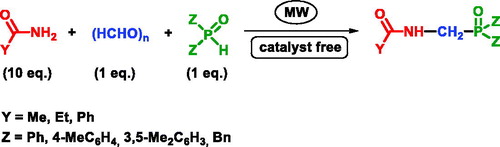
Abstract
A series of acylated α-aminophosphine oxides were synthesized by the microwave-assisted Kabachnik-Fields reaction of a series of carboxylic acid amides, formaldehyde and secondary phosphine oxides. To compensate the lower reactivity of the -NH2 reagents, they had to be used in an excess. The solvolytic condensations furnished the α-aminophosphine oxides in yields of 58-93% after purification by chromatography.
Graphical Abstract
Introduction
The Kabachnik-Fields reaction comprising the condensation of amines, oxo compounds and >P(O)H reagents, e.g. dialkyl phosphites is an important tool towards α-aminophosphonic derivatives [Citation1-3] due to their potential as biologically active substrates.[Citation4-7] Numerous methods were described for the synthesis of α-aminophosphonates, however, the major part of the protocols reported applied a catalyst.[Citation8-16] The senior author of this article, together with a coworker, was who proposed that using the microwave (MW)-assisted solvent-free protocol, there is no need for any catalyst.[Citation17] Hence, cost and environmental burdens may be saved. The green chemical approaches deserve special attention. In this line, the ionic liquid catalyzed or catalyst-free, solvent-free and MW-promoted procedures should be mentioned.[Citation18-26] The authors of this paper belong to those, who tried to extend the scope of the phospha-Mannich reaction to obtain α-aminophosphine oxides.[Citation17,Citation26-28] The “bis” Kabachnik-Fields reaction leading to ZN(CH2P(O)Y2)2 (Z = alkyl or aryl, Y = alkoxy or alkyl/aryl) type of species are also of interest.[Citation25-31]
It was a challenge for us to try the phospha-Mannich condensation using a different kind of carboxylic amides as the -NH2 component (Scheme 1A). The literature survey showed that mostly urethanes were applied as starting materials in the synthesis of acyl-aminophosphonates.[Citation32-40] These preparations requested the use of acetic acid, acetic anhydride or acetyl chloride as the solvent at 25 °C to afford the products in yields of 22-95%. There are three examples, when acetamide or urea derivatives were the starting materials of the Kabachnik-Fields reaction.[Citation41-43] These condensations were performed in toluene, acetic anhydride or acetyl chloride at 40-120 °C. As a matter of fact, the acylated α-aminophosphonates could also be prepared by the acylation of -NH2 derivatives (Scheme 1B). However, the synthesis of the starting materials, unsubstituted α-aminophosphonates by the Kabachnik-Fields reaction utilizing ammonia or its precursors is not easy.[Citation44-46]
It was also obvious for us that the amides exhibit a lower reactivity in the phospha-Mannich condensation than amines.
Results and discussion
We wished to perform the Kabachnik-Fields reaction of a series of carboxylic acid amides (acetamide, propionic acid amide, as well as benzoic acid amide), paraformaldehyde and secondary phosphine oxides under MW conditions. The phospha-Mannich condensation of acetamide, paraformaldehyde and diphenylphosphine oxide served as the model reaction to find the optimum conditions. As earlier, we aimed at the catalyst- and solvent-free protocol.[Citation17] Experimental data were collected in . Reacting the three components at a 1:1:1 molar ratio at 150 °C, there was no reaction after a 2 h’s irradiation (, entry 1). Increasing the amount of acetamide to 5 equivalents, a low conversion of 2% was attained (entry 2). A further increase to 10 equivalents led to a conversion of 11% (, entry 3). An increase in the temperature and reaction time to 180 °C and 3.5 h, respectively, brought the real breakthrough, as in this case the conversion was 61% (entry 4). Following this line, applying a temperature of 200 and 220 °C, the conversion was 93 and 99%, respectively (, entries 5 and 6). One can see that practically a solvolytic-like accomplishment using acetamide in a 10-fold quantity at a relatively high temperature could compensate the lower reactivity of the amide. For comparison purpose, the last reaction was also carried out on conventional heating. A conversion of only 57% could be detected (, entry 7). In the realistic temperature range, the proceeding of the condensation was monitored by 31P NMR measurements. The curves obtained for 180, 200 and 220 °C can be seen in . Our experiments also revealed that there were no side products, the corresponding α-aminophosphine oxide (1a) was formed in a neat reaction. After the work-up procedure including chromatography, product 1a was isolated in a yield of 72%.
