Abstract
Although there is evidence that estradiol has effects in women and in animal models to reduce anxiety and depressive behavior and enhance performance in some cognitive tasks, this is not seen among all individuals. Given the interaction between estradiol and hypothalamic–pituitary–adrenal function, we hypothesized that an individual's prior exposure to stress may mitigate some of the subsequent effects of estradiol. To address this, rats were exposed to gestational stress, or not, to determine if stress exposure during development alters behavioral responses to estradiol in adulthood. If estradiol's effects on anxiety and cognitive behavior are modulated by prior stress experience, then gestationally-stressed rats administered estradiol should have decreased anti-anxiety (open field, elevated plus maze) behavior and cognitive performance in the inhibitory avoidance task. Offspring of dams that were exposed to restraint stress daily on gestational days 14–20, or no such manipulation, were used as adults either intact in behavioral estrus (high estradiol) or diestrus (low estradiol), or ovariectomized (OVX) with empty or estradiol-containing silastic implants. Rats were used for blood collection to determine plasma corticosterone and estradiol concentrations, or were used for behavioral testing. Compared with rats in diestrus or OVX and vehicle-replaced, rats in behavioral estrus and OVX rats with estradiol implants had higher estradiol concentrations, entered more central squares in an open field, spent more time on the open arms of the plus maze, and had a longer latency to crossover to the dark, shock-associated side of the inhibitory avoidance chamber. Gestational stress increased plasma corticosterone but not estradiol levels, decreased plus maze open arm time in cycling rats, and decreased inhibitory avoidance performance. Thus, estradiol and gestational stress can have opposite effects on anxiety and inhibitory avoidance performance.
Introduction
Estradiol has a variety of functional effects to alter mood and cognitive processes, but the nature of these effects is not entirely clear. For instance, verbal memory among surgically- or naturally-menopausal women is enhanced by estradiol-based therapy (as reviewed in Nappi et al. (Citation1999); Janowsky (Citation2002); Sherwin (Citation2003)), but similar improvements are not observed in all studies when different estradiol regimen or memory tasks are utilized (Hogervorst et al. Citation1999, Citation2000; Linzmayer et al. Citation2001). A similar pattern emerges for the effects of estradiol for mood. Estradiol replacement to women enhances mood in some, but not all, studies and some factors that may influence these responses to estradiol for mood and cognition are duration of hypogonadism, age, stage of reproductive life, and psychiatric history (as reviewed in Walf and Frye (Citation2006a)). For instance, a major risk factor for the development of depressive symptoms in the three days following parturition is psychiatric history, such as history of major depression, prior postpartum depression episodes, and/or premenstrual syndrome (Bloch et al. Citation2006). A shorter depression history among pre- or post-menopausal women diagnosed with depression is associated with a favorable response to estradiol-based therapy compared to that seen in women with a longer depression history (Klaiber et al. Citation1979). Thus, individual history of mood and/or anxiety disorders may mitigate effects of estradiol.
Some studies, utilizing animal models, have investigated factors that mitigate estradiol's anti-anxiety and cognitive-enhancing effects. Rats in behavioral estrus, with high endogenous levels of estradiol, demonstrate more anti-anxiety and anti-depressive behavior (Mora et al. Citation1996; Frye et al. Citation2000; Frye and Walf Citation2002) and better performance in the inhibitory avoidance test, than do diestrous rats, with low estradiol levels (Rhodes and Frye Citation2004). Systemic estradiol administration to ovariectomized (OVX) rats that produces behavioral estrus-like, but not higher or lower levels of estradiol, increases anti-anxiety behavior in the plus maze and open field, anti-depressant-like behavior in the forced swim test, and performance in the inhibitory avoidance task (Rachman et al. Citation1998; Frye and Rhodes Citation2002; Frye and Walf Citation2004a,Citationb; Walf and Frye Citation2005a,Citationb). However, exposure to an acute stressor, such as restraint stress, can attenuate anxiolytic and anti-depressant-like effects of estradiol and increase plasma corticosterone levels (Walf and Frye Citation2005a). Indeed, estradiol may have different patterns of effects on hypothalamic–pituitary–adrenal (HPA) axis function depending upon the estradiol regimen utilized and level of the HPA axis that is investigated (adrenal vs. pituitary vs. brain: Figueiredo et al. (Citation2002), (Citation2003); Walf and Frye (Citation2006a,Citationb)). For this reason, we were interested in examining effects of a stressor that produces robust and consistent effects on HPA axis function at the level of the brain, pituitary and adrenals.
