Abstract
Objectives
Alcoholic hepatitis (AH) is a frequent precipitating event for the development of acute-on-chronic liver failure (ACLF), a syndrome characterised by organ failures due to immune dysfunction. The histological features of this complication are not well characterized. We investigated whether ACLF has specific histological characteristics.
Methods
Prospective cohort study in consecutive adult patients admitted between 03-2008 and 04-2021 to a tertiary referral centre with suspected AH. Diagnosis of AH was based on clinical presentation and confirmed by transjugular liver biopsy. All biopsies were assessed by a dedicated liver pathologist, blinded for clinical data and outcome. Diagnosis of ACLF was based on EASL-CLIF criteria. Histological and clinical characteristics of patients with and without ACLF at baseline were compared.
Results
184 patients with biopsy-proven AH were enrolled. Median time from hospital admission to transjugular biopsy was 4.5 days (IQR 2-8). At baseline, ACLF was present in 73 patients (39.7%). Out of the 110 patients without ACLF at baseline, 30 (27.3%) developed ACLF within 28 days (median 7.5 days (IQR 2-20)). At baseline, ductular bilirubinostasis (DB) was the only histological feature significantly more frequently present in patients with ACLF compared to patients without ACLF (50.7% vs. 30.6%, p = 0.003). No clear association between histological features and the development of ACLF later on could be demonstrated.
Conclusions
In this well-defined cohort of patients with biopsy-proven AH, DB was associated with the presence of ACLF. This finding fits with the pathophysiology of this syndrome, which is characterized by systemic inflammation and an increased risk of infections.
Introduction
Alcoholic hepatitis (AH) is an advanced type of alcohol-related liver disease with a high mortality rate [Citation1]. It is a clinical and histological entity with rapid onset of jaundice and liver failure that generally develops in patients with a history of heavy alcohol consumption with or without a background of cirrhosis [Citation2]. It has been shown that severe AH is one of the main precipitating events for the development of acute-on-chronic liver failure (ACLF), a clinical syndrome that is characterized by an acute decompensation of chronic liver disease accompanied by organ failure(s) and a high risk of short-term mortality [Citation3,Citation4]. In patients with ACLF, there is high-grade systemic inflammation and intense immune paralysis, which increases the risk of infections and results in multi-organ failure and death [Citation5–9].
The histological features of ACLF are not well characterized. We demonstrated in the past that ductular bilirubinostasis (DB), described in literature to be an early sign of infection and sepsis, was frequently observed in patients with alcoholic cirrhosis and ACLF [Citation10]. However, this study was performed before well-defined clinical criteria for ACLF were available. Moreover, the control group consisted of patients with chronically decompensated cirrhosis, while AH and ACLF are acute events. Therefore, the applicability of these results is debatable.
The identification of predictors for the development of ACLF is an unmet need. It has been shown that around 23% of the patients with AH develop ACLF within 28 days after diagnosis [Citation11]. Because of the high risk of mortality, it would be of value to identify AH patients at risk for developing ACLF at an early stage when deterioration of the disease might still be prevented [Citation12]. Literature on the role of histology in the prediction of the development of ACLF is lacking. We hypothesized that distinct histological features are present in patients with ACLF or in those at high risk of development of ACLF. If histological features are already present before the clinical syndrome of ACLF develops, these features may have potential as a predictor for the development of ACLF. Therefore, we investigated in this group of patients in more detail the histological characteristics of ACLF and explored whether they contribute to the diagnosis and prediction of ACLF.
Materials and methods
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and approval was obtained by the local ethics committee (UZ Leuven reference number S63917).
Study population
Consecutive adult patients admitted to the liver unit of the tertiary care centre University Hospitals Leuven between March 2008 and April 2021 with a diagnosis of AH were included in a prospective observational dataset. Diagnosis of AH was based on clinical presentation and biochemical values in combination with histological confirmation on transjugular biopsy. Exclusion criteria were: 1) inconclusive biopsy results, 2) concomitant causes of liver disease (e.g. hepatitis B, hepatitis C, etc.), 3) presence of hepatocellular carcinoma, and 4) previous liver transplantation.
