Abstract
Reflux hypersensitivity (RH) is a subtype of gastroesophageal reflux disease. The Rome IV criteria separated RH from the original nonerosive reflux disease subgroup and classified it as a new functional oesophageal disease. Recently, the pathogenesis of RH has become the focus of research. According to the latest research reports, upregulation of acid-sensitive receptors, distribution of calcitonin gene-related peptide-positive nerve fibres, and psychiatric comorbidity have key roles in the pathogenesis of RH. This work reviews the latest findings regarding RH mechanisms.
Introduction
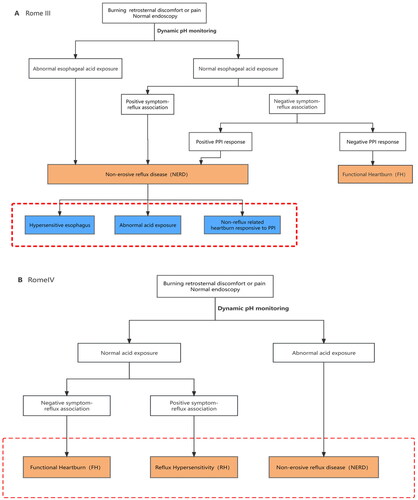
The definition and composition of functional oesophageal diseases have been evolving, and the Rome IV criteria have redefined hypersensitive oesophagus as reflux hypersensitivity (RH) and included it in the category of gut–brain interaction disorders [Citation1] (). RH is defined as patients with typical reflux symptoms (such as heartburn or chest pain), normal endoscopy and physiological oesophageal acid exposure, but positive correlation of symptoms to reflux events [Citation2]. Acid exposure time (AET) was obtained by dynamic reflux monitoring and the Lyon Consensus [Citation3] suggests that AET <4% is physiologic reflux, >6% is pathologic reflux, when AET is between 4% and 6%, reflux monitoring is considered as an auxiliary measure. As for reflux-symptom association, symptom index (SI) and symptom association probability (SAP) are commonly used indicators. When symptoms like heartburn or chest pain appeared within 2 min of the reflux event, then they were considered reflux-related. SI is the proportion of reflux-related symptoms to the total number of reflux symptoms which considered positive when ≥50%. SAP positive is defined in a similar way. Because of the novelty of the Rome IV criteria and the low prevalence of dynamic monitoring techniques for reflux, it is difficult to estimate the population-based prevalence of RH. One study found that among 351 patients with significant heartburn symptoms but negative endoscopic findings, 32% had non-erosive reflux disease (NERD) and 26% had RH [Citation4]. Another study showed that the prevalence of RH and that of NERD were approximately similar among patients with heartburn and negative endoscopic findings [Citation5]. Although RH is now classified as a functional oesophageal disease, the associated symptoms produced by the disease not only reduce the quality of life but also create great financial stress and psychological burden on patients and their families; therefore, it is crucial to explore the pathogenesis associated with RH. However, the pathophysiology of RH has not been fully elucidated. Some studies have shown that the occurrence of RH may be related to the upregulation of acid-sensitive receptors, distributional differences in calcitonin gene-related peptide (CGRP)-positive nerves, and psychiatric comorbidity. During this study, the mechanisms of RH were discussed.
Figure 1. The evolution of reflux hypersensitivity (hypersensitive oesophagus) from Rome III to Rome IV. In Rome III, hypersensitive oesophagus is a subtype of non-erosive reflux disease. However, Rome IV separate hypersensitive oesophagus from NERD as a new functional oesophageal disease. And it should be noted that Rome IV states that RH can partially overlap with GERD.
