Abstract
The chemical composition, sugars profile, total phenolic compounds, phenolic profile and antioxidant activity of ‘Golden Delicious’ apples during storage under controlled atmospheres were evaluated. Total lipids and proteins slightly decreased during storage, while total carbohydrate and mineral content remained constant. The content of total phenolic compounds ranged from 564–748 mg gallic acid equivalents per 100 g of dry apple. Epicatechin, chlorogenic acid, catechin, phloridzin, cyanidin-3-galactoside, quercetin-3-galactoside and quercetin-3-glucoside were the seven major phenolic compounds identified in all samples. Their content was modified by harvest date and in late harvested apples was increased by controlled atmosphere storage. Antioxidant activity, evaluated by ferric reducing antioxidant power (FRAP), radical 2,2-diphenyl-1-picrylhydrazyl scavenger capacity (DPPH) and TROLOX equivalent antioxidant capacity (TEAC), remained unchanged during the whole storage for both harvest dates. The concentration of sucrose decreased, while glucose increased and fructose remained unchanged during the storage time for both harvest dates. The present results suggest that, under the storage conditions used, nutrients and bioactive compounds of apples were maintained for at least 8 months and phenolic profile was modified by harvest date and storage.
Introduction
Consumption of fresh fruits and vegetables is highly recommended due to their content of nutrients such as proteins, sugars, vitamins and minerals. They are also a source of phytochemicals that do not provide calories or nutrients, but are associated with various health benefits (González-Aguilar et al. Citation2008; Rodrigo-García et al. Citation2014). According to Vrhovsek et al. (Citation2004), ‘Golden Delicious’ and ‘Red Delicious’ are the most consumed apple varieties. In Mexico, the state of Chihuahua in the north of the country is the leading producer of ‘Golden Delicious’ apples with a yield of around 15 tonnes per hectare, and 25,000 hectares are used for cultivation (SAGARPA Citation2011).
During storage, fruits are susceptible to deterioration caused by microorganisms, insects or enzymes that can chemically and physically alter the fruit’s structure, resulting in damage that affects subsequent sale (Luck & Jager Citation2000). Controlled atmosphere storage is a well-proven technology for preserving the natural quality of food products in addition to extending the storage life. Storage of fruits and vegetables at low temperatures and low concentration of O2 and CO2 has been used to reduce the metabolic processes in the product (Jayas & Jeyamkondan Citation2002).
Different studies have been conducted to evaluate the characteristics of stored apples. Paster et al. (Citation1995) analysed the growth of microorganisms in stored fruit. Lidster et al. (Citation1983) determined the effect of storage under modified atmosphere on the volatile compounds of apples. Smith et al. (Citation1987) evaluated the effect of modified atmosphere on colour, firmness and sensory properties of apples. However, until recently, the main purpose of storage in controlled atmospheres was to prevent the product from becoming visibly damaged, which would elicit a rejection by the consumer, while less attention was paid to the effect on the content of nutrients. Currently, studies are focused on monitoring the behaviour of nutrients and phytochemicals in products during storage to ensure their integrity as far as possible. In this context, van der Sluis et al. (Citation2001) analysed the effect of storage conditions on the phenolic content and antioxidant activity of four apple cultivars. Likewise, Burda et al. (Citation1990) evaluated the phenolic compounds of three apple cultivars under cold storage. However, these authors focused their studies on certain phenolic compounds and the antioxidant activity of fruits without analysing other components that may be important for their nutritional and sensorial quality.
In view of the high apple production in the region and the extensive use of storage systems with controlled atmospheres, the apple producers are interested in learning more about the effect of storage duration and harvest date on the nutritional quality of controlled atmosphere stored apple fruit. The aim of this study was to assess changes in key nutrients and phenolic phytochemicals during industrial storage of ‘Golden Delicious’ apples harvested on two different dates and stored under modified atmosphere for 8 months, which is the maximal storage time used for commercialisation of these apples in the Mexican market.