The ethical committee provided exemption for written informed consent due to the fact at time of initiation of the study no informed consent was required for observational studies. Because of the results found in our previous study [Citation10], in 2008 biopsies for all suspected patients with ASH were incorporated in the local protocol of the University Hospitals Leuven as standard of care.
Study design and objectives
Demographic, clinical, and analytical data were collected from date of biopsy (time zero) until 1 year after biopsy. At the time of biopsy, information was collected on clinical symptoms and complications of AH (i.e. gastroesophageal varices, hepatic encephalopathy, ascites, infection), laboratory values and clinical scores (i.e. Child Pugh score, Model for End-stage Liver Disease (MELD) score, Maddrey Discriminant Function (mDF), CLIF-C AD score, CLIF-C ACLF score). Of note, no data is available on alcohol consumption before hospitalization, transaminases or phosphatidylethanol levels. Moreover, during the transjugular biopsy procedure, the hepatic venous pressure gradient was measured as described previously [Citation13].
All transjugular liver biopsies were reviewed by an expert liver pathologist (T.A.R.). The pathologist was blinded for clinical parameters and outcomes. Review of the biopsies included the assessment of the presence of cirrhosis, presence of either parenchymal or ductular bilirubinostasis, grade of macrovesicular steatosis, presence of ballooning, Mallory bodies and lobular neutrophil infiltration. Histological grading was assessed according to the recently proposed SALVE grading system for alcohol-related liver disease [Citation14]. Furthermore, information on the use of corticosteroids, development of infection and use of antibiotic therapy, development of ACLF, liver transplantation and survival were collected.
Patients with a mDF ≥32 and in the absence of contraindications received a corticosteroid treatment according to current guidelines [Citation1]. Patients with clinical complications of decompensated cirrhosis, such as ascites, spontaneous bacterial peritonitis or variceal bleeding, were treated according to international guidelines [Citation1,Citation15].
Definitions
Clinical diagnosis of AH was based on the criteria of the European Association for the Study of the Liver (EASL) guidelines [Citation1]. Clinical diagnosis of severe AH was defined as a mDF ≥32. ACLF was defined according to the EASL-CLIF consortium criteria proposed by Moreau et al. [Citation3] Grade of ACLF was based on the number and type of organ failures as defined by the EASL-CLIF consortium criteria [Citation3]. Infection was defined as having a polymorphonuclear cell count in ascites fluid ≥250/mm3 or a positive ascitic fluid culture; having a positive blood culture; having a positive urinary culture; having lesions on chest radiography suspected for pneumonia.
Histological diagnosis of AH was based on presence of hepatocyte ballooning and/or Mallory bodies in combination with presence of lobular neutrophil infiltration and satellitosis. Histological severity of AH was graded according to severity of lobular neutrophil infiltration on histology and was subdivided into two classes: (i) mild AH and (ii) moderate to severe AH.
Statistical analysis
Normally distributed data are reported as mean ± standard and not normally distributed data as median and interquartile range. Categorical variables are reported as number and percentage. Group comparisons were performed using Student’s t-test, Mann–Whitney U-test or Chi-squared test, according to the type of data. Correlations between variables were analysed using Chi-squared test or Spearman’s ρ, according to the type of data. To adjust for possible confounding factors in the correlations a multivariate logistic regression was performed.
Mortality rates were estimated using Kaplan–Meier analysis censoring patients receiving liver transplantation (LT).
To investigate ACLF development, a subgroup analysis was performed in patients without ACLF at baseline. A competing risk analysis was used, censoring patients who died or received a liver transplantation without the development of ACLF. Moreover, corticosteroid treatment was used as time-dependent variable. To estimate clinical and histological predictors for progression of AH to ACLF within 28 and 90 days a cause-specific univariate and multivariate Cox proportional hazard model was used. A multivariable model was built based on predefined variables which were chosen according to expert knowledge and literature search. A p-value of < 0.05 was considered to be statistically significant. For all statistical analyses, the R-software environment version 4.1.1 was used.
Results
Patient characteristics
A total of 184 cases met the inclusion criteria and were included. In 16 patients (8.7%) a new episode of AH occurred after >1 year of follow up and also this new episode of AH was taken in consideration. Median follow-up time was 365 days (interquartile range [IQR] 83.5-365). Median time from hospital admission to transjugular biopsy (time zero) was 4.5 days (IQR 2-8).
