Abstract
Background: Gaps in the knowledge of general practitioners (GPs) in medical genetics prevent the effective utilization of genetic services and increase the risk of liability. Educators recommend that genetics should be integrated into existing teaching programs but the effectiveness of these types of programs is unknown.
Aim: The objective of this study was to provide a 2-year educational program for GPs untrained in genetics and to document its impact on genetic knowledge and referrals to a genetic counselor (GC).
Methods: Eight genetic lectures series were given at quarterly intervals. Family practice residents received additional training in a genetics clinic, and participated in monthly seminars and bi-annual journal clubs. A pretest–post-test study design (n = 143) was used to evaluate the genetic knowledge of GPs. Post-test scores [mean (%) ± SD; 76.1 ± 16.8] showed significant improvement compared to pretest scores (61.9 ± 19.1). The majority of participants (81%) indicated that the program would have an impact on their clinical practice. The number of referrals to a GC from GPs untrained in genetics did not change over the 2-year period of the program.
Conclusion: The results suggest that an integrated educational program in genetics can enhance physicians’ knowledge but may not alter referral patterns to a GC.
Introduction
The primary care setting is expected to become the initial clinical encounter setting for the evaluation and management of heritable disorders in the general population as the identifiable genetic components of both human disease and well-being are increasingly characterized (Greendale & Pyeritz Citation2001; Subramanian et al. Citation2001). Guttmacher et al. (Citation2001) advocate primary care as the appropriate venue for the integration of genomic medicine into mainstream medical practice because of its inherent longitudinal, family-based attention to preventive care. The effective utilization of regional and local genetic services requires that general physician practitioners (GPs) understand current medical genetic principles as they relate to the recognition and management of heritable disorders in the primary care population. While the precise role of GPs in this new era of genetic services is unclear (Hayflick & Eiff Citation1998; Greendale & Pyeritz Citation2001), there remains a critical gap in training and knowledge in medical genetics among GPs (Lapham et al. Citation2000; Burke et al. Citation2002; Suther & Goodson Citation2004). A legal opinion by Howlett et al. (Citation2002) suggests that a lack of an adequate background in medical genetics is detrimental to the management of inherited disorders and increases the risk of liability. Specific lapses in knowledge and management, as well as a self-expressed lack of understanding in medical genetics have been proposed as significant barriers for effective provision of genetic services (Hayflick & Eiff Citation1998; Lapham et al. Citation2000; Burke et al. Citation2002). Burke et al. (Citation2002) recommend that genetic education should be integrated into existing teaching programs and employ a case-based teaching format that incorporates the clinical and social dimensions of genetic disorders. However, time and schedule limitations, as well as turnover in personnel, significantly impede the implementation of targeted educational efforts to further physicians’ knowledge in genetics (Suther & Goodson Citation2004). In this report we describe an education and training program in genetics that follows the curriculum guidelines endorsed by the American Academy of Family Physicians (http://www.aafp.org/PreBuilt/curriculum/medicalgenetics.pdf). Pretest and post-test knowledge measurements, satisfaction surveys, and tracking genetic counseling referrals were used to assess the utility of the program. The results suggest that an educational program in genetics that is integrated into existing teaching programs is effective in increasing GPs’ knowledge but does not necessarily result in an increase in genetic counseling utilization.
Methods
Educational setting, patient demographics, and study participants
The Mid-Hudson Family Health Institute is a not-for-profit organization located in Ulster County, New York, USA. It provides primary care to patients and trains family practitioners in a residency program approved by the Accreditation Council for Graduate Medical Education. The institute has several practice locations in rural as well as urban settings and provides primary and specialty care to approximately 60 000 patients annually in a population of 177 749 people (US Census 2000). Forty-five per cent of the patients are medically indigent and either uninsured or underinsured. Financial constraints limit the access to genetic counseling at other medical centers. An ICD-based record review showed that 3% (n = 800) of the indigent population has a personal or family history of birth defects or mental retardation which require genetic counseling. The recognition of this need was the impetus by the faculty to obtain federal funding for genetic counseling services and to institute an educational program in clinical genetics. The program participants were 36 GPs (14 family practitioners, two pediatricians, two internal medicine practitioners, and 18 family practice residents) who had not received formal medical genetics training beyond basic medical school education. This research project was exempt from United States Department of Health and Human Service regulations (45 CFR Part 46) because it involved the use of educational tests, and surveys that were devoid of personal identifiers.