Pathogenesis of RH
Upregulation of acid-sensitive receptors
Transient receptor potential vanilloid
Transient receptor potential vanilloid 1 (TRPV1) is a subfamily of the transient receptor ion channel family and a nonselective ligand-gated cation channel receptor that is commonly known as the capsaicin receptor. TRPV1 is a key injury receptor in mammals and a major acid receptor in oesophageal epithelial cells; furthermore, it is expressed in both neuronal and epithelial cells [Citation6]. TRPV1 usually labels unmyelinated and slow-conducting class C nerve fibres, which synthesize and release substance P, CGRP, and neurokinin, which are neurotransmitters that have important roles in pain signalling pathways [Citation7]. It has been suggested that TRPV1 may be crucial to the oesophageal hypersensitivity response [Citation8]. Through in vitro experimental studies, Cheng et al. [Citation9] demonstrated that acid exposure promotes the activation of TRPV1 in the feline oesophageal mucosa and induces the synthesis and release of sensory transmitters (such as CGRP and substance P), thereby inducing neurogenic inflammation. By creating a mouse model of acid reflux, Zhang et al. [Citation10] discovered that TRPV1 expressions in the upper oesophagus and lower oesophagus of mice were significantly different, and that the density of TRPV1 in the lower oesophageal epithelium was significantly higher than that in the upper oesophagus, which is usually the site of reflux perception. Therefore, it can be assumed that there is a positive correlation between the perception of reflux and density of TRPV1. Ustaoglu et al. [Citation8] studied human oesophageal epithelial cells and found that patients with NERD had considerably higher TRPV1 expression on superficial sensory nerves than those with Barrett’s oesophagus or erosive reflux disease. In 2021, the function of TRPV1 in the distal oesophageal mucosa of patients with RH was first evaluated at Shanghai Tongji Hospital [Citation11]. The TRPV1-positive cell density was moderately correlated and highly correlated with heartburn and chest pain, respectively; additionally, it was found that TRPV1-positive cell density decreased significantly in patients after distal oesophageal mucosal radiofrequency ablation, whereas symptoms such as heartburn and chest pain significantly improved. These results suggest that symptom improvement after RF ablation is closely associated with downregulation of TRPV1, and that higher TRPV1 afferent fibre density may lead to significant heartburn and chest pain symptoms, thus partially explaining the refractory symptoms of patients with RH in the setting of physiologic reflux, suggesting that alterations in distal oesophageal TRPV1 may be important pathophysiological factors and therapeutics target for the treatment of RH. However, the exact mechanism by which the increased sensitivity or overexpression of TRPV1 receptors occurs is not yet fully understood.
Acid-sensing ion channels
It has been pointed out that the same stimulus can interact with multiple nociceptors, as shown that extracellular protons can activate not only TRPV1 but also Acid-sensing ion channels (ASICs) [Citation12]. ASICs are proton-gated trimeric channels that belong to a family of epithelial amiloride-sensitive sodium channels that are expressed in both the peripheral and central nervous systems and have a key role in the transduction of nociceptive and pain signals [Citation13, Citation14]. ASICs have six subtypes, including ASIC1a and ASIC1b, ASIC2a and ASIC2b, ASIC3, and ASIC4 [Citation15]. Among the ASICs, ASIC1b and ASIC3 are the most sensitive to protons [Citation16]. By establishing a mouse model, Bielefeldt et al. [Citation17] found that ASIC3 and TRPV1 ion channel knockout mice did not respond to acidification in the lumen of the oesophagus. Another study found that proton-evoked membrane currents were significantly reduced in dorsal root ganglia or sensory nerve fibres from TRPV1-deficient mice. However, the responses induced by proton were not completely eliminated, which may be mediated by ASICs [Citation12]. To confirm the possibility that ASIC3 upregulation contributes to enhanced pain perception, Han et al. [Citation18] performed an animal study and found that intrathecal injection of ASIC3-specific inhibitors in mice with NERD resulted in the inactivation of ASIC3, thereby producing significant analgesia. This result further supports the role of ASIC3 in the perception of a chemical stimulus. Therefore, several investigators have suggested that visceral hypersensitivity associated with NERD is partially mediated by ASIC3 upregulation. It can be hypothesized that acid exposure disrupts the intercellular junctions of the oesophageal mucosa and increases oesophageal permeability, thus making it easy for acid to penetrate the submucosa of the oesophagus to reach acid-sensing nociceptive receptors, such as ASIC1 and ASIC3, thereby leading to heartburn, chest pain, and other obvious reflux symptoms. The pathophysiological mechanisms underlying the high expression of ASIC3 in the oesophageal epithelial cells of patients with NERD require further investigation. However, the identification and development of specific antagonists of ASICs may result in an effective pharmacological strategy for the treatment of NERD and RH cases that do not respond to conventional acid suppression therapy.