In , the demographic and clinical characteristics at baseline are shown. Mean age of the study population was 51.1 ± 10.6 and about 53% of the population was male. 152 (82.6%) patients presented with a clinical diagnosis of severe AH. ACLF was present in 73 patients (39.7%) at baseline and within this group of patients 22 patients (30.1%) were diagnosed with ACLF grade I, 34 (46.6%) with grade II and 16 (21.9%) grade III, in 1 patient (1.4%) the grade of ACLF was unknown. As expected, patients with ACLF were more severely ill as reflected in a higher MELD score; they had higher levels of bilirubin (median 19.6 mg/dl (IQR 13–27.2) vs. 9 mg/dl (IQR 5.3–14.9)) and creatinine (median 1.3 mg/dl (IQR 0.7–1.8) vs. 0.7 mg/dl (IQR 0.5–0.9)) and presented more frequently with hepatic encephalopathy (65.8% vs. 19.8%) and ascites (94.5% vs. 73.9%). Moreover, they had a higher level of white-cell count (median 10.9 × 109/L (IQR 7.6–16.1) vs. 8.6 × 109/L (IQR 5.3–11.9)) and more frequently a proven infection (52.1% vs. 27%).
Table 1. Demographical and clinical characteristics of the total population at baseline and a comparison of the patients with and without ACLF at baseline.
During 1 year of follow-up, 72 patients (65.5%) received corticosteroid therapy and the mean starting time of corticosteroid treatment after transjugular biopsy was 2.9 ± 4.4 days with a mean duration of corticosteroid treatment of 24.1 ± 9.7 days. Of note, 9 patients (4.9%) received corticosteroids before transjugular biopsy was performed. In total 12 patients (6.5%) received a liver transplantation within 1 year of follow-up. The median time to from diagnosis to transplantation was 188.5 days (IQR 112.75–228.25). The 90-day and 1-year transplant-free mortality rates of the complete cohort were 25.2% and 36% respectively. When differentiating between patients with and without ACLF at baseline, the 28-day and 90-day transplant-free mortality rates were 0.9% and 14.5% respectively for the group of patients without ALCF at baseline, and 20.5% and 41.1% for the group with ACLF at baseline (Supplementary Figure 1).
Histological features
The histological characteristics of the study group are shown in . The majority of patients was diagnosed with a histological moderate/severe grade AH (81.5%). No difference in histological grade of AH was found between the group with and without ACLF at baseline, nor was there a difference in the SALVE activity score between the groups. DB () was the only histological marker found to be significantly more frequently present in patients with ACLF compared to patients without ACLF (53.4% vs. 30.6%, p = 0.003).
Figure 1. (A) ductular bilirubinostasis: at the edge of a portal tract (PT), a ductule with a bilirubins plug in its lumen is seen (arrow) vs (B) parenchymal bilirubinostasis: at a distance of the PT bilirubin droplets in the cytoplasm of hepatocytes are seen. Original magnification x400.
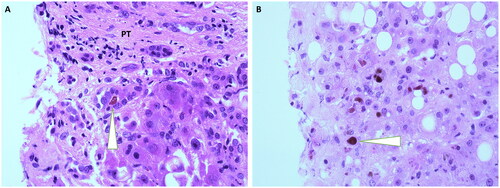
Table 2. Histological characteristics of the total population at baseline and a comparison of the patients with and without ACLF at baseline.
Association of ductular bilirubinostasis and clinical and biochemical features
Associations between DB and clinical and biochemical features are shown in . In patients with ACLF at baseline, presence of DB was not correlated to grade of ACLF or specifically liver failure (defined by the CLIF-C ACLF criteria as bilirubin levels ≥12 mg/dL) (p = 0.7 and p = 0.6, respectively). Presence of DB was significantly correlated with serum bilirubin levels (median bilirubin level 10.3 mg/dl (IQR 5.4.-18.8) vs 15.1 mg/dl (IQR 8.1–24.7) in patients without and with DB respectively, p = 0.005). However, in multivariate logistic regression bilirubin levels did not influence the association between the presence DB and the presence of ACLF (DB: OR 2.09, 95% CI 1.07–4.1, p = 0.03; bilirubin: OR 1.1, 95% CI 1.06–1.14, p < 0.001). Moreover, in patients with DB a trend towards a higher c-reactive protein (CRP) level was observed, although this did not reach statistical significance (median CRP 25.6 mg/L (IQR 10.8–42.9) vs 32.6 mg/L (IQR 14.7–57.9) in patients without and with DB respectively, p = 0.07).