Stress during gestation, referred to as prenatal stress (PNS), is an animal model of a mood disorder, such as depression, which is characterized by dysregulation of each level of the HPA axis. Gestational stress can produce a persistent enhancement of stress responsiveness of offspring (Weinstock Citation2001; Kofman Citation2002; Newport et al. Citation2002), but this is not observed in all studies even when a behavioral effect of gestational stress occurs (Szuran et al. Citation2000; Fujioka et al. Citation2001; Cannizzaro et al. Citation2006; Richardson et al. Citation2006). When gestational stress does alter HPA axis function, compared with control animals there can be increased corticotropin releasing factor expression (Ward et al. Citation2000; as reviewed in Weinstock (Citation2005)), increased adrenocorticotropin levels in response to acute stress (Jezova et al. Citation2002), a phase shift in the circadian rhythm of corticosterone secretion (Koehl et al. Citation1999), increased basal corticosterone levels (Peters Citation1982; Fride et al. Citation1986; Ward et al. Citation2000), and increased levels, and/or duration, of corticosterone secretion after stressor exposure (Peters Citation1982; McCormick et al. Citation1995; Vallee et al. Citation1997), and adrenal hypertrophy (as reviewed in Weinstock (Citation2005)). Gestational stress alters glucocorticoid and mineralocorticoid receptor expression in the brain (as reviewed in Weinstock (Citation2005)). PNS rats also demonstrate behavioral differences as adults. For instance, behavioral inhibition, such as reduced exploratory behavior, more freezing, and more anxiety to novel situations is typical in PNS compared to non-PNS rodents (Pohorecky and Roberts Citation1991; Poltyrev et al. Citation1996; Vallee et al. Citation1997; Drago et al. Citation1999; Morgan et al. Citation1999).
In the present study, the possibility that some of estradiol's effects for affective processing may be modulated by individual stress history was investigated using gestational stress. Female rat offspring that were stressed during gestation or not were used for blood collection, or were tested in behavioral estrus or diestrus, or were OVX and administered vehicle or estradiol. Rats from each condition were tested in the open field and elevated plus maze, and then in the inhibitory avoidance task the following week. We hypothesized that: gestational stress would alter HPA axis function and this would be modulated by estradiol; female rats with higher estradiol levels, produced by endogenous estrous cycle changes or estradiol administration to OVX rats, would have decreased anxiety behavior and increased cognitive performance; and that gestational stress would attenuate effects of estradiol.
Materials and methods
The animal procedures were approved by the Institutional Animal Care and Use Committee at SUNY Albany and performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80 23, revised 1978).
Animals and housing
Adult (55 + days old), female Long–Evans rats (n = 199) were obtained from the breeding colony in the Social Sciences Building at SUNY-Albany (original stock from Taconic Farms, Germantown, NY). Rats were group-housed (4–5 per cage) in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1°C) in the Laboratory Animal Care Facility. Rats were maintained on a 12/12 h reversed light cycle (lights off at 8:00 am) with continuous access to Purina Rat Chow and tap water ad libitum.
Induction of prenatal stress or control condition
The gestational stress induction protocol utilized was similar to that previously employed (Frye and Orecki Citation2002a,Citationb; Frye and Wawrzycki 2003). Female rats (n = 60) were cycled through two normal, 4–5 day estrous cycles and then mated. Pregnant rats were randomly assigned to be restraint stressed for 45 min daily (n = 34, stress condition) or not stressed (n = 26, control condition) from gestational day 14–20. Rats were restraint stressed under a single 60-W light in a cylindrical plexiglas restrainer (15.25 cm long × 7.6 cm diameter). An experimenter monitored rats during this time. Rats in the non-stressed, control condition remained undisturbed in their home cages. To control for possible litter effects, only 1–3 pups from each litter in the gestational stress (n = 34) or control (n = 26) conditions were utilized, and these were distributed across experimental groups to minimize cohort/litter effects. Cursory analyses did not reveal differences in behavior of rats related to litter/maternal origin.
Determination of behavioral estrus and diestrus condition
Adult female rats from the PNS and control litters were cycled to determine behavioral estrus and diestrus condition, as per previously published methods (Frye and Walf Citation2002). Briefly, intact adult female rats were vaginally-masked and placed in an empty aquarium with a sexually-experienced male rat. Behavioral estrus condition was determined when rats displayed a typical lordosis response following mounting by a male. Rats that were in behavioral estrus 2 days prior and did not demonstrate lordosis in response to male mounting were considered to be in diestrus.
Ovariectomy and estrogen replacement
Adult female rats from PNS and control litters were OVX under xylazine (12 mg/kg i.p.; Bayer Corp., Shawnee Mission, KS) and ketamine (80 mg/kg i.p.; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia 1 week before behavioral testing. During surgery, a cohort of rats was subcutaneously implanted with a silastic capsule (1.57 mm inner diameter, 3.18 mm outer diameter, 20 mm length) containing crystalline 17β-estradiol (Steraloids, Newport, RI).