Program description
The educational program in medical genetics was developed by a board-certified genetic counselor (GC), a specialist with 3 years of fellowship training in molecular biology and clinical neurogenetics, a medical social worker, and family practice. The educational strategy was based on an evidence-based approach using multifaceted activities such as seminars, didactic lectures, journal clubs, and direct patient contact in a genetics clinic (Davis et al. Citation1995). The curriculum was designed on the recommended curriculum guidelines endorsed by the American Academy of Family Physicians (http://www.aafp.org/PreBuilt/curriculum/medicalgenetics.pdf) and integrated into the existing program. Specific emphasis was placed on gathering genetic family-history information, including an appropriate multi-generational family history. The ethical, legal, privacy, and confidentiality issues were also stressed in the context of families referred for genetic counseling consultation. The curriculum included the role of genetics in the diagnosis, prevention, and treatment of common disorders (e.g. chromosomal abnormalities, familial variants, oncology, geriatric disorders, metabolic disorders, skeletal/connective tissue abnormalities, cardiopulmonary, hematologic disorders, gastrointestinal abnormalities, neuromuscular disorders, neural tube defects, craniofacial abnormalities, psychiatric disorders, prenatal abnormalities, and dysmorphic syndromes). The educational program was organized into 60-minute quarterly lectures given to the faculty physicians and family practice residents (8 hours over 2 years). The educational lectures featured Microsoft® PowerPoint® presentations and covered principles of general medical genetics (). After these lectures, the GC provided 45-minute working didactic seminars on a monthly basis for the family practice residents that expanded on these topics (). Bi-annual journal clubs were focused on reviewing genetic articles pertinent to these topics.
Table 1. Quarterly topics for faculty physicians and family practice residents
Table 2. Monthly didactic seminars for family practice residents
Case-based approaches during the lectures were included with emphasis on clinical utility and the development of appropriate attitude and skills related to the effective management of genetic issues encountered in general medical practice. PGYII and PGYIII residents also rotated through a bi-weekly clinic where they had opportunities to observe and participate in genetic evaluations and genetic counseling sessions.
Knowledge measurements
Genetic knowledge and the attitude toward the curriculum were monitored by a pretest–post-test study design and Likert scales. Knowledge regarding genetic principles was assessed by multiple choice questions adapted from the lecture series and the American Academy of Family Physicians (AAFP) curriculum guidelines. Examples of the types of questions that were determined to assess knowledge are found in the Appendix. A pretest and post-test was administered to each participant before and after each lecture. The presentations were preceded by a 10-question test of the material to be covered in the presentation. The same test was then administered to participants immediately following the presentation. Participants at the end of each presentation were asked to complete a five-point Likert scale as part of the post-test which evaluated the presentation according to usefulness, understanding, impact, and quality. Likert scale results were collected for the eight quarters and combined into single pools for statistical analysis.
Tracking of genetic counseling referrals
GPs directly referred patients to the GC for genetic counseling services. The number of total patients that were referred to a GC was tracked on a quarterly basis beginning 6 months prior to the beginning of the lecture series. The number of patients referred to the GC by GPs who attended the educational program was compared to the referrals from a genetic specialist and a perinatologist who were not study participants. It is important to note that the referrals to the perinatologist and the genetic specialist were from physicians outside the institute that did not participate in the educational program. The patient population and number of referring providers remained constant throughout the study period.