Table 3. Association of ductular bilirubinostasis and clinical and biochemical features.
Clinical and histological features associated with development of ACLF
To analyse features associated with development of ACLF, a subgroup-analysis was performed in patients without ACLF at baseline. Since one patient had missing follow-up data, 110 patients were included in this analysis. Out of these, 30 patients (27.3%) developed ACLF within 28 days after biopsy (characteristics are shown in supplementary table 1) and 39 (35.1%) within 90 days. One patient was censored due to dying without developing ACLF within 90 days. The median time to development of ACLF was 7.5 days (IQR 2-20). Since most events occurred within the first 28 days, we focused in the following analysis on the 28-day follow-up data. Results of a univariate cause-specific Cox regression analysis for the development of ACLF within 28 days, with all main characteristics at baseline as covariates, are shown in . Regarding clinical features, a higher age (Cause-specific Hazard Ratio [HRcs] 1.1, 95% Confidence Interval [CI] 1-1.1, p = 0.016), a higher creatinine concentration (HRcs 7.2, 95% CI 2.6–19.8, p < 0.001), a higher MELD score (HRcs 1.2, 95% CI 1.1–1.3, p = 0.004) and a higher CLIF-C AD score (HRcs 1.1, 95% CI 1–1.1, p < 0.001) were associated with the development of ACLF within 28 days. Regarding histological characteristics, no associations with the development of ACLF within 28 days were found. A multivariate model was built, and the CLIF-C AD score had the highest performance in predicting ACLF development within 28 days (). To investigate whether corticosteroid treatment was associated with the development of ACLF, a univariate analysis with corticosteroid treatment as time-dependent variable was performed. No significant relation between corticosteroid treatment and the development of ACLF within 28 days was observed (HRcs 0.87, 95% CI 0.44–1.71, p = 0.7).
Table 4. Uni- and multivariate cause-specific Cox proportional hazard model for association of development of ACLF within 28 days.
Discussion
In this prospective cohort study in patients diagnosed with biopsy-proven alcoholic hepatitis, we observed that ductular bilirubinostasis is a histological marker of the presence of ACLF but not for the development of ACLF.
The baseline characteristics and clinical outcomes of our study group are in line with the data previously published and therefore the results of this study are applicable for patients with AH in general. To illustrate this; within the group of patients without ACLF at baseline, 27.3% developed ACLF within 28 days after biopsy. This finding is in line with previous observation that around 23% of the patients with AH (mostly not biopsy-proven) develop ACLF within 28 days after diagnosis [Citation11]. The 90-day transplant-free mortality rate in this cohort of patients with AH was 25%, which is also in line with the literature [Citation16].
Although several large studies have been conducted in patients with ACLF and alcoholic cirrhosis, the histological features of ACLF are not well characterized. Histology is the golden standard to confirm the diagnosis of AH [Citation1,Citation10,Citation17–20]. However, the guidelines from the American Association for the Study of Liver Disease (AASLD) and EASL differ in their recommendations for the use of biopsy [Citation1,Citation21]. Moreover, a percutaneous liver biopsy bears a risk in this patient group, and the safer option of transjugular biopsy is not widely available [Citation22,Citation23]. For this reason, liver biopsies are not routinely performed, making it difficult to study the role of histology in the management of patients with AH and ACLF. In our third-line centre, all patients with suspicion of AH systematically underwent a transjugular biopsy. Therefore, we were able to study histology in more detail in a large prospective cohort of patients with AH and ACLF.
In a prior study, we found that DB was frequently observed in patients with alcoholic cirrhosis and ACLF [Citation10]. However, this study was performed before the EASL-CLIF criteria were defined [Citation24], resulting in the use of a non-uniform definition of ACLF. Moreover, the specificity of this finding with regard to the role of acute decompensation could not be assessed, since the control group only consisted of chronically decompensated patients and finally the study was not performed in a homogeneous group of patients with AH. Since in our study cohort almost 40% of the patients admitted to the hospital with AH already had developed ACLF we created a homogenous group of patients with AH in which we were able to compare the characteristics of patients with and without ACLF.