Materials and methods
Collection and storage of plant material
Samples of ‘Golden Delicious’ apples were provided by Agropecuaria La Norteñita, the largest apple producer in Mexico, located in the city of Cuauhtemoc in the state of Chihuahua in northern Mexico (28°25′N and 106°52′W). In this region, the optimal harvest months for apple are September (early harvest [EH]) and October (late harvest [LH]); therefore, the effect of storage was analysed in samples from both harvest seasons. Both collections were stored at 4 °C under a controlled atmosphere (2% CO2 and 2% O2). Nine samples (each consisting of 20–25 randomly selected whole apples) were taken from each collection, one at harvest time and then one after each month of storage. For analysis, samples were transported in dark plastic bags to the laboratory. Once there, all apples in the sample were sliced, frozen at −80 °C, lyophilised in a Labconco Freeze dry/shell freeze system (Kansas City, MO, USA), ground, homogenised and stored in a deep freeze Revco ExF (Thermo Scientific, Marietta, OH, USA) at −80 °C until analysis.
Chemical analysis
Chemical analysis was performed according to Association of Official Analytical Chemists (AOAC) International techniques (Williams Citation2005). Total protein content was calculated by multiplying the total nitrogen quantified by the Kjeldahl method by the factor 6.25. The total mineral content was calculated from the incineration of the samples at 525 °C to constant weight. Total lipids were extracted in Soxhlet apparatus for 6 h using ethyl ether as solvent extractor. Total carbohydrates were calculated by difference (100 – Σ [protein + lipid + total mineral]). All analyses were performed in triplicate.
Total phenolic content
Phenolic compounds were extracted with 80% methanol, according to the protocol of Alvarez-Parrilla et al. (Citation2010). Specifically, 0.1 g of each sample was weighed into screw-cap tubes. Then 5 mL of the solvent was added, sonicated for 15 min and centrifuged at 4000 rpm for 15 min. This process was performed twice and the extracts were placed quantitatively in 10 mL volumetric flask. A 100 µL sample of the above extract was taken and 500 µL of the Folin-Ciocalteu reagent was added and allowed to stand for 2 min. Thereafter, 400 µL of Na2CO3 was added and incubated at 50 °C for 15 min. Finally, the mixture was cooled in an ice bath, 250 µL was collected and placed in a microplate well and absorbance was measured at 740 nm in a Bio-Rad xMark Plus. Data were obtained using Microplate Manager 6.0 (Bio-Rad, Tokyo, Japan) computer software. A calibration curve was created using gallic acid as a standard and the results expressed in mg gallic acid equivalents (GAE) per 100 g of dry sample. All reagents were obtained from Sigma (St Louis, MI, USA) unless stated otherwise.
Profile of phenolic compounds using HPLC
Individual phenolic compounds were quantified using high-performance liquid chromatography (HPLC), following the protocol of de la Rosa et al. (Citation2011) with minor modifications. Phenolic compounds were extracted as described in the previous section. One millilitre of the extract was filtered through a 0.45 µm membrane, and 20 µL was injected into the HPLC system. The equipment was a Perkin Elmer chromatograph Series 200 with a quaternary pump, column oven and diode array detector (Shelton, CT, USA). A C18 Phenomenex column (5 µm, 250 × 46 mm, Torrance, CA, USA) was used, the absorbance was monitored at 280 and 320 nm, and characteristic absorption spectra were collected. A binary mobile phase was used: solvent A was methanol:acetonitrile (95:5) and solvent B 1% phosphoric acid. The gradient programme was: 2.5 min, 100% B; 5.5 min, 70% B; 22.5 min, 20% B; and 25.5 min, 100% B. Identification of individual phenolic compounds was achieved by comparison of retention times and spectra with those of standards (epicatechin, chlorogenic acid, caffeic acid, p-coumaric acid, catechin, phloridzin, cyanidin-3-galactoside, quercetin-3-galactoside, quercetin-3-glucoside, and procyanidins B1 and B2, all from Sigma, St Louis, MI, USA). Calibration curves were made with all standards in order to quantify individual phenolic compounds in apple samples.