Statistical methods
Power analysis estimated that a sample size of 150 tests had a power of 0.90 to detect a 10% difference in test scores (alpha = 0.05, sigma = 20). The scores on each pretest and post-test were based on the percentage of correct questions answered by the participants. Pretest, post-test, and Likert scale scores from each of the eight quarterly lectures were combined into single pools for statistical analysis using the JMP™ statistical software package release 5.0.1.2 (JMP, Cary, NC, USA). The data was entered as continuous values to calculate the normal distribution of scores, quantiles, and moments. The paired two-tailed student t-test was used to compare pretest and post-test values. The number of genetic counseling sessions per quarter was graphed as an overlay plot.
Results
Likert scale attitude measurements
The physicians’ scores on the Likert scale (n = 82) agreed or strongly agreed that the eight lectures were useful in clinical practice (89%), easily understandable (94%), and were of good quality (96%). The majority (81%) agreed or strongly agreed that the information would have an impact on their medical practice.
Genetic knowledge scores
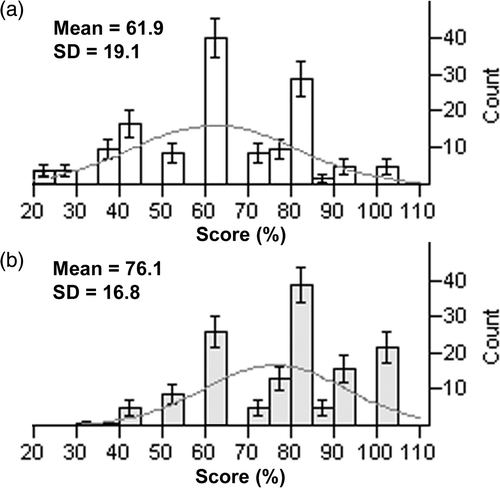
Fifty per cent of expected total (n = 288) of both pretest and post-test sets were complete for individual study participants. The attending physicians’ scores (n = 68) did not differ (p > 0.05) from the scores of the family practice resident (n = 75) for the eight lectures. The scores from both groups were combined (n = 143) and the pretest and post-test scores were plotted and fitted to a normal distribution (). The pretest scores [61.9 ± 19.1 (58.8–65.1); mean (%) ± SD (95% confidence interval)] showed an improvement (p < 1 × 10–10) as compared to the post-test scores [76.1 ± 16.8 (73.3–78.9)] for the entire sample. The median pretest score was 60% with the lower quartile at 50% and the upper quartile at 80%. The median post-test score was 80% with the lower quartile at 60% and the upper quartile at 90%. compares the distribution of scores and shows a shift of the post-test scores to higher values.
Figure 1. Normal distribution of genetic knowledge test scores. (A) Pretests show lower mean scores as compared to (B) post-test scores (n = 143) after an educational program in genetics for primary care physicians. The rectangles represent the count of individuals who received a particular test score (%). The curves represent the normal distribution of the scores. The bars at the top of the rectangles show the standard error of the mean.
Quarterly genetic counseling referrals
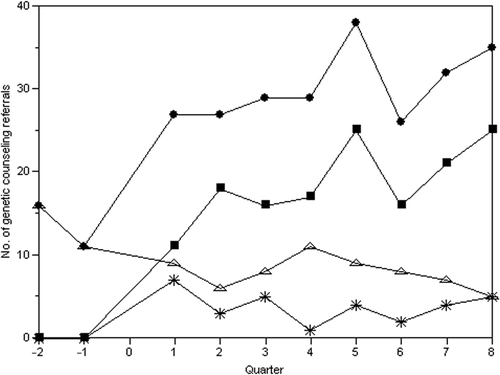
shows the number of referrals to a GC before, during, and after the 2-year educational program. The number of referrals to a GC was between 11 and 16 patients for the two quarters immediately preceding the lecture series. This number increased during the first year of the lecture series and reached 26–39 patients per quarter during the second year (). The number of referrals was analyzed by provider type because specialists in perinatology and genetics who did not participate in the educational program also requested genetic counseling services. shows that most genetic counseling referrals were from these specialists. Seven referrals were made by the GPs during the first quarter prior to the lecture series and remained between one and seven referrals over the 2-year study period. Five per cent of the referrals were initiated by the pediatricians and 95% from the other GPs.