In the present study, we found that DB is a marker of ACLF in patients with AH. However, with a specificity of 69.1% this finding is not sufficient to use for the diagnosis of ACLF in clinical practice. DB is the descriptive term for the presence of bile plugs in dilated ductules with often damaged epithelial cells. It has been reported to be related to portal-tract inflammation and considered as an early sign of sepsis [Citation10,Citation25,Citation26]. Therefore, this is an interesting finding, considering that in patients with ACLF there is high-grade systemic inflammation and intense immune paralysis, which increases the risk of infections [Citation5–9]. In our study, we observed a higher level of CRP and white-cell count and more infections in patients with ACLF. Moreover, we also observed a trend towards a higher CRP level in patients with DB, although not statistically significant. A direct correlation between the presence of DB and the presence of infection/positive blood culture could not be found in this cohort. With DB being a marker for ACLF, one could hypothesize that this histological change may be the result of the altered gut permeability and consequently systemic inflammation found in decompensated cirrhosis, especially since it is known that the severity of the systemic inflammation is correlated with the severity of decompensation and ACLF [Citation4,Citation27].
Since ACLF is associated with a very high short-term mortality rate, early recognition is important [Citation28]. Therefore, considering the association of presence of DB and ACLF in this cohort at baseline, we subsequently investigated DB as potential predictor of development of ACLF in patients without ACLF at baseline. Both in univariate and multivariate analysis, DB was not associated with short-term ACLF development. Neither could we detect any other histological marker, such as the histological severity of AH to predict ACLF development.
A general limitation of histological studies in patients with alcohol-related liver disease is the lack of a uniform agreement on the assessment of the biopsies. Several grading systems have been proposed in the past years, but to date none has been widely and uniformly adopted [Citation14,Citation17,Citation18]. In our study, we chose to follow the SALVE grading system, which was recently developed and validated by the SALVE Histopathology Group (SHG) [Citation14]. Besides DB, none of the other histological characteristics used in the SALVE grading system was found to be associated with ACLF. However, we cannot exclude the possibility that other histological characteristics which have not been investigated in the current study, may also be associated with ACLF. Another limitation is introduced by using the moment of transjugular biopsy as time point zero. The median time from hospital admission to transjugular biopsy was 4.5 days. Since the time period between precipitating event and ACLF development is known to be very short (i.e. most frequently within 28 days) [Citation29] the chosen timepoint zero may lead to a time bias. However, for the purpose of this study this was the only correct way to handle the data.
In conclusion, in this well-defined, large cohort of patients admitted to the hospital with AH, we show that ductular bilirubinostasis is associated with the presence of ACLF. This finding fits with the pathophysiology of this syndrome characterized by systemic inflammation and an increased risk of infections. Moreover, it shows that ACLF is not only a clinical entity, but also is reflected in the histological findings. Despite this, besides the well-known clinical predictors for the development of ACLF, no histological marker for prediction of the development of ACLF was identified. The findings of this study indicate that liver biopsy has no role in the diagnosis of ACLF or in the prediction of ACLF development. Further research is needed to investigate the place of biopsies in patients with ACLF in clinical practice.
Financial support statement
L.VM. was supported by the Research Foundation of the Flemish Government (FWO) [grant number 1110121 N].
| Abbreviations | ||
| AH | = | Alcoholic hepatitis |
| ACLF | = | Acute-on-chronic liver failure |
| DB | = | Ductular bilirubinostasis |
| CLIF-C | = | CLIF Consortium |
| CLIF-C AD | = | CLIF Consortium Acute Decompensation |
| MELD | = | Model for end-stage liver disease |
| mDF | = | Maddrey Discriminant Function |
| EASL | = | European Association for the Study of the Liver |
| LT | = | Liver transplantation |
| IQR | = | Interquartile range |
| HRcs | = | Cause-specific Hazard Ratio |
| CI | = | Confidence interval |
| AASLD | = | Association for the Study of Liver Disease |
| SHG | = | SALVE Histopathology Group |
Supplemental Material
Download Zip (23 KB)Acknowledgements
We would like to thank Jolien Derdeyn for her contribution to the set-up of the dataset. We would like to thank Natalie van den Ende for her administrative help during this study. Preliminary data of this study have been presented during the following international meetings; during the International Liver Congress in 2022 in the form of a poster presentation and during the United European Gastroenterology Week in 2022 in the form of an oral presentation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360(26):2758–2769. doi: 10.1056/NEJMra0805786.