Antioxidant activity
Antioxidant activity was analysed by techniques of ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and TROLOX equivalent antioxidant activity (TEAC) (Alvarez-Parrilla et al. Citation2011). To determine FRAP, a standard curve was performed using 3 mM FeSO4 as a source of Fe+2, which forms a coloured complex with 2,4,6-tripyridyl-s-triazine (TPTZ). The solutions were made in 80% methanol. A 100 µL sample of the extract was mixed with 300 µL of water and vortexed. Then 24 µL of the mixture was placed in the microplate well with 180 µL of TPTZ working solution (a mixture of 0.3 M acetate buffer, pH 3.6; 10 mM TPTZ solution in 40 mM HCl and 20 mM FeCl3, at a 10:1:1 ratio) and absorbance was read at 595 nm every 30 s for 30 min. Results were expressed as mmol Fe2+ 100 g−1 dry fruit. In the DPPH assay, a 60 mM DPPH solution was prepared in methanol; 5 µL of the extract was added to 195 µL of the DPPH solution and the absorbance read at 517 nm every minute for 1 h. TROLOX was used as standard and results were expressed as mmol TROLOX equivalents 100 g−1 dry fruit. To generate the ABTS•+ radical on the TEAC assay, ABTS and potassium persulphate were mixed in distilled water and allowed to react for 16 h. A suitable volume of this solution was dissolved in ethanol to give an absorbance of about 0.7 at 734 nm. Subsequently, 190 µL of the radical was mixed with 10 µL of sample and incubated for 1 min. Then, the absorbance was measured every 30 s for 6 min. TROLOX was used as standard and results were expressed as mmol TROLOX equivalents 100 g−1 dry fruit.
Sugars profile
The content of glucose, sucrose and fructose in apples was determined by HPLC, following the method reported by Smith et al. (Citation1987). Approximately 3 g of sample was weighed and 20 mL of ultrapure water was added. The mixture was placed in a water bath at 70 °C for 15 min, then filtered and centrifuged at 6000 rpm. A 1 mL sample of the supernatant was filtered through a 0.45 µm membrane filter and 20 µL injected into the HPLC system. Acetonitrile:water (80:20 v/v) was used as mobile phase under isocratic conditions, at a flow of 1 mL min−1 in the HPLC Perkin Elmer system mentioned above. Separation was achieved using a Luna 5u NH2 100 Å (5 µm, 250 × 46 mm, Phenomenex, Torrance, CA, USA) column, and a refractive index detector Perkin Elmer Series 200 was used for quantification. A standard curve was made using pure standards of glucose, sucrose and fructose (Sigma, St Louis, MI, USA).
Statistical analysis
All results are expressed in dry matter unless stated otherwise. Data were analysed using Statgraphics Centurion XVI (StatPoint Technologies Inc, Warrenton, VA, USA). Results of all analysis are expressed as mean ± standard deviation of at least three replicates of each sample. In addition, an analysis of variance (ANOVA) and the Tukey test (P < 0.05) were used to compare results of different harvests and storage times.
Results and discussion
Chemical analysis
shows the initial and final values of mineral content, protein, lipids and carbohydrates of EH and LH apples stored at 4 °C under modified atmosphere during 8 months. Harvest date and storage duration did not affect total carbohydrates or mineral content (). Total protein and lipid content decreased at the end of storage in apples from both harvests. Lipid content was also lower in LH apples in comparison with EH, both at the beginning and the end of storage.
Table 1 Effect of harvest date and storage duration on chemical composition of ‘Golden Delicious’ apple samples at harvest and after 8 months of controlled atmosphere storage at 4 °C.
The reduction in lipids during storage under controlled atmosphere may be explained by the effect of low O2 concentrations on lipid metabolism, as described by Brackmann et al. (Citation1993) who found that storing ‘Golden Delicious’ apples under low oxygen conditions for up to 8 months reduced the content of fatty acids and volatile compounds derived from fatty acid metabolism. Production of volatile compounds derived from fatty acids could also help explain the lower lipid levels in LH apples. Villatoro et al. (Citation2008) observed higher levels of lipoxygenase activity and volatile biosynthesis in apples harvested in October compared with those harvested in September, and it has been described that fatty acids are major precursors of aroma volatiles in many apple varieties, including ‘Golden Delicious’. Even though no reports have been published about the effects of controlled atmosphere storage on total protein content, several studies have shown a decrease in the biosynthesis of many enzymes associated with quality loss (Galvis Sanchez & Morais Citation2002) which could have an effect on the total protein content in the fruit.