Figure 2. Overlay plot of quarterly referrals for genetic counseling. The graph shows the total number of genetic counseling referrals (•) during the two quarters (6 months) before the genetic educational program and over the 2-year period of the program. The number of genetic counseling referrals from primary care physicians who participated in the program (*) remained the same during the 2-year period. The number of referrals from specialists in perinatology (▪) and genetics (Δ) who did not participate in the educational program accounted for most of the genetic counseling referrals. It is important to note that all of the referrals from the perinatologist were from prenatal visits and were referred directly from the perinatologist to the genetic counselor. The number of referrals to the genetic counselor from the perinatologist seemed to increase despite their non-participation in the genetic educational program.
Discussion
Data from the Human Genome Project has advanced the field of molecular medicine at such a staggering pace that general physicians are usually not prepared to deal with the potential application of this information to clinical practice. In this report we describe an education and training program in medical genetics for practicing providers at a community family health institute. The results suggest that this type of program can effectively enhance genetic knowledge but does not increase the utilization of genetic counseling services. Several variables may have influenced the genetic knowledge scores including the lecture content, format, setting, presenter style, the difficulty of the pretest and post-test questions, and the number, experience, interest and motivation of the participants. Although pretest–post-test designs are used to investigate the effects of most educational programs (Siddell et al. Citation2003; Wilkes et al. Citation2003) some education researchers require validation by using a two-group pretest–post-test design with a control group that receives no training intervention and a group that receives the training (Zapp Citation2001; Gall et al. Citation2003). It was anticipated that family practice residents would not perform as well on the testing compared to GPs with more experience. However, the results indicated no significant difference between resident and faculty performance. Detecting the differences in the knowledge base of residents and faculty was hindered by the relatively small number of test scores. Family practice residents also received additional training and education from their monthly genetic seminar. The reasons why the educational program did not increase the utilization of genetic counseling services are uncertain. The baseline of seven consults per quarter from the 36 GPs participating in the program did not increase to the level of a single geneticist or perinatologist over the 2-year period of the program. Several explanations for this disparity are that the density of appropriate referral candidates is lower in GPs’ practices, and that the referral process itself may have been cumbersome and costly for the GPs. The referral process usually involves written justification for the service and increases GPs’ practice costs by involving an increase in administrative support. It is also possible that even if GPs improve their knowledge base in current genetic medicine and understand the importance of genetics in clinical care, this may not lead to an increase in the utilization of genetic counseling services. Alternatively, the improved genetic education of the GPs may have resulted in a better selection of referrals and thus did not increase the quantity but rather enhanced the reasons for genetic counseling services.
Burke et al. (Citation2002) recommend that genetic education should be integrated into existing teaching programs and employ a case-based teaching format that incorporates the clinical and social dimensions of genetic disorders. The present study included an educational program with an emphasis on elements of case management, counseling, legal, ethical, and family dynamic issues associated with genetic testing. These components were targeted to GPs to appeal to their sense of holistic and comprehensive care. Suther and Goodson (Citation2004) stressed the importance of this aspect of training since the GP's perception of the compatibility of genomic medicine with current practices is a strong predictor of their likelihood to adopt genomic medicine. In addition, the training effort was organized in a manner to meet the varied schedules and backgrounds of the GPs by being integrated into existing programs and schedules. In the future, the program can be easily modified and updated to reflect new developments, shifting priorities, and the needs or interests of the participants without requiring significant additional time commitment. Although the long-term objective of the educational program was to promote an increase in the utilization of genetic services, changing GPs practice patterns is a complex process that involves the primary care team, the organization in which they work, and convincing providers of the clinical utility of the change (Collins & McKusick Citation2001).
