Abstract
An infrequent observation of assessing hematoxylin and eosin sections is the blue staining of mucins (for example those in goblet cells). This is believed to be due to a low concentration of alum and high pH of the hematoxylin staining solution. This study examines the incidence of blue mucin in various sites of the gastrointestinal tract using a low alum, high pH hematoxylin solution. The results are compared with a conventional hematoxylin solution, iron alum celestine blue method and an alcian blue (pH 2.5)-periodic acid-Schiff (AB-PAS) stain to characterize the type of mucin demonstrated. This study is the first to offer evidence that blue-stained mucin with low alum, high pH hematoxylin corresponds with carboxylated mucins as shown by the AB-PAS stain in the gastrointestinal tract. Iron alum celestine blue was also found to stain the mucin of a proportion of rectal biopsies and appendix as well as the carboxylated mucin of one duodenal biopsy.
Introduction
The hematoxylin and eosin (H&E) procedure is the ‘bread and butter’ stain in histopathology.Citation1 The diagnosis as well as other histochemical tests all follow on from a good H&E.Citation2 As John ChanCitation2 has remarked ‘It is extraordinary that the hematoxylin–eosin (H&E) stain, introduced more than a century ago, has stood the test of time as the standard stain for histologic examination of human tissues’ Anecdotal mucin staining by some hematoxylins has been recorded in the literature and this study was designed to investigate the occurrence of mucin staining in the gastrointestinal tract.
Methods
Formalin-fixed paraffin embedded sections of biopsies of the gastrointestinal tract were analyzed. This included six gastric, seven duodenal, five appendix, and six rectal biopsies. Five lung biopsies containing neutral mucin were also included. A modified Gill’s hematoxylin solutionCitation3 with a quarter concentration of aluminum sulfate and no added acetic acid was found to stain some mucins. It has also been noted that the iron alum celestine blue (CB) nuclear stain also stains some mucins and this technique was included with an eosin counterstain.Citation4,5 Two counterstains were compared, a classic alcoholic eosin–phloxine and an aqueous eosin–erythrosin. The aqueous eosin-erythrosin solution is used by a few of the largest histopathology laboratories in Australia though the author has failed to secure a reference for this solution.
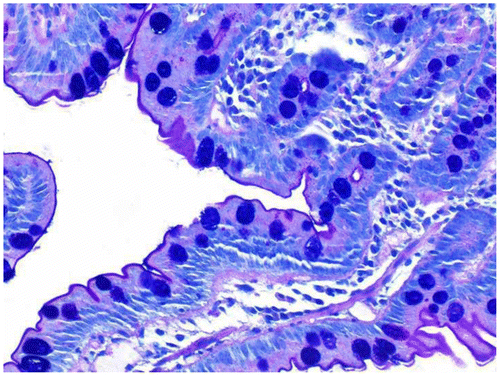
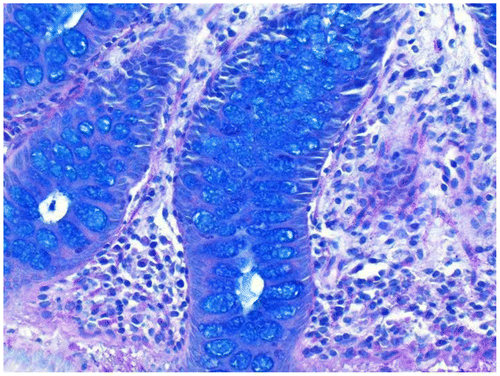
To further characterize the mucins, an Alcian blue (pH 2.5) – periodic acid Schiff (PAS), based on the method of MowryCitation6 was also done on all cases. The two modified Gill’s hematoxylins used were based on the Graham modificationCitation3 and are given in Table . For the working solution, 1 ml of solution B was added to 49 ml of solution A.
Table 1 Composition of modified Gill’s hematoxylin solutions
Iron alum celestine blueCitation4: for stock solution A: dissolve 1 g of celestine blue B (CI 51050) in 100 ml of distilled water. For stock solution B, dissolve 4 g of iron alum (ammonium iron sulfate, FeNH4(SO4)2·12H2O) in 100 ml of distilled water. These two stock solutions are mixed in equal proportions, resulting in blue–black staining solution. The recipes for the eosin–phloxine solutionCitation7 and the eosin–erythrosin solution are given in Table .