Total phenol content
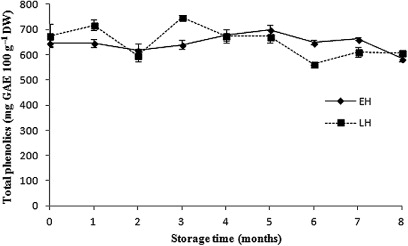
The values of total phenolic compounds ranged from 564 to 748 mg GAE 100 g−1 DW (). The total phenolic content showed a greater degree of dispersion in LH relative to EH. Samples from both harvest periods showed a slight decrease (approximately 10%) after 8 months of storage. The highest value of these phytochemicals was found in the third month in LH apples, while the lowest amount was observed in the seventh and eighth months in the LH and EH apples, respectively. Stratil et al. (Citation2007), Sun et al. (Citation2002) and Wolfe et al. (Citation2003) reported a higher content of phenolics in fresh apples compared with this study. In contrast, the values obtained by Imeh & Khokhar (Citation2002) and Proteggente et al. (Citation2002) are similar to those obtained in our investigation. This indicates that factors such as variety, crop type and environmental conditions may be involved in the amount of phenolic compounds in fresh apples. Furthermore, Proteggente et al. (Citation2002) determined the concentration of polyphenols in bananas, peas, cauliflower, tomato, leek and lettuce, all values below those reported in our study. As to the effect of storage on the total phenolic content, our results are in agreement to those reported by van der Sluis et al. (Citation2001), who observed that the concentration of apple phenols was kept almost constant during storage.
Profile of phenolic compounds using HPLC
The seven major phenolic compounds identified in the samples were (in order of abundance): epicatechin, chlorogenic acid, catechin, phloridzin, cyanidin-3-galactoside (C-3-Gal), quercetin-3-galactoside (Q-3-Gal) and quercetin-3-glucoside (Q-3-Glu) (). All of these compounds were identified in apples from both harvest dates and storage times; however, their contents and behaviour during storage were different between harvests. At harvest, LH apples showed higher contents of all phenolic compounds except Q-3-Gal. At the end of the storage time (8 months in a controlled atmosphere of 2% O2, 2% CO2), EH showed a decrease in the contents of epicatechin, chlorogenic acid and phloridzin, while the other compounds remained statistically similar to those observed at harvest time. In contrast, controlled atmosphere storage of LH apples produced an increase in the content of all phenolic compounds. An increase in some phenolic acids, flavonoids and anthocyanidins during controlled atmosphere storage has been reported (Burda et al. Citation1990; Napolitano et al. Citation2004; Hoang et al. Citation2011) and involvement of phenylalanine ammonia lyase (PAL) activation has been suggested. However, the same authors have observed an inconsistent behaviour in some individual phenolic compounds during storage, and also differences depending on the part of the fruit in which they are measured. Ju et al. (Citation1996) reported that some phenolic compounds decreased more rapidly in early harvested apples, in agreement with our results for EH samples.
Table 2 Phenolic profile of ‘Golden Delicious’ apple samples at two harvest seasons and after 8 months of storage at 4 °C under controlled atmosphere.
Antioxidant activity
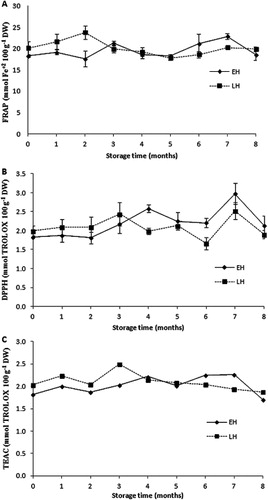
FRAP, DPPH and TEAC values ranged from 17.62–23.78 mM Fe+2 100 g−1 DW, 1.8–2.9 mmol of TROLOX 100 g−1 DW and 1.7–2.3 and 1.9–2.5 mmol TROLOX 100 g–1 DW, respectively (). No significant differences were found between initial and final antioxidant activity values by any of the methods used. Despite the slight decrease observed on phenolic compounds determined by the Folin-Ciocalteu method, the antioxidant activity was constant; this may be due to the presence of other compounds that contribute to antioxidant activity, such as vitamins and procyanidins (van der Sluis et al. Citation2001; Enriquez et al. Citation2010).
