Citation4,Citation6]. The incidence of thyroid cancer is very low therefore the interpretation of our results is uncertain. Although the SIR for breast cancer was increased, the estimate did not reach the level of statistical significance; therefore, the findings at 20 years of follow-up were not replicated [Citation13]. Fewer lung cancers than expected were observed in female patients with UC. The proportion of patients with a history of smoking was lower than that of the background population in the same period, which might have helped diminish the incidence of cancers attributable to tobacco use [Citation39].
Strengths and limitations
The strengths of this prospective population-based study are the well-defined inclusion criteria systematically enforced by dedicated specialists, the extensive clinical characterization and the long follow-up time. Thus design misclassification, selection, and observer bias were minimized.
Screening for PSC after 20 years of follow-up contributed to an accurate estimation of the prevalence of PSC by detecting cases that would have otherwise remained subclinical. Norwegian national health registers and the national identity number facilitate data collection and analysis and allow the drawing of matched controls.
The Cancer Registry of Norway has very good data completeness for most cancer types and precisely characterized quality indicators [Citation40]. Accordingly, there were a few patients lost to follow-up. A limitation of our cohort is its moderate size, which limits the statistical power, especially in the subgroup analyses. Rare events inevitably lead to less accurate estimates. The long follow-up period partially compensated for the moderate cohort size by increasing the cumulative number of cancer cases.
No further prospective and systematic clinical data were collected after 20 years of follow-up, it was not possible to associate recent medical treatment or precise cumulative doses with the outcomes. Therefore, our definition of TP prescription is binary and does not make assumptions about cumulative doses. Furthermore, as a pre-biologic era cohort, inhibitors of TNF-α were introduced during the second decade after diagnosis and only 3.2% of patients had been prescribed TNF-α inhibitors 20 years after diagnosis. Therefore no association between TNF-α inhibitors and the outcomes could be tested in this study [Citation13].
Clinically relevant data for the controls were not available and consequently could not be adjusted for, for example, cancer risk factors such as smoking. Lead-time bias may have led to the apparent overestimation of the 5-year survival rate for patients with CRC, although it is unlikely since the survival may be attributed to diagnosis at comparatively early stages, and we did not either observe a difference in mortality due to CRC in the IBSEN study [Citation23].
Conclusion
In this long-term study, the overall risk of cancer or CRC in patients with UC was not significantly higher than in the general population. However, since the incidence of CRC is high in the Norwegian population, CRC continues to be an important issue, and patients with UC will still need appropriate surveillance.
In agreement with consensus knowledge, patients with UC were at a higher risk of biliary tract cancers than the general population, especially when PSC was present, and male patients showed a high risk of developing hematologic malignancies. The absolute risk for biliary tract cancer and hematologic cancer is substantial and needs careful consideration in clinical practice.
Author contributions
B F-A, MCS, ØH, L-P J-J and BM: study conception, design, collection and interpretation of the data, manuscript writing and critical revision. B F-A and MCS: statistical analyses. All authors approved the final manuscript.
Acknowledgments
The authors thank the following members of the Inflammatory Bowel South-Eastern Norway (IBSEN) study group for participating in this study: Aida Kapic Lunder, Morten Vatn, and Jørgen Jahnsen, Akershus University Hospital; Iril Kempski-Monstad, Marte Lie Høivik and Camilla Solberg, Oslo University Hospital, Ole Høie, Sørlandet Hospital Trust, Arendal, Magne Henriksen, Kalnes Hospital Trust, Tomm Bernklev and Gert Huppertz-Haus, Telemark Hospital Trust, Skien.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article cannot be shared publicly due to the restrictions imposed by the ethics committee, the registers, and the national legal framework.
Additional information
Funding
References
- Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372(15):1441–1452. doi: 10.1056/NEJMra1403718.
- Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015;9(11):945–965. doi: 10.1093/ecco-jcc/jjv141.
- Lo B, Zhao M, Vind I, et al. The risk of extra-intestinal cancer in inflammatory bowel disease: a systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2021;19(6):1117–1138.e19. doi: 10.1016/j.cgh.2020.08.015.
- Jussila A, Virta LJ, Pukkala E, et al. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol. 2013;48(12):1405–1413. doi: 10.3109/00365521.2013.846402.
- Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395(10218):123–131. doi: 10.1016/S0140-6736(19)32545-0.
- Burisch J, Lophaven S, Munkholm P, et al. Surgery, cancer and mortality among patients with ulcerative colitis diagnosed 1962-1987 and followed until 2017 in a Danish population-based inception cohort. Aliment Pharmacol Ther. 2022;55(3):339–349. doi: 10.1111/apt.16677.
- Adami HO, Bretthauer M, Emilsson L, et al. The continuing uncertainty about cancer risk in inflammatory bowel disease. Gut. 2016;65(6):889–893. doi: 10.1136/gutjnl-2015-311003.
- Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22(20):4794–4801. doi: 10.3748/wjg.v22.i20.4794.
- Trivedi PJ, Crothers H, Mytton J, et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology. 2020;159(3):915–928. doi: 10.1053/j.gastro.2020.05.049.
- Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO pathogenesis scientific workshop (III). J Crohns Colitis. 2014;8(1):31–44. doi: 10.1016/j.crohns.2013.04.006.
- Lirhus SS, Hoivik ML, Moum B, et al. Incidence and prevalence of inflammatory bowel disease in Norway and the impact of different case definitions: a nationwide registry study. Clin Epidemiol. 2021;13:287–294. doi: 10.2147/CLEP.S303797.