Behavioral testing
As adults, behaviorally-tested rats were tested on two occasions in the same stress and hormone condition. During their first week of testing, rats were tested for their exploratory and anxiety responses in the open field and elevated plus maze. During week 2, rats were trained and tested in the inhibitory avoidance task. Behavioral tasks were not counterbalanced to minimize the effects that inhibitory avoidance training (i.e. exposure to the shock stimulus) would have on behavior in the open field and elevated plus maze.
Testing took place in the dark-phase of the rats' reversed light cycle. Rats in behavioral estrus or diestrus were tested within an hour of determination of cycle phase on both testing occasions. All OVX rats were tested one week after OVX and vehicle treatment or estradiol replacement in the open field and elevated plus maze, and then tested the next week in the inhibitory avoidance task.
Open field
Rats were placed in a brightly-lit open field (76 × 57 × 35 cm) and observed for 5 min while the number of central and peripheral squares entered was recorded (Frye et al. Citation2000; Walf and Frye Citation2005a,Citationb). Central entries are considered an index of anti-anxiety behavior.
Elevated plus maze
Testing in the elevated plus maze was done using previously published methods (Frye et al. Citation2000; Walf and Frye Citation2005a,Citationb). The elevated plus maze utilized was matte black with two open arms (50 cm long × 10 cm wide) and two closed arms (with 30 cm high walls) elevated 50 cm from the ground. The time spent by rats in the closed or open arms of this maze was recorded for 5 min with open arm duration considered to be an index of anti-anxiety behavior.
Inhibitory avoidance
The inhibitory avoidance task consisted of a two compartment, stainless steel chamber divided in half by a guillotine door. One compartment was painted white and brightly-lit from above and the other was painted black and dark. First, rats were habituated for 2 min on the white side of the box. During training, rats were placed in the white side for 1 min and then the door was lifted. Latency to move to the dark side was recorded, with a maximum latency of 20 min. When rats moved to the dark-side of the chamber, the door was closed and a mild shock (0.2 mA, 5 s duration) was administered to their feet. During testing (4 h later) rats were placed in the white chamber for 1 min, the door was lifted and the latency to move to the dark side was recorded (maximum latency was 5 min). A longer latency to crossover to the dark, shock-associated side of the chamber indicates improved performance in this task (Frye and Rhodes Citation2002).
Blood collection
In a non-tested group of adult female rats from the PNS or control litters, blood was collected from rats immediately after removal from the home cage (basal) during the early dark-phase (between 09:00–11:00 h) of their light cycle. Blood was collected, following rapid decapitation, in chilled glass test tubes containing EDTA (saturated in saline; 10 μl). Blood was then centrifuged at 4°C (3000g for 20 min) and plasma was decanted into chilled eppendorf tubes and stored at − 80°C.
Radioimmunoassay
Estradiol radioimmunoassay
Plasma estradiol concentrations were determined with radioimmunoassay according to previously published methods (Frye and Bayon Citation1999; Walf and Frye Citation2005a). Briefly, estradiol was extracted twice with ether by snap freezing. Ether was evaporated and pellets were reconstituted in PBS (pH = 7.4). The standard curve was prepared in duplicate (12.5–1000 pg/0.1 ml). Standards were added to PBS, with estradiol antibody (Dr Niswender, #244, Colorado State University, Fort Collins, CO), and [3H] estradiol (NET-317, 51.3 ci/mmol; Perkin Elmer). Assay tubes were incubated at room temperature for 50 min. Dextran-coated charcoal was used to separate bound and free steroid after a 10 min incubation on ice and centrifugation at 3000g for 10 min. Supernatant was pipetted into a glass scintillation vial with scintillation cocktail and counted using a Tri-Carb 2000CA liquid scintillation analyzer. Unknowns were interpolated from the standard curve using assay zap, a program for RIA analyses. The minimum detection of this assay is 12.5 pg/tube. The inter- and intra-assay coefficients of variance were 0.08 and 0.10, respectively.
Corticosterone radioimmunoassay
Plasma corticosterone was measured according to previously published methods (Frye and Bayon Citation1999; Walf and Frye Citation2005a). Briefly, corticosterone was extracted from plasma (sample volume of 5 μl) by heating tubes at 60°C for 30 min. Samples were incubated for 45 min at room temperature with 3H CORT (NET 182: specific activity = 48.2 ci/mmol; Perkin Elmer) and a 1:20,000 dilution of antibody (Endocrine Sciences: #B3–163). Dextran-coated charcoal was used to separate bound and free following a 15 min incubation on ice and centrifugation at 3000g for 10 min. Supernatant was decanted into a glass scintillation vial with scintillation cocktail and then counted using a Tri–Carb 2000CA liquid scintillation analyzer. Unknowns were interpolated from the standard curve using assay zap. The minimum detection of this assay is 15 pg/tube. The inter- and intra-assay reliability coefficients were 0.05 and 0.08, respectively.