Figure 1. Time dependence of the conversion of acetamide, paraformaldehyde, and Ph2P(O)H to α-aminophosphine oxide (1a) at a 10:1:1 molar ratio.

Table 1. Optimization of the Kabachnik-Fields reaction of acetamide, paraformaldehyde, and diphenylphosphine oxide. 
In the next stage of our work, acetamide was also reacted in Kabachnik-Fields reactions utilizing other secondary phosphine oxides, such as bis(4-metylphenyl)phosphine oxide, bis(3,5-dimetylphenyl)phosphine oxide and dibenzylphosphine oxide at the conditions elaborated above. The corresponding α-aminophosphine oxides (1a-d) were obtained in yields of 72-93% (, entries 1-4). Then, propionic acid amide and benzamide were also converted to the corresponding α-aminophosphine oxides, 2a-d and 3a-d, respectively (, entries 5-8 and 9-12, respectively). As can be seen, the yields fell in the range of 57-91%, and the phopha-Mannich condensation of benzoic acid amide required a somewhat higher temperature of 240 °C. All compounds prepared are new, and were characterized by 31P, 13C and 1H NMR spectral data, as well as HRMS.
Table 2. Kabachnik-Fields reaction of amides, paraformaldehyde, and secondary phosphine oxides. 
Conclusions
In summary, an efficient, catalyst-free MW-assisted method was developed for the selective synthesis of acylated α-aminophosphine oxides by the phospha-Mannich reaction of carboxylic acid amides, paraformaldehyde and secondary phosphine oxides. All together, 12 new derivatives were prepared in good to high yields, which form a new family of compounds.
Experimental
General procedure for the synthesis of acylated α-aminophosphine oxides(1a-d, 2a-d and 3a-d)
A mixture of 5.0 mmol of the amide (0.29 g of acetamide, 0.37 g of propionamide or 0.61 g of benzamide), 0.50 mmol (0.02 g) of paraformaldehyde and 0.50 mmol of the secondary phosphine oxide (0.11 g of diphenylphosphine oxide, 0.12 g of di(p-tolyl)phosphine oxide, 0.13 g of bis(3,5-dimetylphenyl)phosphine oxide or 0.12 g of dibenzylphosphine oxide) was heated at 220 °C or 240 °C in a closed vial in a CEM Discover microwave reactor equipped with a pressure controller for 3.5 h. The crude product so obtained was dissolved in dichloromethane, and the excess of amide was removed by extraction with 3 × 15 mL of water.
The combined organic phases were dried (Na2SO4). Evaporation of the solvent left a residue that was passed through a 1 cm silica gel layer using 10% methanol in dichloromethane as the eluent to provide products 1a-d, 2a-d and 3a-d as white crystals. Most of the aminophosphine oxides were recrystallized from acetone. Characterization data and copies of 31P, 1H, and 13C NMR spectra for the compounds synthesized were collected in the Supporting Information.
Supplemental Material
Download PDF (2 MB)Additional information
Funding
References
- Keglevich, G.; Bálint, E. The Kabachnik-Fields Reaction: Mechanism and Synthetic Use. Molecules. 2012, 17, 12821-12835. DOI: 10.3390/molecules171112821.
- Bálint, E.; Tripolszky, A.; Tajti, Á. Synthesis of α-Aminophosphonates by the Kabachnik-Fields Reaction and by the Pudovik Reaction, In: Organophosphorus Chemistry - Novel Developments, Keglevich, G., Ed. de Gruyter: Germany, 2018; pp 108-147.
- Kafarski, P.; Górniak, M. G.; Andrasiak, I. Kabachnik-Fields Reaction under Green Conditions - A Critical Overview. Curr. Green Chem. 2015, 2, 218-222. DOI: 10.2174/2213346102666150109203606.
- Kukhar, V. P.; Hudson, H. R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Wiley: Chichester, 2000.
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955-5980. DOI: 10.1021/jm200587f.
- Forlani, G.; Berlicki, L.; Duo, M.; Dziedziola, G.; Giberti, S.; Bertazzini, M.; Kafarski, P.; Synthesis Evaluation, o. Effective Inhibitors of Plant δ1-Pyrroline-5-Carboxylate Reductase. J. Agric. Food Chem. 2013, 61, 6792-6798. DOI: 10.1021/jf401234s.