Several studies have identified that patients’ interest in a genetic evaluation was the greatest factor prompting a genetics referral by GPs (Hayflick & Eiff Citation1998; Sifri et al. Citation2003). For example, a marketing campaign for breast and ovarian cancer genetic testing markedly increased the number genetic counseling referrals for genetic testing (Mouchawar et al. Citation2005). Such advertisements increase patients’ interest and these concerns are conveyed to their GPs. While many of these patients may not be appropriate for referral to a medical geneticist or even an oncologist, risk evaluation and genetic counseling is usually appropriate. It may be that patient education and awareness of the relevance of genetic information to their own health is a critical component in terms of the use of genetic counseling services. Nevertheless, GPs need the proper knowledge and skills to assess and manage this level of genetic healthcare. Although, inadequate knowledge is a barrier to physicians in utilizing genetic services (Emery et al. Citation1999; Lapham et al. Citation2000), the current economic situation in medicine also prevents access (Rothstein & Hoffman Citation1999). The under- or uninsured population is at particular risk for not receiving these services. Future studies that analyze patient demographics, cost-effectiveness, insurance coverage, and patient outcome as they relate to genetic services utilization will provide measurements of the long-term effectiveness of genetic education programs such as the one described in this study.
The results of this study suggest that specialists are an important source for genetic counseling referrals. shows that genetic counseling at a prenatal clinic accounted for the increase in referrals seen during the study period, even though the perinatologist at this clinic did not attend the lecture program. One explanation for this observation is that obstetrics training recognizes the importance of reproductive genetics, and integrates well-accepted standards and protocols for patient management. Therefore, physicians with specialized reproductive training are more likely to understand the utility of genetic counseling services and refer patients.
The present study suggests that a 2–year educational program in genetics does not change the number of referrals by practicing GPs to a GC despite increasing general genetic knowledge. It is intuitive that GPs must exhibit core competencies in genetics to be effective clinicians in the age of genomic medicine. Although the utilization of genetic services is driven by patient awareness and the genetic knowledge of GPs, more research is needed to analyze the quality of the clinical indications for referrals before and after a genetic education program.
In summary, few studies have evaluated the effectiveness of particular education strategies to address the recognized need for increased general physician education in genetics. The results of this study suggest that a flexible, targeted training program in genetics when adapted to the interests and needs of GPs in an institutional setting can effectively enhance the knowledge of physicians. The reasons that educating GPs in genetics is not linked to an increase in genetic counseling referrals in this study are unclear. Patient demographics, insurance coverage, and the genetic knowledge base of patients themselves are factors that may impede referrals to a GC. Perhaps increasing community awareness of genetics may provide the impetus for patients to ask their GPs about inherited disorders in their families. The utilization of genetic counseling services may be dependent upon patient-driven inquiries rather than GP-initiated referrals. Although didactic teaching accompanied by a pretest–post-test design is a common method for teaching, it may be inadequate in some settings. Future studies should assess whether a program of this nature is effective in teaching medical genetics to GPs.
Acknowledgements
This study was funded by a 3-year federal training grant award to the Mid-Hudson Family Health Institute by the US Department of Health and Human Services, HRSA, Bureau of Health Professions’ Training in Primary Care Medicine Grant Program (D58HP00392). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services, HRSA.
Additional information
Notes on contributors
Joseph J. Higgins
JOSEPH J. HIGGINS, MD, the program director, is a professor of pediatrics and neurology at Weill–Cornell Medical College. Formerly, he was Director of Clinical Neurogenetics at the New York State Department of Health and Chief, Neurogenetics Unit at the National Institute of Neurological Disorders and Stroke in Bethesda, Maryland, USA.