Procedure
Adult rats (n = 32) from PNS and control litters were not behaviorally-tested and utilized for measurement of plasma estradiol and/or corticosterone. For these measures, there were non-stressed rats in behavioral estrus (n = 4) or diestrus (n = 3) and gestationally-stressed rats in behavioral estrus (n = 3) or diestrus (n = 2) and non-stressed OVX rats administered estradiol (n = 6) or vehicle (n = 4) and gestationally-stressed OVX rats administered estradiol (n = 4) or vehicle (n = 5).
Another group of rats from PNS and control litters were behaviorally tested and left intact and used at different estrous cycle stages (n = 53) or OVX and administered vehicle or estradiol (n = 54). Non-stressed intact rats were tested as behavioral estrous (n = 12) or diestrous (n = 13) and gestationally-stressed rats were tested as behavioral estrous (n = 14) or diestrous (n = 14). Non-stressed OVX rats were administered estradiol (n = 12) or vehicle (n = 13) and gestationally-stressed OVX rats were administered estradiol (n = 14) or vehicle (n = 15).
Statistical analyses
Two-way analyses of variance (ANOVAs) were used to examine effects of hormone and gestational stress condition on plasma estradiol and corticosterone levels and behavior in the open field, elevated plus maze, and inhibitory avoidance task. Separate ANOVAs were used to determine effects of endogenous fluctuations in, and exogenous administration of, estradiol for these measures. ANOVAs were followed by Fisher's post hoc tests to determine differences among groups, when appropriate. The α level for statistical significance was P ≤ 0.05.
Results
Plasma estradiol levels
As indicates, rats in behavioral estrus had significantly higher plasma estradiol concentrations than did diestrous rats [F(1,8) = 234.8, P < 0.01]. In OVX rats, estradiol administration via silastic capsules significantly increased plasma estradiol concentrations compared to empty capsules [F(1,16) = 8.5, P < 0.01]. There was no difference in estradiol concentrations between non-stressed and gestationally-stressed rats.
Table I. Plasma concentrations (pg/ml; mean±SEM) of estradiol in rats that were gestationally-stressed or not, and intact at different stages of the estrous cycle, or OVX and administered estradiol or vehicle via subcutaneous silastic capsules.
Plasma corticosterone levels
As indicates, gestationally-stressed rats in behavioral estrus did not have significantly higher plasma corticosterone concentrations than did non-stressed or gestationally-stressed diestrous rats. Gestational stress significantly increased plasma corticosterone concentrations of OVX rats [F(1,16) = 5.3, P ≤ 0.03], but there was no significant main effect of estradiol administration.
Table II. Plasma concentrations (μg/dl; mean±SEM) of corticosterone in rats that were gestationally-stressed or not, and intact at different stages of the estrous cycle or OVX and administered estradiol or vehicle via subcutaneous silastic capsules.
Open field
Rats in behavioral estrus made significantly more total [F(1,49) = 8.7, P < 0.01; and central [F(1,49) = 4.0, P < 0.05; , top] entries than did diestrous rats. There was no main effect of gestational stress for these measures in the open field.
Table III. Total entries made in the open field (mean±SEM) in rats that were gestationally-stressed or not, and intact in different stages of the estrous cycle or OVX and administered estradiol or vehicle via subcutaneous silastic capsules.
Figure 1 The mean ( ± SEM) number of entries made into the center squares of the open field by gestationally-stressed (PNS) and non-stressed control rats that were in diestrous or behavioral estrous (top) or OVX and administered vehicle or estradiol (E2; bottom). *Above bar indicates significant increases compared to diestrous (top) or vehicle-administration (bottom; P < 0.05).
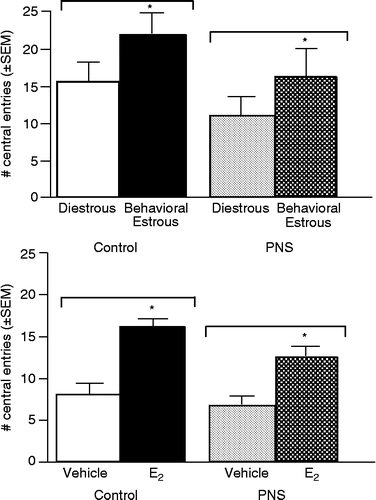
Among OVX rats, estradiol significantly increased total [F(1,50) = 4.2, P < 0.05; and central [F(1,50) = 16.8, P < 0.01; , bottom] entries compared to vehicle. There was no effect of gestational stress on central entries and no significant interactions, but gestational stress decreased total entries made in the open field [F(1,50) = 6.3, P < 0.02].
Elevated plus maze
Rats in behavioral estrus had increased open arm time compared to diestrous rats [F(1,49) = 34.4, P < 0.01]. Gestationally-stressed rats had decreased open arm time compared to non-stressed rats [F(1,49) = 6.9, P < 0.01]. The interaction of these variables did not reach statistical significance (, top).