Sugar profile
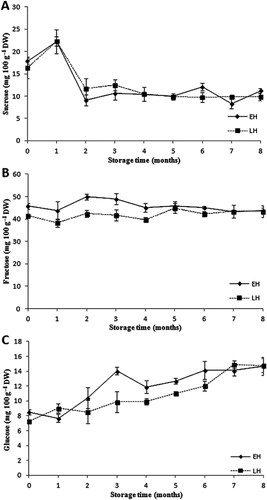
The contents of the major sugars (sucrose, fructose and glucose) and their change during storage under controlled atmosphere and low temperature are shown in . The concentration of sucrose increased in the first month of storage from 17 to 23 mg 100 g−1 DW and then decreased to 10 mg 100 g−1 DW for the rest of time in both EH and LH apples. Fructose concentrations remained constant during the whole storage time; the average value was 45 mg 100 g−1 DW. However, glucose content increased gradually from 8 to 15 mg 100 g−1 DW for EH and LH apples, during the 8 month storage period. The behaviour of individual sugars during storage could be explained in terms of sucrose and fructose metabolism (Gerhardt et al. Citation1987). Róth et al. (Citation2007) reported high fructose content in apples representing 55%–70% of total sugars, followed by sucrose and glucose, which behaved similarly to the results presented in this study. Several post-harvest treatments that delay the ageing process also allow the products to maintain higher levels of sugars; however, the presence of saccharolytic enzymes can be associated with a decrease in the sucrose concentration (Hasperué et al. Citation2014). A decline in the concentration of sucrose accompanied by an increase in the glucose content of apples could be explained by the activity of saccharolytic enzymes such as neutral and vacuolar invertase (Zhu et al. Citation2013). The content of the three sugars and their behaviour during storage was the same for both harvest dates.
Conclusions
The storage conditions evaluated in the present study (4 °C and controlled atmosphere of 2% CO2 and 2% O2) are appropriate to maintain the concentration of phytochemicals and nutrients in ‘Golden Delicious’ for at least 8 months of storage. However, slight decreases in lipid, protein and sucrose contents were observed and lipid and individual phenolic compounds were different between early and late harvests. Individual phenolic compounds were increased by storage only in LH apples. These results suggest that a late harvest could have beneficial effects on the phytochemical content of ‘Golden Delicious’ apples.
Acknowledgements
Funding of this work by the Mexican National Council of Science and Technology (CONACYT, Project CONACYT-Chihuahua 2005-C01-22028) and CONACYT/Universidad Autonoma de Ciudad Juarez internal projects is gratefully acknowledged. Authors also wish to thank ‘La Norteñita’ Group Company, research assistant Karla Salazar, and bachelor students Esly Shalom Jimenez-Rodríguez and Blanca Erika Torres-Diaz.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alvarez-Parrilla E, de la Rosa LA, Amarowicz R, Shahidi F 2011. Antioxidant activity of fresh and processed jalapeño and serrano peppers. Journal of Agricultural and Food Chemistry 59: 163–173.10.1021/jf103434u
- Alvarez-Parrilla E, de La Rosa LA, Legarreta P, Saenz L, Rodrigo-García J, González-Aguilar GA 2010. Daily consumption of apple, pear and orange juice differently affects plasma lipids and antioxidant capacity of smoking and non-smoking adults. International Journal of Food Sciences and Nutrition 61: 369–380.10.3109/09637480903514041
- Brackmann A, Streif J, Bangerth F 1993. Relationship between a reduced aroma production and lipid metabolism of apples after long-term controlled-atmosphere storage. Journal of the American Society of Horticultural Sciences 118: 243–247.
- Burda S, Oleszek W, Lee CY 1990. Phenolic compounds and their changes in apples during maturation and cold storage. Journal of Agricultural and Food Chemistry 38: 945–948.10.1021/jf00094a006
- Enriquez C, Almonacid S, Chiffelle I, Valenzuela T, Araya M, Cabezas L et al. 2010. Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chilean Journal of Agricultural Research 70: 523–536.10.4067/S0718-58392010000400001
- Galvis Sánchez AC, Morais AMMB 2002. Effects of controlled atmosphere (CA) storage on pectinmethylesterase (PME) activity and texture of ‘Rocha’ pears. Journal of the Science of Food and Agriculture 82: 143–145.
- Gerhardt R, Stitt M, Heldt HW 1987. Subcellular metabolite levels in spinach leaves. Plant Physiology 83: 399–407.10.1104/pp.83.2.399
- González-Aguilar G, Robles-Sánchez RM, Martínez-Téllez MA, Olivas GI, Alvarez-Parrilla E, de la Rosa LA 2008. Bioactive compounds in fruits: health benefits and effect of storage conditions. Postharvest Review 4: 1–10.
- Hasperué J, Lemoine ML, Chaves A, Martínez G 2014. Effect of time of day for harvest and postharvest treatments on the sugar metabolism of broccoli (Brassica oleracea var. italica). Agricultural and Food Science 23: 48–59.