Proteinase-activated receptor 2
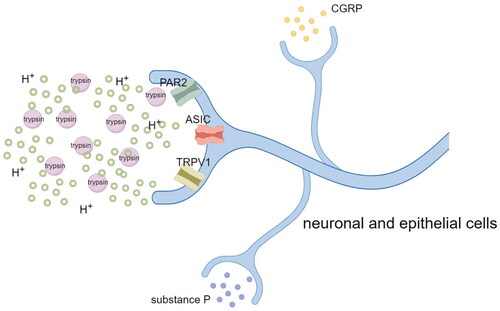
Proteinase-activated receptor 2 (PAR2) is a member of the G-protein-coupled receptor superfamily that is activated by serine proteases or mast cell-derived trypsin and expressed in gastrointestinal epithelial cells, peripheral neurons, and spinal afferent nerves [Citation6, Citation19]. Acid exposure promotes high expression of PAR2 in oesophageal squamous epithelial cells [Citation20, Citation21], which are highly susceptible to contact with substances such as trypsin in the regurgitated material, thereby leading to massive PAR2 activation. PAR2 activation mediates neurogenic inflammation by releasing large amounts of IL-8, IL-1β, and tumour necrosis factor-α, and it triggers nociception by inducing the release of neuropeptides such as substance P and CGRP [Citation22, Citation23] (). Additionally, Dai et al. [Citation24] found that PAR2 activation enhances the activity of the transient receptor potential A1 ion channel, thus leading to the amplification of nociception. Therefore, the selection of appropriate receptor antagonists as therapeutic targets for patients with RH (especially those with RH that does not respond to acid suppression therapy) may be an effective therapeutic approach that deserves further investigation.
Figure 2. Acid-sensitive receptors (nociceptors) such as TRPV1, ASICs, and PAR2 expressed by peripheral neurons or oesophageal epithelial cells are activated by protons or trypsin. Activation of nociceptors not only causes sensory nerve endings to release neurotransmitters such as CGRP and substance P to mediate neurogenic inflammation, but also transmits the information to the dorsal horn of the spinal cord (and from the dorsal horn of the spinal cord to the brain) thus leading to the occurrence of pain. (Figure by figdraw.).
Distributional differences in CGRP-positive nerves
It has been reported that the distribution of nerve fibres in the oesophageal mucosa may have a role in RH [Citation25]. Afferent nerve fibres in the distal oesophagus are generally located in the deeper mucosal epithelial layers and are usually not readily accessible to noxious reflux material [Citation26]. A prospective study by Woodland et al. (which included 13 patients with NERD, 11 patients with erosive reflux disease, 16 patients with Barrett’s oesophagus, and 10 healthy volunteers) found no significant differences in the distribution of CGRP-positive nerves in the oesophageal mucosa of patients with erosive reflux disease or Barrett’s oesophagus when compared to healthy volunteers; however, on the contrary, the CGRP-positive nerve fibres were significantly more superficial in NERD, both the proximal and distal oesophageal mucosa [Citation25]. CGRP is a neuropeptide that is widely distributed in the human nervous system and participates in the transmission of nociceptive information and the formation of nociception in the peripheral and central nervous systems; furthermore, it is a marker of nociceptive sensory nerve fibres. Therefore, Woodland et al. [Citation25] suggested that acid hypersensitivity of patients with NERD (when RH is a subgroup of NERD) may be partially explained by the increased proximity of their afferent nerves to the oesophageal lumen and greater exposure to toxic substances in the refluxate, which may caused by the injury of the mucosal integrity. As mentioned by the numerous studies, the key factor in the pathophysiology of NERD is the impaired integrity of the oesophageal mucosa resulting in dilated intercellular spaces (DIS) [Citation26–29]. The conclusions of Nikaki et al. [Citation30] and Ustaoglu et al. [Citation8] were consistent with those of Woodland et al. [Citation25], thereby successfully confirming their results.
The mechanism underlying the more superficial nerve location in NERD is unknown; however, it may involve mechanical forces pushing the mucosal nerves toward the surface because of basal cell hyperplasia or refluxate-induced synthesis of neurotrophic factors in the superficial epithelium. However, this has not been proven.
Psychiatric comorbidity
Based on clinical data, it is not uncommon for patients with RH to experience anxiety or depression, and their levels of anxiety or depression are significantly greater than those of patients with significant oesophageal mucosal injury, such as reflux oesophagitis [Citation31]. Many studies have shown that RH is strongly associated with psychosomatic factors such as anxiety and depression [Citation2, Citation31, Citation32]. Jansson et al. [Citation33] performed a large, population-based, case-control study and found that there was a strong dose–response association between anxiety and depression and the risk of reflux; specifically, compared to patients without anxiety or depression, the risk of reflux increased 3.2-fold for patients with anxiety, 1.7-fold for patients with depression, and almost 4-fold for patients with severe anxiety and depression. Sharma et al. [Citation34] first reported that anxiety amplifies central oesophageal sensitivity in humans; furthermore, their study found that acute stress increases the permeability of the oesophageal wall, thereby making it easier for gastric acid to reach oesophageal nociceptive receptors. It has been hypothesised that stress may cause changes in oesophageal mucosal permeability through degranulation, tight junctions of the mast cells, or redistribution of bridging grains [Citation35].