- Sienczyk, M.; Oleksyszyn, J. Irreversible Inhibition of Serine Proteases - design and in Vivo Activity of Diaryl Alpha-aminophosphonate Derivatives. Curr. Med. Chem. 2009, 16, 1673-1687. DOI: 10.2174/092986709788186246.
- Lee, S.; Park, J. H.; Kang, J.; Lee, J. K. Lanthanide Triflate-catalyzed Three Component Synthesis of α-Amino Phosphonates in Ionic Liquids. A Catalyst Reactivity and Reusability Study. Chem. Commun. 2001, 2001, 1698-1699. DOI: 10.1039/b104967b.
- Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. A Facile and Highly Efficient Route to α-Amino Phosphonates via Three-Component Reactions Catalyzed by Mg(ClO4)2 or Molecular Iodine. Org. Biomol. Chem. 2006, 4, 1663-1666. DOI: 10.1039/B602536F.
- Bhattacharya, A. K.; Kaur, T. An Efficient One-pot Synthesis of α-Amino Phosphonates Catalyzed by Bismuth Nitrate Pentahydrate. Synlett. 2007, 2007, 745-748. DOI: 10.1055/s-2007-970762.
- Bhagat, S.; Chakraborti, A. K. An Extremely Efficient Three-component Reaction of Aldehydes/Ketones, Amines, and Phosphites (Kabachnik − Fields Reaction) for the Synthesis of α-Aminophosphonates Catalyzed by Magnesium Perchlorate. J. Org. Chem. 2007, 72, 1263-1270. DOI: 10.1021/jo062140i.
- Zhang, Y.; Zhu, C. Gold Complex-Catalyzed C-P Bond Formation by Kabachnik-Fields Reactions. Catal. Commun. 2012, 28, 134-137. DOI: 10.1016/j.catcom.2012.08.001.
- Mulla, S. A. R.; Pathan, M. Y.; Chavan, S. S.; Gampleb, S. P. Sarkarb, D. Highly Efficient One-pot Multi-component Synthesis of α-Aminophosphonates and Bis-α-aminophosphonates Catalyzed by Heterogeneous Reusable Silica Supported Dodecatungstophosphoric Acid (DTP/SiO2) at Ambient Temperature and Their Antitubercular Evaluation against Mycobactrium Tuberculosis. RSC Adv. 2014, 4, 7666-7672. DOI: 10.1039/c3ra45853a.
- Lewkowski, J.; Moya, M. R.; Wrona-Piotrowicz, A.; Zakrzewski, J.; Kontek, R.; Gajek, G. Synthesis, Fluorescence Properties and the Promising Cytotoxicity of Pyrene-Derived Aminophosphonates. Beilstein J. Org. Chem. 2016, 12, 1229-1235. DOI: 10.3762/bjoc.12.117.
- Shady, A. A.; Bakr, S. M. A.; Khidre, M. D. Synthesis of Various Schiff Bases Containing Isoxazole Ring and Their Applications with Thioglycollic Acid and Diverse Phosphorus Reagents. J. Heterocyclic Chem. 2017, 54, 71-79. DOI: 10.1002/jhet.2541.
- Li, N.; Wang, X.; Qiu, R.; Xu, X.; Chen, J.; Zhang, X.; Chen, S.; Yin, S. Air-stable Zirconocene Bis(perfluorobutanesulfonate) as a Highly Efficient Catalyst for Synthesis of α-Aminophosphonates via Kabachnik-Fields Reaction under Solvent-free Condition. Catal. Commun. 2014, 43, 184-187. DOI: 10.1016/j.catcom.2013.10.013.
- Keglevich, G.; Szekrényi, A. Eco-friendly Accomplishment of the Extended Kabachnik-Fields Reaction; a Solvent- and Catalyst-free Microwave-assisted Synthesis of α- Aminophosphonates and α-Aminophosphine Oxides. Lett. Org. Chem. 2008, 5, 616-622. DOI: 10.2174/157017808786857598.
- Yadav, J. S.; Reddy, B. V. S.; Sreedhar, P. An Eco-friendly Approach for the Synthesis of α-Aminophosphonates Using Ionic Liquids. Green Chem. 2002, 4, 436-438. DOI: 10.1039/B203934F.
- Boroujeni, K. P.; Shirazi, E. R.; Doroodmand, M. M. Synthesis of α-Aminophosphonates Using Carbon Nanotube Supported Imidazolium Salt-Based Ionic Liquid as a Novel and Environmentally Benign Catalyst. Phosphorus, Sulfur, Silicon Relat. Elem. 2016, 191, 683-688. DOI: 10.1080/10426507.2015.1072182.