Table 2 Composition of cytoplasmic counterstains
The staining protocols were the same for the nuclear and cytoplasmic stains. After oven drying for 30 min at 65 °C, 4 μm sections were de-waxed in two changes of xylene (5 min each) and rehydrated to water with graded alcohols. Sections were stained in either of the Gill’s hematoxylins or the iron alum celestine blue solution for 5 min. After rinsing the sections in water, the hematoxylins were differentiated in 1% hydrochloric acid in 70% ethanol, if required. Sections were then blued in diluted lithium carbonate, rinsed in water, counter stained in one of the eosin solutions for 2 min, dehydrated in ethanol, cleared in xylene and cover slipped in a resinous mountant.
Results
The results of the stains on various tissues are given in Table . It was evident the blue staining with Gill’s B (low concentration of alum and no added acid) only occurred in those cases where carboxylated mucins, as shown by blue mucin staining with the AB–PAS, was present (Figures ). When glacial acetic acid was added to Gill’s B (1 ml per 50 ml stain solution), there was an appreciable decrease in acidic mucin staining. When the routine Gill’s A hematoxylin was used (more alum and acid added), blue mucin staining was rarely seen but weakly in one duodenal biopsy. It is interesting to note that iron alum celestine blue also stained the mucin of a proportion of rectal biopsies and appendix as well as the carboxylated mucin of one duodenal biopsy (Figure ). Both cytoplasmic counter stains stained equally well, allowing tinctorial separation of muscle, collagen, and red blood cells. Paneth cell and eosinophil granules were clearly stained.Citation1
Table 3 Mucin staining results
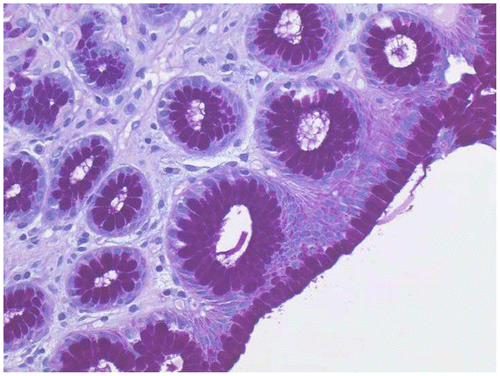
Figure 1 Stomach biopsy stained with Gill’s B hematoxylin and eosin–phloxine, ×40. Mucin is unstained.
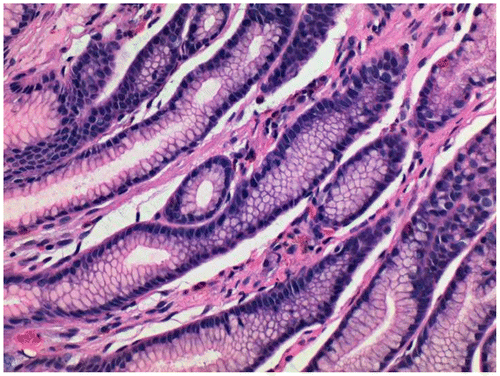
Figure 2 Duodenal biopsy stained with Gill’s B hematoxylin and eosin–phloxine, ×40. Mucin is stained blue.
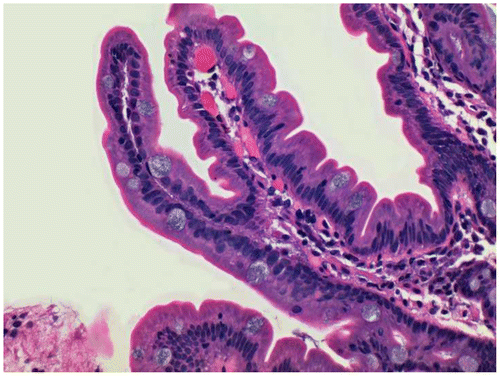
Figure 3 Rectal biopsy stained with Gill’s B hematoxylin and eosin–phloxine, ×40. Mucin is stained blue.