References
- Burke W, Acheson L, Botkin J, Bridges K, Davis A, Evans J, Frias J, Hanson J, Kahn N, Kahn R, et al. Genetics in primary care: A USA faculty development initiative. Commun Genet 2002; 5: 138–146
- Collins FS, McKusick VA. Implications of the human genome project for medical science. J Am Med Assoc 2001; 285: 540–544
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. J Am Med Assoc 1995; 274: 700–705
- Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract 1999; 16: 426–445
- Gall M, Gall J, Borg W. Educational Research: an introduction7th. Pearson Education Inc, Boston, MA 2003
- Greendale K, Pyeritz RE. Empowering primary care health professionals in medical genetics: how soon? How fast? How far?. Am J Med Genet 2001; 106: 223–232
- Guttmacher AE, Jenkins J, Uhlmann WR. Genomic medicine: who will practice it? A call to open arms. Am J Med Genet 2001; 106: 216–222
- Hayflick SJ, Eiff MP. Role of primary care providers in the delivery of genetic services. Commun Genet 1998; 1: 18–22
- Howlett MJ, Avard D, Knoppers BM. Physicians and genetic malpractice. Med Law 2002; 21: 661–680
- Lapham EV, Kozma C, Weiss JO, Benkendorf JL, Wilson MA. The gap between practice and genetics education of health professionals: HuGEM survey results. Genet Med 2000; 2: 226–231
- Mouchawar J, Hensley-Alford S, Laurion S, Ellis J, Kulchak-Rahm A, Finucane ML, Meenan R, Axell L, Pollack R, Ritzwoller D. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: a naturally-occurring experiment. Genet Med 2005; 7: 191–197
- Rothstein MA, Hoffman S. Genetic testing, genetic medicine, and managed care. Wake Forest Law Rev 1999; 34: 849–888
- Siddell E, Marinelli K, Froman RD, Burke G. Evaluation of an educational intervention on breastfeeding for NICU nurses. J Hum Lact 2003; 19: 293–302
- Sifri R, Myers R, Hyslop T, Turner B, Cocroft J, Rothermel T, Grana J, Schlackman N. Use of cancer susceptibility testing among primary care physicians. Clin Genet 2003; 64: 355–360
- Subramanian G, Adams MD, Venter JC, Broder S. Implications of the human genome for understanding human biology and medicine. J Am Med Assoc 2001; 286: 2296–2307
- Suther S, Goodson P. Texas physicians’ perspective of genomic medicine as an innovation. Clin Genet 2004; 65: 368–377
- Wilkes G, Lasch KE, Lee JC, Greenhill A, Chiri G. Evaluation of a cancer pain education module. Oncol Nurs Forum 2003; 30: 1037–1043
- Zapp L. Use of multiple teaching strategies in the staff development setting. J Nurses Staff Dev 2001; 17: 206–212
Appendix: Types of questions used to determine genetic knowledge
The normal human chromosome complement consists of
Thousands of individual chromosomes
Hundreds of individual chromosomes
46 chromosomes
12 chromosomes
Two chromosomes (X for female, Y for male)
A father and his son have the same inherited single-gene disorder. The most likely mode of inheritance for this disorder is:
X-linked
Autosomal dominant
Autosomal recessive
Can’t be determined from this limited information
Adenine, thymine, guanine and cytosine refer to?