Figure 2 The mean ( ± SEM) time (seconds) spent in the open arms of the elevated plus maze by gestationally-stressed (PNS) and non-stressed control rats that were in diestrus or behavioral estrus (top) or OVX and administered vehicle or estradiol (E2; bottom). *Above bar indicates significant increases compared to diestrous (top) or vehicle-administration (bottom; P < 0.05). *Above grouped bars indicates difference from control rats (P < 0.05).
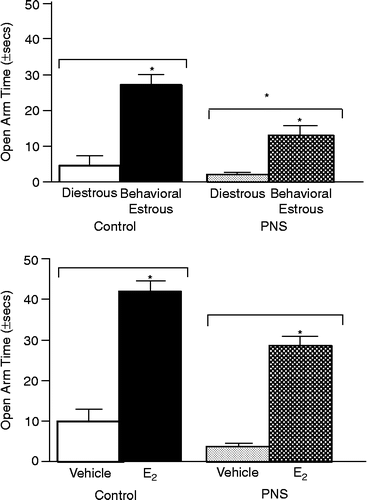
Estradiol significantly increased open arm time among OVX rats compared to vehicle administration [F(1,50) = 35.8, P < 0.01] and there was no significant effect of gestational stress or an interaction between these variables (, bottom).
Inhibitory avoidance
Rats in behavioral estrus performed better in this task than did rats in diestrus [F(1,49) = 4.7, P ≤ 0.03]. Gestational stress significantly decreased inhibitory avoidance crossover latencies [F(1,49) = 3.9, P ≤ 0.05]. There was no significant interaction (, top).
Figure 3 The mean ( ± SEM) latency (seconds) to enter the dark, shock-associated side of the inhibitory avoidance chamber of gestationally-stressed (PNS) and non-stressed control rats that were in diestrus or behavioral estrus (top) or OVX and administered vehicle or estradiol (E2; bottom). *Above bar indicates significant increases compared to diestrous (top) or vehicle-administration (bottom; P < 0.05). *Above grouped bars indicates significant difference from control rats (P < 0.05).
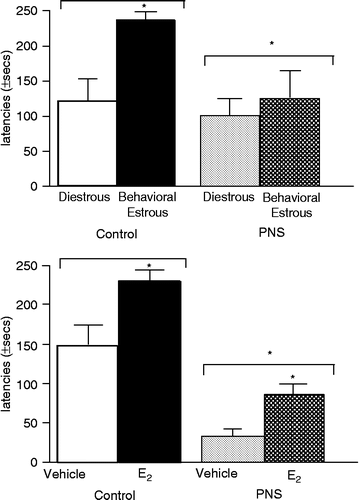
Among OVX rats, estradiol significantly increased open arm time compared to that seen with vehicle administration [F(1,50) = 4.7, P ≤ 0.03]. Gestationally-stressed rats had reduced crossover latencies in the inhibitory avoidance task compared to non-stressed controls [F(1,50) = 25.0, P < 0.01]. There was no significant interaction (, bottom).
Discussion
The present results supported our hypothesis that estradiol and gestational stress would produce different effects on anxiety and cognitive behavior. Estradiol, in physiological levels as were observed in behavioral estrus rats or OVX rats administered estradiol via subcutaneous silastic capsules, decreased anxiety in the open field and elevated plus maze and enhanced performance in the inhibitory avoidance task. Although gestational stress did not alter estradiol levels, increases in plasma corticosterone levels were observed in OVX gestationally-stressed compared to non-stressed rats. Gestational stress decreased open arm time of intact, cycling rats and produced performance deficits in cycling and OVX rats in the inhibitory avoidance task. Thus, these data suggest that estradiol has anti-anxiety and cognitive-enhancing effects and stress exposure during gestation produces alterations in HPA axis function, elevated plus maze behavior, and performance deficits in the inhibitory avoidance task.