- Yu J, Refsum E, Perrin V, et al. Inflammatory bowel disease and risk of adenocarcinoma and neuroendocrine tumors in the small bowel. Ann Oncol. 2022;33(6):649–656. doi: 10.1016/j.annonc.2022.02.226.
- Hovde O, Hoivik ML, Henriksen M, et al. Malignancies in patients with inflammatory bowel disease: results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2017;11(5):571–577.
- Moum B, Ekbom A, Vatn MH, et al. Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in South Eastern Norway. Gut. 1997;40(3):328–332. doi: 10.1136/gut.40.3.328.
- Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol. 2009;44(4):431–440. doi: 10.1080/00365520802600961.
- Monstad IL, Solberg IC, Cvancarova M, et al. Outcome of ulcerative colitis 20 years after diagnosis in a prospective population-based inception cohort from South-Eastern Norway, the IBSEN study. J Crohns Colitis. 2021;15(6):969–979. doi: 10.1093/ecco-jcc/jjaa232.
- Klepp P, Kisiel JB, Smastuen MC, et al. Multi-target stool DNA test in the surveillance of inflammatory bowel disease: a cross-sectional cohort study. Scand J Gastroenterol. 2018;53(3):273–278. doi: 10.1080/00365521.2018.1424935.
- Lunder AK, Hov JR, Borthne A, et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology. 2016;151(4):660–669 e4. doi: 10.1053/j.gastro.2016.06.021.
- Adamo M, Groves C, Dickie L, et al. SEER Program Coding and Staging Manual 2023. National Institutes of Health National Cancer Institute; 2022.
- Cancer registry of Norway statistics bank [Internet]. Accessed 1 June 2022. Available from https://sb.kreftregisteret.no/.
- Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12(2):265–273 e1. doi: 10.1016/j.cgh.2013.03.034.
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912.
- Follin-Arbelet B, Småstuen MC, Hovde Ø, et al. Mortality in patients with inflammatory bowel disease: results from 30 years of follow-up in a Norwegian inception cohort (the IBSEN study). J Crohns Colitis. 2023;17(4):497–503. doi: 10.1093/ecco-jcc/jjac156.
- Bye WA, Nguyen TM, Parker CE, et al. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2017;9(9):CD000279.
- Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: practice guidelines and recent developments. World J Gastroenterol. 2019; 25(30):4148–4157. doi: 10.3748/wjg.v25.i30.4148.
- Nasjonalt kvalitetsregister for tykk- og endetarmskreft, Årsrapport 2020. [Norwegian colorectal cancer registry, annual report 2020]. Cancer registry of Norway; 2021.
- Lundberg FE, Birgisson H, Johannesen TB, et al. Survival trends in patients diagnosed with Colon and rectal cancer in the nordic countries 1990-2016: the NORDCAN survival studies. Eur J Cancer. 2022;172:76–84. doi: 10.1016/j.ejca.2022.05.032.
- Castano-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014; 39(7):645–659. doi: 10.1111/apt.12651.
- Bergquist A, Weismuller TJ, Levy C, et al. Impact on follow-up strategies in patients with primary sclerosing cholangitis. Liver Int. 2023;43(1):127–138. doi: 10.1111/liv.15286.
- Yu J, Refsum E, Helsingen LM, et al. Risk of hepato-pancreato-biliary cancer is increased by primary sclerosing cholangitis in patients with inflammatory bowel disease: a population-based cohort study. United European Gastroenterol J. 2022;10(2):212–224. doi: 10.1002/ueg2.12204.
- Erichsen R, Olen O, Sachs MC, et al. Hepatobiliary cancer risk in patients with inflammatory bowel disease: a scandinavian population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2021;30(5):886–894. doi: 10.1158/1055-9965.EPI-20-1241.
- Sorensen JO, Nielsen OH, Andersson M, et al. Inflammatory bowel disease with primary sclerosing cholangitis: a Danish population-based cohort study 1977-2011. Liver Int. 2018;38(3):532–541. doi: 10.1111/liv.13548.
- Muller M, Broseus J, Feugier P, et al. Characteristics of lymphoma in patients with inflammatory bowel disease: a systematic review. J Crohns Colitis. 2021;15(5):827–839. doi: 10.1093/ecco-jcc/jjaa193.
- Lopez A, Mounier M, Bouvier AM, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(8):1324–1329. doi: 10.1016/j.cgh.2014.02.026.
- Huang SZ, Liu ZC, Liao WX, et al. Risk of skin cancers in thiopurines-treated and thiopurines-untreated patients with inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34(3):507–516. doi: 10.1111/jgh.14533.
- Solitano V, D’Amico F, Correale C, et al. Thiopurines and non-melanoma skin cancer: partners in crime in inflammatory bowel diseases. Br Med Bull. 2020;136(1):107–117. doi: 10.1093/bmb/ldaa033.
- Khan N, Patel D, Trivedi C, et al. Incidence of acute myeloid leukemia and myelodysplastic syndrome in patients With inflammatory bowel disease and the impact of thiopurines on their risk. Am J Gastroenterol. 2021;116(4):741–747. doi: 10.14309/ajg.0000000000001058.
- Pasternak B, Svanstrom H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177(11):1296–1305. doi: 10.1093/aje/kws375.
- Statistics Norway. Percentage daily smokers and occasional smokers, by sex and age (per cent) 1973 – 2021; 2022. [accessed 2022 June 1] Available from https://www.ssb.no/en/statbank/table/05307.
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi: 10.1016/j.ejca.2008.10.037.