Low doses of tricyclic antidepressants or 5-hydroxytryptamine reuptake inhibitors can significantly reduce noncardiac chest pain and have a positive therapeutic effect on patients with symptoms such as heartburn and reflux [Citation36, Citation37]. A prospective study by Viazis et al. [Citation38] was the first to report the use of selective serotonin reuptake inhibitors for patients with oesophageal allergies. Their study clearly demonstrated that, compared to placebo, citalopram (a selective serotonin reuptake inhibitor) significantly improved the symptoms of heartburn and reflux caused by oesophageal allergy. Ostovaneh et al. [Citation39] compared the efficacy of omeprazole and fluoxetine for the treatment of heartburn experienced by patients with negative endoscopic results and found that fluoxetine was significantly more effective than omeprazole and placebo for improving heartburn symptoms of patients with physiological acid exposure. During an 8-week, double-blind, placebo-controlled trial, imipramine resulted in a significant improvement in the quality of life of patients with RH [Citation40]. During another randomised, controlled trial of patients with noncardiac chest pain, a comparison of sertraline treatment and placebo treatment showed that oesophageal pain associated with oesophageal allergy was significantly reduced after 8 weeks of sertraline treatment [Citation41]. Coss-Adame et al. [Citation42] concluded that promethazine, trazodone, citalopram, sertraline, venlafaxine, and paroxetine significantly improved the symptoms of oesophageal chest pain caused by oesophageal allergy.
The hypersensitivity to physiological acid reflux occurring in RH may be attributable to the peripheral sensitisation of the oesophageal nerves, resulting in an enhanced response to intraluminal stimuli or altered neuromodulation in the central nervous system [Citation43]. Furthermore, noncardiac chest pain associated with reflux may be related to changes in the central level of pain perception as well as the visceral hypersensitivity reaction [Citation44]. Antidepressants may improve nociceptive sensitisation in the peripheral nervous system and central nervous system via the following two mechanisms: the interference of antidepressants with the transmission of nociception to the spinal cord through a complex downstream projection system in the nucleus accumbens and the interference of antidepressants with pain-related brain circuits through their monoaminergic effects [Citation45]. Therefore, anxiolytics or antidepressants are promising treatment options for most patients with hypersensitivity to reflux. Furthermore, it may be more beneficial to avoid empirical proton pump inhibitors and use anxiolytics or antidepressants instead.
3 Conclusion
RH is a functional oesophageal disease that seriously affects the quality of life; however, its mechanism remains to be elucidated. Upregulation of acid-sensitive receptors, distributional differences in CGRP-positive nerve fibres, and Psychiatric comorbidity are potential factors associated with its mechanism. Since there are few reports of acid-sensitive receptor-specific antagonists for clinical use, there are currently no suitable therapeutic targets in this area, which is a direction that we can focus on exploring in future studies. However, unlike the former, neuromodulators (including tricyclic antidepressants, 5-hydroxytryptamine reuptake inhibitors, norepinephrine reuptake inhibitors, etc.) have a positive effect on RH with psychiatric comorbidity and have been clinically demonstrated [Citation40, Citation46]. Unfortunately, not many clinical trials with neuromodulators have been reported, so more controlled trials should be conducted to evaluate the therapeutic benefits of such drugs for RH in the future. In addition, it has also been suggested that RH may be associated with impaired integrity of oesophageal mucosal [Citation47], which makes proton pump inhibitor perhaps one of the effective treatments as well. So far, both the diagnosis and treatment of RH are still immature, which require us to actively carry out relevant clinical trials and continuously research and explore.
Acknowledgments
We thank the patients who provided the data for above studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schmulson MJ, Drossman DA. What is new in rome IV. J Neurogastroenterol Motil. 2017;23(2):151–163. doi: 10.5056/jnm16214.
- Aziz Q, Fass R, Gyawali CP, et al. Functional esophageal disorders. Gastroenterology. 2016;150(6):1368–1379. doi: 10.1053/j.gastro.2016.02.012.