The present data, showing that gestational stress increases basal corticosterone levels in OVX rats, confirm previous investigations on how stress during development can produce alterations in neuroendocrine and behavioral responses in adulthood. Although other studies have shown changes in levels of progestins and prolactin with gestational stress, there were no apparent differences in plasma estradiol concentrations between PNS and control rats in the present results, which suggests that the behavioral differences observed may be more likely due to changes in HPA axis function, particularly in OVX rats that have a disrupted hypothalamic–pituitary–gonadal axis (Kinsley and Bridges Citation1987; Kinsley et al. Citation1989; Frye and Walf Citation2004a,Citationb). Female rodents may be more sensitive to HPA axis changes and behavioral disturbances produced by gestational stress and it is possible that estradiol modulates such effects. However, we did not find significant interactions between gestational stress and the estradiol milieu to support this notion in the present study. In other studies, compared to males, females exposed to gestational stress have greater HPA axis reactivity (Weinstock et al. Citation1992; McCormick et al. Citation1995; Koehl et al. Citation1999; Szuran et al. Citation2000). Gestational stress attenuates estradiol's anti-depressant-like effects in the forced swim test in OVX rats (Frye and Wawrzycki Citation2003) and socio-sexual behavior (Frye and Orecki Citation2002a,Citationb). Latencies to withdraw paws from a heat source in the hot-plate task, a measure of nociceptive sensitivity, are decreased by gestational stress in female, but not male, mice; this effect was abrogated by OVX and restored by estradiol administration (Sternberg Citation1999). Similar anti-anxiety effects have been observed in non-stressed OVX rats administered estradiol (Walf and Frye Citation2006a,Citationb). However, in the present study effects of gestational stress to alter anxiety measures were only observed among intact, cycling rats in the elevated plus maze. Thus, estradiol can reduce anxiety behavior while gestational stress produces a different pattern of effects, compared to estradiol, for these measures.
Gestational stress can alter cognitive performance in adulthood. In the present study, PNS rats had poorer performance in the inhibitory avoidance task, as indicated by decreased crossover latencies, compared to non-stressed rats. Gestational stress produces decrements in cognitive performance in the delayed alternation, Morris water maze, and inhibitory avoidance tasks compared to that observed in non-stressed rodents (Lordi et al. Citation1997, Citation2000; Lemaire et al. Citation2000). Higher endogenous estradiol levels or administration of estradiol to OVX rodents improves performance in these spatial and non-spatial tasks in some but not all studies, and this may be related to task demand (Chesler and Juraska Citation2000; Sandstrom and Williams Citation2001; Galea et al. Citation2001, Citation2002; Frye and Rhodes Citation2002; Holmes et al. Citation2002; Rhodes and Frye Citation2005). Indeed, performance in the inhibitory avoidance task may reflect motor differences, which were observed in the present study in cycling gestationally-stressed rats for total open field entries, and/or greater fear. Total entries made in the open field were also increased in rats with higher estradiol level in the present study. Estradiol has known effects to alter motor behavior and this factor needs to be taken into consideration when interpreting the present results.
There are sex differences in the effects of stress to alter cognitive performance, suggesting that endogenous estradiol may mitigate these effects. For instance, acute stressor exposure in adulthood produces more robust performance deficits among female rodents than males (Wood and Shors Citation1998; Shors et al. Citation1998, Citation1999; Wood et al. Citation2001; Shors and Leuner Citation2003). Chronic stress during adulthood facilitates learning in female rats (Bowman et al. Citation2002; Luine Citation2002). However, the present study did not reveal an interaction between the effects of estradiol and gestational stress for performance in the inhibitory avoidance task. Studies that have utilized a single restraint stress session in pregnant rats did not find differences in cognitive tasks due to gestational stress exposure (Vallee et al. Citation1997; Fujioka et al. Citation2001; Meunier et al. Citation2004). Together, these data suggest that there may be different effects of stress for cognitive performance depending on timing of stressor exposure.
Likely central targets for the observed neuroendocrine and behavioral responses are the amygdala and hippocampus. The circuitry of the stress response involves activation of both of these brain structures (Herman et al. Citation2003), which are critical for inhibitory avoidance performance (Izqueirdo and Medina Citation1993) and regulate affective behavior. Estradiol infusion or implants in the hippocampus or amygdala increase open field central entries and plus maze open arm time (Frye and Walf Citation2004a,Citationb; Walf and Frye Citation2006a,Citationb) and increase crossover latencies in the inhibitory avoidance task (Frye and Rhodes Citation2002). Stress during the last week of gestation, as was utilized in this study, occurs at a time when the limbic system is developing and affective and cognitive behaviors may be particularly altered (Weinstock Citation2001). Gestational stress alters the adult hippocampus. Neurogenesis in the dentate gyrus of gestationally-stressed rats is reduced compared to non-stressed rats (Lemaire et al. Citation2000). Gestationally-stressed female rats show more cell loss in the hippocampus than do gestationally-stressed males or non-stressed controls (Schmitz et al. Citation2002). Future studies will investigate whether stress-induced changes in the hippocampus and/or amygdala may contribute to the behavioral effects observed with these manipulations.