Lab test to order for patient suspected to have inherited genetic disorder
The nucleotide components of DNA
Four non-essential amino acids
Most common molecular components in the human body after H2O
All of the following are absolute indications to refer a prenatal patient for genetic counseling EXCEPT:
Either parent is a carrier of a balanced chromosomal rearrangement
Parental consanguinity
History of one prior pregnancy ending in miscarriage
Family history of cystic fibrosis
Diagnostic, accurate predictive genetic testing for Alzheimer's disease is
Currently available; but appropriate for only a small few families
Currently available; but only in private ‘clinics’ outside the United States
Currently not available: there are no single gene mutations that cause Alzheimer's
Not possible, since Alzheimer's disease is not genetic
The underlying cause of 30–50% of mental retardation in children can’t be identified. The most common identifiable cause of mental retardation is
Environmental or teratogenic
Complications of prematurity
Chromosomal abnormalities
Familial mental retardation
Shaken baby syndrome
At intake, a pregnant 29-year-old patient informs you that she and her partner have a baby girl with cystic fibrosis, an inherited autosomal recessive disorder. The chance that this new baby will have cystic fibrosis is:
75%
50%
25%
1 in 3360
At intake, a pregnant 29-year-old patient informs you that her first baby died after 2 weeks because of a chromosome abnormality. Genetic counseling is appropriate for this patient:
To reduce her anxiety so that she can have a healthy pregnancy
So that an amniocentesis can be arranged quickly and pregnancy termination arranged if the baby turns out to be affected
The couple can learn what may have caused it and what testing options are available for this pregnancy
The couple can be better educated about genetics and chromosomes
At intake, a pregnant 29-year-old patient informs you that her sister has sickle-cell anemia. It would be best to ask her first
If she is sure, since only boys can get sickle-cell anemia
If she has been tested for being a sickle-cell carrier
To go see her doctor right away
If anyone in her husband's family has ever had sickle-cell anemia
The American College of Obstetrics and Gynecology (ACOG) recommends that preconception or prenatal genetic screening be offered to all Ashkenazi Jewish individuals for which disorder(s)?
Cystic fibrosis
Canavan disease
Tay–Sachs disease
All of the above
All of the following are part of newborn genetic screening in New York State except
Urea cycle disorders
Hypothyroidism
Phenylketonuria (PKU)
Congenital adrenal hyperplasia (CAH)
Cystic fibrosis (CF)
Hemophilia is a human bleeding disorder caused by a sex-linked recessive mutation. Who would you expect to be affected by the hemophilia disorder?
Females and males in equal numbers
Primarily females with a few rare males
Primarily males with a few rare females
Adults over the age of 50
A 25-year-old patient expresses concern about a strong family history of breast and ovarian cancer. Her father's mother had breast cancer and died of ovarian cancer. His sister was recently diagnosed with breast cancer at age 43. She wants that new genetic test she heard about. You counsel her that genetic testing at this time is not a good idea because of which of the following?
It's best to first test a family member who already has cancer
It's on her father's side of the family
You’re too young for now to get breast or ovarian cancer
Very little cancer is actually inherited so you probably don’t have a cancer gene
An asymptomatic female patient of yours is identified with a genetic mutation in the BRCA1 gene. Which of the following is true?
She will undoubtedly develop breast or ovarian cancer
Her risk of developing breast cancer is higher than the general population
There are no environmental factors that influence the effect of the BRCA1 mutation
Surveillance is the most effective preventative measure in this case
Discussing a young boy's behavioral problems with his mother, you note during your PE he has a somewhat long face and large ears. Reviewing the patient's family history, you note that his brother was diagnosed with autistic-like behaviors and is receiving special ed. services in school. You write a note to consider a genetic referral for suspicion of
William syndrome
Down syndrome
Velocardiofacial syndrome
Fragile X syndrome
Turner syndrome
You’ve been monitoring a patient for a strong maternal history of colon cancer. During a routine GYN exam, she corrects a note in her chart that a maternal aunt actually had endometrial cancer and not cervical cancer. This raises your index of suspicion to recommend genetic counseling for which hereditary colon cancer syndrome?
Familial juvenile polyposis
Familial colitis
HNPCC (hereditary non-polyposis colon cancer)
FAP (familial adenomatous polyposis)
Which recessive disease gene has the highest prevalence in the general Caucasian population?
Hexosaminidase A (Tay–Sachs disease)
HFE (hereditary hemochromatosis)
CFTR (cystic fibrosis)
FMR1 (fragile X syndrome)
Beta-globin (sickle-cell trait/anemia)
The harmful effects of prenatal alcohol exposure are restricted to which weeks of pregnancy?
0–2 weeks embryologic age
First trimester
Second trimester
Third trimester
Throughout pregnancy
Answers
c
b
b
c
a
c
c
c
d
c
a
c
a
b
d
c
b
e