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67(7):1351–1362. doi: 10.1136/gutjnl-2017-314722.
- Gao F, Gao Y, Chen X, et al. Comparison of oesophageal function tests between Chinese non-erosive reflux disease and reflux hypersensitivity patients. BMC Gastroenterol. 2017;17(1):67. doi: 10.1186/s12876-017-0624-7.
- Savarino E, Zentilin P, Tutuian R, et al. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol. 2012;47(2):159–168. doi: 10.1007/s00535-011-0480-0.
- Altomare A, Luca Guarino Sara Emerenziani MP, Cicala M, et al. Gastrointestinal sensitivity and gastroesophageal reflux disease. Ann N Y Acad Sci. 2013;1300(1):80–95. doi: 10.1111/nyas.12236.
- Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. doi: 10.1002/cne.20794.
- Ustaoglu A, Sawada A, Lee C, et al. Heartburn sensation in nonerosive reflux disease: pattern of superficial sensory nerves expressing TRPV1 and epithelial cells expressing ASIC3 receptors. Am J Physiol Gastrointest Liver Physiol. 2021;320(5):G804–G815. doi: 10.1152/ajpgi.00013.2021.
- Cheng L, De La Monte S, Ma J, et al. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G135–43. doi: 10.1152/ajpgi.90386.2008.
- Zhang Z, Wu X, Zhang L, et al. Menthol relieves acid reflux inflammation by regulating TRPV1 in esophageal epithelial cells. Biochem Biophys Res Commun. 2020;525(1):113–120. doi: 10.1016/j.bbrc.2020.02.050
- Jiang YX, Dong ZY, Wang JW, et al. Efficacy of endoscopic radiofrequency ablation for treatment of reflux hypersensitivity: a study based on Rome IV criteria. Gastroenterol Res Pract. 2022;2022:4145810–4145818. doi: 10.1155/2022/4145810.
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019.
- Yu Y, Chen Z, Li WG, et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68(1):61–72. doi: 10.1016/j.neuron.2010.09.001.
- Deval E, Gasull X, Noël J, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128(3):549–558. doi: 10.1016/j.pharmthera.2010.08.006.
- Vick JS, Askwith CC. ASICs and neuropeptides. Neuropharmacology. 2015;94:36–41. doi: 10.1016/j.neuropharm.2014.12.012.
- Yagi J, Wenk HN, Naves LA, et al. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99(5):501–509. doi: 10.1161/01.RES.0000238388.79295.4c.
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G130–8. doi: 10.1152/ajpgi.00388.2007.
- Han X, Zhang Y, Lee A, et al. Upregulation of acid sensing ion channels is associated with esophageal hypersensitivity in GERD. Faseb J. 2022;36(1):e22083. doi: 10.1096/fj.202100606R.
- Rayees S, Rochford I, Joshi JC, et al. Macrophage TLR4 and PAR2 signaling: role in regulating vascular inflammatory injury and repair. Front Immunol. 2020;11:2091. doi: 10.3389/fimmu.2020.02091.
- Kandulski A, Wex T, Mönkemüller K, et al. Proteinase-activated receptor-2 in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2010;105(9):1934–1943. doi: 10.1038/ajg.2010.265.
- Ma J, Altomare A, Guarino M, et al. HCl-induced and ATP-dependent upregulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G635–45. doi: 10.1152/ajpgi.00097.2012.
- Winkelsett L, Malfertheiner P, Wex T, et al. Mucosal Two-Step pathogenesis in gastroesophageal reflux disease: repeated weakly acidic stimulation and activation of Protease-Activated receptor-2 on mucosal interleukin-8 secretion. Digestion. 2018;98(1):19–25. doi: 10.1159/000486480.
- Yoshida N, Kuroda M, Suzuki T, et al. Role of nociceptors/neuropeptides in the pathogenesis of visceral hypersensitivity of nonerosive reflux disease. Dig Dis Sci. 2013;58(8):2237–2243. doi: 10.1007/s10620-012-2337-7.
- Dai Y, Wang S, Tominaga M, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117(7):1979–1987. doi: 10.1172/JCI30951.
- Woodland P, Shen Ooi JL, Grassi F, et al. Superficial esophageal mucosal afferent nerves may contribute to reflux hypersensitivity in nonerosive reflux disease. Gastroenterology. 2017;153(5):1230–1239. doi: 10.1053/j.gastro.2017.07.017.