- Eshghi, H.; Mirzaei, M.; Hasanpour, M.; Mokaber-Esfahani, M. Benzimidazolium Dicationic Ionic Liquid as an Efficient and Reusable Catalyst for the Synthesis of α-Aminophosphonates and Bis (α-aminophosphonates) under Solvent-Free Condition. Phosphorus, Sulfur, Silicon Relat. Elem. 2015, 190, 1606-1620. DOI: 10.1080/10426507.2015.1012199.
- Kumar, M. A.; Park, Y.-K.; Lee, K. D. Synthesis and Antiproliferative Activity of Novel α-Aminophosphonates. Chem. Pharm. Bull. 2012, 60, 1531-1537. DOI: 10.1248/cpb.c12-00676.
- Ranu, B. C.; Hajra, A. A Simple and Green Procedure for the Synthesis of α-Aminophosphonate by a One-pot Three-component Condensation of Carbonyl Compound, amine and Diethyl Phosphite without Solvent and Catalyst. Green Chem. 2002, 4, 551-554. DOI: 10.1039/B205747F.
- Kabachnik, M. I.; Zobnina, E. V.; Beletskaya, I. P. Catalyst-free Microwave-Assisted Synthesis of α-Aminophosphonates in a Three-component System: R1C(O)R2-(EtO)2P(O)H-RNH2. Synlett. 2005, 2005, 1393-1396. DOI: 10.1055/s-2005-868519.
- Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Microwave-Assisted Solvent-free and Catalyst-free Kabachnik-Fields Reactions for α-Amino Phosphonates. Tetrahedron Lett. 2006, 47, 1125-1127. DOI: 10.1016/j.tetlet.2005.12.027.
- Bálint, E.; Fazekas, E.; Pinter, G.; Szollosy, A.; Holczbauer, T.; Czugler, M.; Drahos, L.; Körtvélyesi, T.; Keglevich, G. Synthesis and Utilization of the Bis(>P(O)CH2)amine Derivatives Obtained by the Double Kabachnik-Fields Reaction with Cyclohexylamine; quantum Chemical and X-ray Study of the Related Bidentate Chelate Platinum Complexes. Curr. org. Chem. 2012, 16, 547-554. DOI: 10.2174/138527212799499822.
- Bálint, E.; Takács, J.; Drahos, L.; Juranovič, A.; Kočevar, M.; Keglevich, G. Oxides by the Microwave‐Assisted Kabachnik-Fields Reactions of 3‐amino‐6‐methyl‐2 H‐pyran‐2‐Ones. Heteroatom Chem. 2013, 24, 221-225. DOI: 10.1002/hc.21086.
- Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. Synthesis and Use of α-Aminophosphine Oxides and N,N-bis(phosphinoylmethyl)amines - A Study on the Related Ring Platinum Complexes. J. Organomet. Chem. 2016, 801, 111-121. DOI: 10.1016/j.jorganchem.2015.10.029.
- Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.; Czugler, M.; Keglevich, G. Synthesis and Utilization of Optically Active α-Aminophosphonate Derivatives by Kabachnik-Fields Reaction. Tetrahedron. 2017, 73, 5659-5667. DOI: 10.1016/j.tet.2017.07.060.
- Prishchenko, A. A.; Livantsov, M. V.; Novikova, O. P.; Livantsova, L. I.; Petrosyan, V. S. Synthesis of Bis‐ and Tris‐organophosphorus Substituted Amines and Amino Acids with PCH2N Fragments. Heteroatom Chem. 2010, 21, 430-440. DOI: 10.1002/hc.20616.
- Cherkasov, R. A.; Garifzyanov, A. R.; Talan, A. S.; Davletshin, R. R.; Kurnosova, N. V. Synthesis of New Liophilic Functionalized Aminomethylphosphine Oxides and Their Acid-base and Membrane-Transport Properties Toward Acidic Substrates. Russ. J. Gen. Chem. 2009, 79, 1835-1849. DOI: 10.1134/S1070363209090114.
- Keglevich, G.; Szekrényi, A.; Szöllősy, Á.; Drahos, L. Synthesis of Bis(phosphonatomethyl)-, Bis(phosphinatomethyl)-, and Bis(phosphinoxidomethyl)amines, as Well as Related Ring Bis(phosphine) platinum Complexes. Synth. Commun. 2011, 41, 2265-2272. DOI: 10.1080/00397911.2010.501478.