The present findings that estradiol has anti-anxiety and cognitive-enhancing effects and gestational stress can produce performance deficits in the inhibitory avoidance task are intriguing, but there are some limitations of these findings that should be considered. First, only basal corticosterone levels in plasma were measured in a small group of non-tested animals so the effects of this prenatal manipulation and estradiol status on other indices of HPA axis reactivity, such as adrenocorticotropin and/or corticosteroid-binding globulin levels, which also can be altered by estradiol, in tested and non-tested rats is of interest (Young et al. Citation2001; Figueiredo et al. Citation2002; McCormick et al. Citation2002). As well, direct manipulation of these other targets was not examined. Estradiol may alter distribution of brain glucocorticoid receptors (Patchev et al. Citation1995), which are important for the expression of affective behavior as revealed by knockout/knockin studies (Wei et al. Citation2004; Boyle et al. Citation2006). Second, other receptor mechanisms by which estradiol influences affective and cognitive performance are also of interest. Whether estradiol's effects occur via actions at two well-described isoforms of the estrogen receptor (ER), ERα or ERβ, which are expressed in different regions of the brain, and may drive HPA axis function in opposing ways, are not clear (Miller et al. Citation2004; Suzuki and Handa Citation2004, 2005; Lund et al. Citation2005). Administration of an ERβ-specific ligand reduces corticosterone levels in OVX rats following exposure to the elevated plus maze (Lund et al. Citation2005) and ERβ may be the receptor target of estradiol's effects to reduce anxiety/depression (Walf and Frye 2006). Indeed, a possibility to investigate in the future is that stressor exposure, during gestation and/or later in life, may alter expression of these potential receptor targets of estradiol and therefore the functional response to estradiol.
In summary, the present data suggest that estradiol can reduce anxiety behavior and enhance cognitive performance and that there are long-term neuroendocrine and behavioral consequences to stress exposure during gestation. Rats in a hormonal milieu associated with higher estradiol levels (behavioral estrous or OVX and estradiol-replaced) had reduced anxiety behavior in the open field and/or elevated plus maze and improved performance in the inhibitory avoidance task. Gestational stress increased plasma corticosterone levels in OVX rats, without producing changes in plasma estradiol levels, decreased open arm time on the plus maze in intact rats and decreased inhibitory avoidance performance. Together, these data support a role for estradiol and/or prior stress exposure to alter affective behavior and cognitive performance.
Acknowledgements
This research was supported by grants from National Science Foundation (IBN 98-96263, IBN03-16083, DBI-0091343). Technical assistance provided by S. Jeddi, Z. Orecki, M. Rhodes, K. Sumida, and J. Wawrzycki is greatly appreciated.
References
- Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry 2006; 28: 3–8
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 2002; 113: 401–410
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 2006; 26: 1971–1978
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: Interaction with a brief, daily maternal separation. Behav Brain Res 2006; 169: 128–136
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav 2000; 38: 234–242
- Drago F, Di Leo F, Giardina L. Prenatal stress induces body weight deficit and behavioural alterations in rats: The effect of diazepam. Eur Neuropsychopharmacol 1999; 9: 239–245
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology 2002; 143: 2534–2540
- Figueiredo HF, Dolgas CM, Herman JP. Dissociation of ACTH and corticosterone responses to stress in female rats treated with estrogen. Horm Behav 2003; 44: 48–49
- Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav 1986; 37: 681–687
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α, 5α-THP and 3α-Diol. Neuroendocinol 1999; 11: 839–847
- Frye CA, Orecki ZA. Prenatal stress produces deficits in socio-sexual behavior of cycling, but not hormone-primed, Long–Evans rats. Pharmacol Biochem Behav 2002a; 73: 53–60
- Frye CA, Orecki ZA. Prenatal stress alters reproductive responses of rats in behavioral estrus and paced mating of hormone-primed rats. Horm Behav 2002b; 42: 472–483
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res 2002; 956: 285–293
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 2002; 41: 306–315
- Frye CA, Walf AA. Estrogen and/or progesterone systemically or to the amygdala can have anxiety, fear, and pain reducing effects in ovariectomized rats. Behav Neurosci 2004a; 118: 306–313
- Frye CA, Walf AA. Hippocampal 3alpha,5alpha-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav 2004b; 78: 531–540
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav 2003; 44: 319–326
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav 2000; 67: 587–596
- Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y, Nakamura S. Mild prenatal stress enhances learning performance in the non-adopted rat offspring. Neuroscience 2001; 103: 301–307
- Galea LA, Lee TT, Kostaras X, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase ”depressive-like“ behaviors in the female meadow vole. Physiol Behav 2002; 277: 217–225
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res 2001; 126: 115–126
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol 2003; 24: 151–180
- Hogervorst E, Boshuisen M, Riedel W, Willeken C, Jolles J. 1998 Curt P. Richter Award. The effect of hormone replacement therapy on cognitive function in elderly women. Psychoneuroendocrinology 1999; 24: 43–68
- Hogervorst E, Willliams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: A meta-analysis. Neuroscience 2000; 101: 485–512
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci 2002; 116: 928–934
- Izquierdo I, Medina JH. Role of the amygdala, hippocampus and entorhinal cortex in memory consolidation and expression. Braz J Med Biol Res 1993; 26: 573–589