- Woodland P, Sifrim D. Oesophageal mucosal barrier: a key factor in the pathophysiology of non-erosive reflux disease (NERD) and a potential target for treatment. Gut. 2014;63(5):705–706. doi: 10.1136/gutjnl-2013-305101.
- Sawada A, Rogers B, Visaggi P, et al. Effect of hiatus hernia on reflux patterns and mucosal integrity in patients with non-erosive reflux disease. Neurogastroenterol Motil. 2022;34(11):e14412. doi: 10.1111/nmo.14412.
- Triantos C, Koukias N, Karamanolis G, et al. Changes in the esophageal mucosa of patients with non erosive reflux disease: how far have we gone?. World J Gastroenterol. 2015;21(19):5762–5767. doi: 10.3748/wjg.v21.i19.5762.
- Kataria J, Rivera D, Grin A, et al. The role of histology in the diagnosis of non-erosive reflux disease: a systematic review and meta-analysis. Neurogastroenterol Motil. 2023;35(12):e14631. doi: 10.1111/nmo.14631.
- Nikaki K, Woodland P, Lee C, et al. Esophageal mucosal innervation in functional heartburn: closer to healthy asymptomatic subjects than to non-erosive reflux disease patients. Neurogastroenterol Motil. 2019;31(9):e13667. doi: 10.1111/nmo.13667.
- Yang XJ, Jiang HM, Hou XH, et al. Anxiety and depression in patients with gastroesophageal reflux disease and their effect on quality of life. World J Gastroenterol. 2015;21(14):4302–4309. doi: 10.3748/wjg.v21.i14.4302.
- Yamasaki T, Fass R. Reflux hypersensitivity: a new functional esophageal disorder. J Neurogastroenterol Motil. 2017;23(4):495–503. doi: 10.5056/jnm17097.
- Jansson C, Nordenstedt H, Wallander MA, et al. Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population-based study. Aliment Pharmacol Ther. 2007;26(5):683–691. doi: 10.1111/j.1365-2036.2007.03411.x.
- Sharma A, Van Oudenhove L, Paine P, et al. Anxiety increases acid-induced esophageal hyperalgesia. Psychosom Med. 2010;72(8):802–809. doi: 10.1097/PSY.0b013e3181f5c021.
- Farré R, De Vos R, Geboes K, et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007;56(9):1191–1197. doi: 10.1136/gut.2006.113688.
- Voulgaris T, Lekakis V, Vlachogiannakos J, et al. Efficacy of citalopram or amitriptyline versus no treatment in patients with functional chest pain. Ann Gastroenterol. 2023;36(1):6–11. doi: 10.20524/aog.2023.0759.
- Fass R, Zerbib F, Gyawali CP. AGA clinical practice update on functional heartburn: expert review. Gastroenterology. 2020;158(8):2286–2293. doi: 10.1053/j.gastro.2020.01.034.
- Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2012;107(11):1662–1667. doi: 10.1038/ajg.2011.179.
- Ostovaneh MR, Saeidi B, Hajifathalian K, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: double-blind placebo-controlled trial. Neurogastroenterol Motil. 2014;26(5):670–678. doi: 10.1111/nmo.12313.
- Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, et al. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol. 2016;111(2):217–224. doi: 10.1038/ajg.2015.413.
- Varia I, Logue E, O’connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140(3):367–372. doi: 10.1067/mhj.2000.108514.
- Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224–1245. doi: 10.1016/j.cgh.2013.08.036.
- Hollerbach S, Bulat R, May A, et al. Abnormal cerebral processing of oesophageal stimuli in patients with noncardiac chest pain (NCCP). Neurogastroenterol Motil. 2000;12(6):555–565. doi: 10.1046/j.1365-2982.2000.00230.x.
- Min YW, Rhee PL. Esophageal hypersensitivity in noncardiac chest pain. Ann N Y Acad Sci. 2016;1380(1):27–32. doi: 10.1111/nyas.13182.
- Ruffle JK, Coen SJ, Giampietro V, et al. Preliminary report: parasympathetic tone links to functional brain networks during the anticipation and experience of visceral pain. Sci Rep. 2018;8(1):13410. doi: 10.1038/s41598-018-31522-2.
- Törnblom H, Drossman DA. Psychotropics, antidepressants, and visceral analgesics in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2018;20(12):58. doi: 10.1007/s11894-018-0664-3.
- Patel A, Wang D, Sainani N, et al. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44(8):890–898. doi: 10.1111/apt.13777.