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437.e9., 37 e1-9. doi: 10.1053/j.gastro.2013.02.042.
- Trebicka J, Fernandez J, Papp M, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097–1108. doi: 10.1016/j.jhep.2020.11.019.
- Korf H, Du Plessis J, van Pelt J, et al. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut. 2019;68(10):1872–1883. doi: 10.1136/gutjnl-2018-316888.
- Bernsmeier C, van der Merwe S, Périanin A. Innate immune cells in cirrhosis. J Hepatol. 2020;73(1):186–201. doi: 10.1016/j.jhep.2020.03.027.
- Van der Merwe S, Chokshi S, Bernsmeier C, et al. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1(Suppl 1):S82–S100. doi: 10.1016/j.jhep.2020.11.029.
- Albillos A, Martin-Mateos R, Van der Merwe S, et al. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19(2):112–134. doi: 10.1038/s41575-021-00520-7.
- Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2(1):16041. doi: 10.1038/nrdp.2016.41.
- Katoonizadeh A, Laleman W, Verslype C, et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010;59(11):1561–1569. doi: 10.1136/gut.2009.189639.
- Sersté T, Cornillie A, Njimi H, et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J Hepatol. 2018;69(2):318–324. doi: 10.1016/j.jhep.2018.02.022.
- Jalan R, Pavesi M, Saliba F, et al. The CLIF consortium acute decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62(4):831–840. doi: 10.1016/j.jhep.2014.11.012.
- Maleux G, Willems E, Fieuws S, et al. Prospective study comparing different indirect methods to measure portal pressure. J Vasc Interv Radiol. 2011;22(11):1553–1558. doi: 10.1016/j.jvir.2011.08.003.
- Lackner C, Stauber RE, Davies S, et al. Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J Hepatol. 2021;75(4):810–819. doi: 10.1016/j.jhep.2021.05.029.
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024.
- Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372(17):1619–1628. doi: 10.1056/NEJMoa1412278.
- Mookerjee RP, Lackner C, Stauber R, et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55(5):1103–1111. doi: 10.1016/j.jhep.2011.02.021.
- Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146(5):1231–1239. e1-6. doi: 10.1053/j.gastro.2014.01.018.
- Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618. doi: 10.1016/j.jhep.2016.11.011.
- Spahr L, Rubbia-Brandt L, Genevay M, et al. Early liver biopsy, intraparenchymal cholestasis, and prognosis in patients with alcoholic steatohepatitis. BMC Gastroenterol. 2011;11(1):115. doi: 10.1186/1471-230X-11-115.
- O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307–328. doi: 10.1002/hep.23258.
- Filingeri V, Francioso S, Sforza D, et al. A retrospective analysis of 1.011 percutaneous liver biopsies performed in patients with liver transplantation or liver disease: ultrasonography can reduce complications? Eur Rev Med Pharmacol Sci. 2016;20(17):3609–3617.
- Forrest EH, Gleeson D. Is a liver biopsy necessary in alcoholic hepatitis? J Hepatol. 2012;56(6):1427–1428; author reply 8-9. doi: 10.1016/j.jhep.2011.12.028.
- Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012.
- Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and "cholangitis lenta. Hum Pathol. 1982;13(1):19–24. doi: 10.1016/s0046-8177(82)80134-2.
- Crawford JM, Boyer JL. Clinicopathology conferences: inflammation-induced cholestasis. Hepatology. 1998;28(1):253–260. doi: 10.1002/hep.510280133.
- Arroyo V, Angeli P, Moreau R, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74(3):670–685. doi: 10.1016/j.jhep.2020.11.048.
- Hernaez R, Kramer JR, Liu Y, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: a national cohort study from the USA. J Hepatol. 2019;70(4):639–647. doi: 10.1016/j.jhep.2018.12.018.
- Trebicka J, Fernandez J, Papp M, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842–854. doi: 10.1016/j.jhep.2020.06.013.