- Hoang NTT, Golding JB, Wilkes MA 2011. The effect of postharvest 1-MCP treatment and storage atmosphere on ‘Cripps Pink’ apple phenolics and antioxidant activity. Food Chemistry 127: 1249–1256.10.1016/j.foodchem.2011.01.052
- Imeh U, Khokhar S 2002. Distribution of conjugated and free phenols in fruit: antioxidant activity and cultivar variations. Journal of Agricultural and Food Chemistry 50: 6301–6306.10.1021/jf020342j
- Jayas DS, Jeyamkondan S 2002. PH–Postharvest technology: modified atmosphere storage of grains meats fruits and vegetables. Biosystems Engineering 82: 235–251.10.1006/bioe.2002.0080
- Ju Z, Yuan Y, Liu C, Zhan S, Wang M 1996. Relationships among simple phenol, flavonoid, and anthocyanin in apple fruit peel at harvest and scald susceptibility. Postharvest Biology and Technology 8: 83–93.10.1016/0925-5214(95)00062-3
- Lidster PD, Lightfoot HJ, Mc Rae KB 1983. Production and regeneration of principal volatiles in apples stored in modified atmosphere and air. Journal of Food Science 48: 400–402.10.1111/j.1365-2621.1983.tb10751.x
- Luck E, Jager M 2000. Conservación química de los alimentos: características, usos y efectos. Zaragoza, España, Acribia.
- Napolitano A, Cascone A, Graziani G, Ferracane R, Scalfi L, Di Vaio C et al. 2004. Influence of variety and storage on the polyphenol composition of apple flesh. Journal of Agricultural and Food Chemistry 52: 6526–6531.10.1021/jf049822w
- Paster N, Huppert D, Barkai-Golan R 1995. Production of patulin by different strains of Penicillium expansum in pear and apple cultivars stored at different temperatures and modified atmospheres. Food Additives & Contaminants 12: 51–58.10.1080/02652039509374278
- Proteggente AR, Pannala AS, Paganga G, van Buren L, Wagner E, Wiseman S et al. 2002. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radical Research 36: 217–233.10.1080/10715760290006484
- Rodrigo-García J, Alvarez-Parrilla E, López-Díaz DJA, Wall MA 2014. Perspectiva de los alimentos funcionales. In: González-Aguilar GA, González-Córdova AF, Vallejo-Córdoba B, Alvarez-Parrilla E, García HS. eds. Los alimentos funcionales: un nuevo reto para la industria de alimentos. México, DF, AGT Editor.
- de la Rosa LA, Alvarez-Parrilla E, Shahidi F 2011. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). Journal of Agricultural and Food Chemistry 59: 152–162.10.1021/jf1034306
- Róth E, Berna A, Beullens K, Yarramraju S, Lammertyn J, Schenk A et al. 2007. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biology and Technology 45: 11–19.
- SAGARPA 2011. Servicio de Información Agroalimentario y Pesquera. México, DF, Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación.
- Smith SM, Geeson JD, Martin BK, Genge PM, Everson HP 1987. Modified-atmosphere retail packaging of discovery apples. Journal of the Science of Food and Agriculture 40: 165–178.10.1002/jsfa.2740400209
- Stratil P, Klejdus B, Kubáň V 2007. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 71: 1741–1751.10.1016/j.talanta.2006.08.012
- Sun J, Chu YF, Wu X, Liu RH 2002. Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry 50: 7449–7454.10.1021/jf0207530
- van der Sluis AA, Dekker M, de Jager A, Jongen WMF 2001. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. Journal of Agricultural and Food Chemistry 49: 3606–3613.10.1021/jf001493u
- Villatoro C, Altisent R, Echeverría G, Graell J, Lopez ML, Lara I 2008. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of ‘Pink Lady®’ apples. Postharvest Biology and Technology 47: 286–295.10.1016/j.postharvbio.2007.07.003
- Vrhovsek U, Rigo A, Tonon D, Mattivi F 2004. Quantitation of polyphenols in different apple varieties. Journal of Agricultural and Food Chemistry 52: 6532–6538.10.1021/jf049317z
- Williams S ed. 2005. Official methods of analysis. 18th edition. Arlington VA, Association of Official Analytical Chemists (AOAC).
- Wolfe K, Wu X, Liu RH 2003. Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry 51: 609–614.10.1021/jf020782a
- Zhu Z, Liu R, Li B, Tian S 2013. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chemistry 141: 3323–3328.10.1016/j.foodchem.2013.06.025