- Janowsky JS. The role of ovarian hormones in preserving cognition in aging. Curr Psychiatry Rep 2002; 4: 467–473
- Jezova D, Skultetyova I, Makatsori A, Moncek F, Duncko R. Hypothalamo–pituitary–adrenocortical axis function and hedonic behavior in adult male and female rats prenatally stressed by maternal food restriction. Stress 2002; 5: 177–183
- Kinsley CH, Bridges RS. Prenatal stress reduces estradiol-induced prolactin release in male and female rats. Physiol Behav 1987; 40: 647–653
- Kinsley CH, Mann PE, Bridges RS. Alterations in stress-induced prolactin release in adult female and male rats exposed to stress, in utero. Physiol Behav 1989; 45: 1073–1076
- Klaiber EL, Broverman DM, Vogel W, Kobayashi Y. Estrogen therapy for severe persistent depressions in women. Arch Gen Psychiatry 1979; 36: 550–554
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo–pituitary–adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol 1999; 40: 302–315
- Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 2002; 26: 457–470
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. PNAS 2000; 97: 11032–11037
- Linzmayer L, Semlitsch HV, Saletu B, Bock G, Saletu-Zyhlarz G, Zoghlami A, Gruber D, Metka M, Huber J, Oettel M, Graser T, Grunberger J. Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen–progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung 2001; 51: 238–245
- Lordi B, Protais P, Mellier D, Caston J. Acute stress in pregnant rats: Effects on growth rate, learning, and memory capabilities of the offspring. Physiol Behav 1997; 62: 1087–1092
- Lordi B, Patin V, Protais P, Mellier D, Caston J. Chronic stress in pregnant rats: Effects on growth rate, anxiety and memory capabilities of the offspring. Int J Psychophysiol 2000; 37: 195–205
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress 2002; 5: 205–216
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor β on anxiety-related behaviors. Endocrinol 2005; 146: 797–807
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic–pituitary–adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 1995; 84: 55–61
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress 2002; 5: 235–247
- Meunier J, Gue M, Recasens M, Maurice T. Attenuation by a sigma1 (sigma1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br J Pharmacol 2004; 142: 689–700
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)β isoforms rather than ERα regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci 2004; 24: 10628–10635
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 1996; 21: 609–620
- Morgan KN, Thayer JE, Frye CA. Prenatal stress suppresses rat pup ultrasonic vocalization and myoclonic twitching in response to separation. Dev Psychobiol 1999; 34: 205–215
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: Impact of age in ovariectomized women. Gynecol Obstet Invest 1999; 47: 29–36
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. Am J Psychiatry 2002; 159: 1265–1283
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo–pituitary–adrenal regulation. FASEB J 1995; 9: 419–423
- Peters DA. Prenatal stress: Effects on brain biogenic amine and plasma corticosterone levels. Pharmacol Biochem Behav 1982; 17: 721–725
- Pohorecky LA, Roberts P. Activity in a modified open-field apparatus: Effect of diazepam and prenatal stress. Neurotoxicol Teratol 1991; 13: 129–133
- Poltyrev T, Keshet GI, Kay G, Weinstock M. Role of experimental conditions in determining differences in exploratory behavior of prenatally stressed rats. Dev Psychobiol 1996; 29: 453–462
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. PNAS 1998; 95: 13941–13946
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav 2004; 78: 551–558
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem 2005; 85: 183–191
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology 2006; 147: 2506–2517
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 2001; 115: 384–393
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: Reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry 2002; 7: 810–813
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev 2003; 24: 133–151
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord 2003; 74: 85–96
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport 1998; 9: 419–423
- Shors TJ, Pickett J, Wood G, Paczynski M. Acute stress persistently enhances estrogen levels in the female rat. Stress 1999; 3: 163–171
- Sternberg WF. Sex differences in the effects of prenatal stress on stress-induced analgesia. Physiol Behav 1999; 68: 63–72
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta; expression in the female rat hypothalamus: Differential effects of dexamethasone and estradiol. Endocrinology 2005; 145: 3658–3670
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: Effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav 2000; 71: 353–362
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. J Neurosci 1997; 17: 2626–2636
- Walf AA, Frye CA. Estradiol's effects to reduce anxiety and depressive behavior may be mediated by estradiol dose and restraint stress. Neuropsychopharmacology 2005a; 30: 1288–1301
- Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology 2005b; 30: 1598–1609
- Walf AA, Frye CA. A review and update of: Mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 2006a; 31: 1097–1111
- Walf AA, Frye CA. Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav 2006b, In press
- Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in the rat brain. Physiol Behav 2000; 70: 359–366
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 2001; 65: 427–451
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 2005; 19: 296–308
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic–pituitary adrenal system in the female rat. Brain Res 1992; 595: 195–200
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. PNAS 2004; 101: 11851–11856
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. PNAS 1998; 95: 4066–4071
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 2001; 115: 175–187
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology 2001; 25: 881–891