- Dimitrev, M. E.; Ragulin, V. V. New Opinions on the Amidoalkylation of Hydrophosphorylic Compounds. Tetrahedron Lett. 2010, 51, 2613-2616. DOI: 10.1016/j.tetlet.2010.03.020.
- Skorenski, M.; Oleksyszyn, J.; Sienczyk, M. Efficient Methods for the Synthesis of α-Aminophosphonate Fluoroalkyl Esters. Tetrahedron Lett. 2013, 54, 1566-1568. DOI: 10.1016/j.tetlet.2013.01.039.
- Serim, S.; Baer, P.; Verhelst, S. H. L. Mixed Alkyl Aryl Phosphonate Esters as Quenched Fluorescent Activity-based Probes for Serine Proteases. Org. Biomol. Chem. 2015, 13, 2293-2299. DOI: 10.1039/C4OB02444C.
- Vassiliou, S.; Węglarz-Tomczak, E.; Berlicki, Ł.; Pawełczak, M.; Nocek, B.; Mulligan, R.; Joachimiak, A.; Mucha, A. Structure-Guided, Single-Point Modifications in the Phosphinic Dipeptide Structure Yield Highly Potent and Selective Inhibitors of Neutral Aminopeptidases. J. Med. Chem. 2014, 57, 8140-8151. DOI: 10.1021/jm501071f.
- Huber, T.; Manzenrieder, F.; Kuttruff, C. A.; Dorner-Ciossek, C.; Kessler, H. Prolonged Stability by Cyclization: Macrocyclic Phosphino Dipeptide Isostere Inhibitors of β-Secretase (BACE1). Bioorg. Med. Chem. Lett. 2009, 19, 4427-4431. DOI: 10.1016/j.bmcl.2009.05.053.
- Matziari, M.; Yiotakis, A. Shortcut to Fmoc-Protected Phosphinic Pseudodipeptidic Blocks. Org. Lett. 2005, 7, 4049-4052. DOI: 10.1021/ol051622y.
- Chen, S.; Cowark, J. K. A General Method for the Synthesis of N-protected α-Aminoalkylphosphinic Acids. Tetrahedron Lett. 1996, 37, 4335-4338. DOI: 10.1016/0040-4039(96)00839-8.
- Roos, G. H. P.; Balasubramaniam, S. Synthesis of α-Amino Phosphonates under Diastereocontrol by Imidazolidin-2-one Auxiliaries. Synth. Commun. 1998, 28, 3877-3884. DOI: 10.1080/00397919808004941.
- Yuan, C.; Wang, G. Studies on Organophosphorus Compounds 65, A Facile Synthetic Route to Phosphonopeptides. Phosphorus, Sulfur, Silicon Relat. Elem. 1992, 71, 207-212. DOI: 10.1080/10426509208034513.
- Rozhko, L. F.; Ragulun, V. V. α-Hydroxy-α-aminophosphinic Acids: I. Synthesis of a New Analog of Phenylglycine and Its Enantiomers. Russ. J. Gen. Chem. 2005, 75, 533-536. DOI: 10.1007/s11176-005-0267-1.
- Kaboudin, B.; Afsharinezhad, M. B.; Yokomatsu, T. A Convenient and General Procedure for the Synthesis of α-Ureidophosphonates under Catalyst-free Conditions. Arkivoc. 2012, 4, 44-53. DOI: 10.3998/ark.5550190.0013.405.
- Dai, Q.; Chen, R. Synthesis of Dialkyl α-(p-toluenesulfonureado) phosphonates and Their Quantitative Structure-anti-TMV Activity Relationship. Phosphorus, Sulfur, Silicon Relat. Elem. 1997, 122, 261-267. DOI: 10.1080/10426509708043515.
- Kabachnik, M. I.; Medved, T. Y. New Synthesis of Aminophosphonic Acids. Dokl. Akad. Nauk SSSR. 1952, 83, 689-692.
- Kaboudin, B.; Rahmani, A. Convenient Synthesis of 1-Aminoalkylphosphonates under Solvent-free Conditions. Org. Prep. Proced Int. 2004, 36, 82-86. DOI: 10.1080/00304940409355376.
- Pawłowska, A.; Jean-Noel, V.; Virieux, D.; Pirat, J.-L.; Janiak, A.; Nowicki, M.; Hoffmann, M.; Pluskota-Karwatka, D. Perfluorophenyl Phosphonate Analogues of Aromatic Amino Acids: Synthesis, X-ray and DFT Studies. Tetrahedron. 2018, 74, 975-986. DOI: 10.1016/j.tet.2018.01.019.