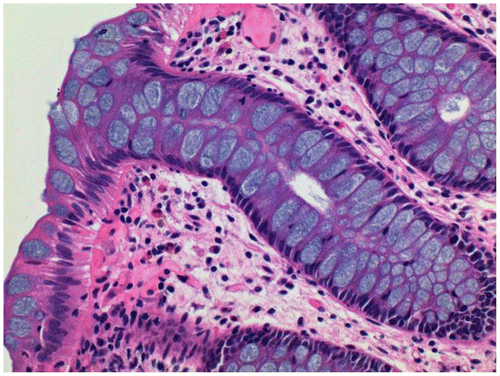
Figure 4 Rectal biopsy stained with iron alum celestine blue and eosin-erythrosin, x40. Mucin is stained blue.
Discussion
Comments are sometimes made about unexpected mucin staining with strong haemalum solutions. Within the context of the comments is the inference that this is an undesirable thing.Citation8 From the literature, there are many instances where the color of mucin with a H&E has been used to support various diagnoses.Citation2, 9–12
Hayashi and coworkersCitation13 found that minimal deviation adenocarcinoma of the uterine cervix usually shows a gastric phenotype resembling foveolar epithelium of the stomach and mucin is of neutral type, appearing pink in the H&E stain. Thus, the color of the cytoplasm can provide an important clue to the diagnosis because normal endocervical epithelial cells contain a mixture of acidic and neutral mucins, and show blue intracytoplasmic mucin.Citation2, 9
EpsteinCitation10 found the presence of blue-tinged (acidic) mucin in the lumens of prostatic glands is also a clue that would raise a suspicion of carcinoma because prostatic cells normally contain only neutral mucin (pink).Citation2, 10 Chandrasoma and colleaguesCitation11 studied epithelial types in columnar cell lined esophagus. They found one criterion for the differentiation of goblet cells from columnar cells with distended mucin vacuoles (‘pseudo-goblet cells’) was that goblet cells had a single round vacuole, usually containing basophilic material compared to pseudo-goblet cells where vacuoles were often multiple, clear, and not perfectly round. Panarelli and YantissCitation12 also found that in Barrett’s esophagus, goblet cells containing large cytoplasmic vacuoles of blue mucin compressed the nucleus and cytoplasmic membranes of adjacent cells. Background foveolar epithelial cells contained an apical cap of neutral mucin (appear pink to clear). Pseudo-goblet cells, unlike goblet cells, however, were pink due to an abundance of neutral mucin and were associated with similar-appearing foveolar epithelial cells. It is commonly believed hematoxylins with high hematoxylin to mordant ratios will stain goblet cells. For example, the approximate ratio of hematoxylin to alum for Harris’s hematoxylin is 0.05; Mayer’s is 0.06 while Gill’s (A) is 0.11. Gill’s (B) used above has a ratio of 0.45. The results support this with Gills B (ratio of 0.45) staining acidic mucins of the gastrointestinal tract. Presence of acidic, carboxylated mucin was confirmed with demonstration of alcian blue positive mucin following the AB–PAS stain. Sections of stomach and lung, known to be rich in PAS positive, neutral mucins did not stain blue with Gill’s B hematoxylin. Mayer used the ability of low-alum hematoxylin in his Mucihaematein method for staining mucin. This technique is a progressive method using sodium iodate as the oxidizer and aluminum chloride as a mordant to stain mucin.Citation14
An acid (usually acetic) is often used to lower the pH of the hematoxylin staining solution. The acid separates the mordant from the hematein and reduces the working concentration of aluminum–hematein, the combination which produces the ultimate blue color. A feature of a hematoxylin solution with a high pH is the staining of mucin, especially in the alimentary tract. Lowering the pH of the staining solution to about 2.5 will decrease this mucin staining. When glacial acid was added to Gill’s B, there was an appreciable decrease in acidic mucin staining. An interesting finding was Catalano and Lillie’s iron alum celestine blueCitation4 also stained acidic mucins to some degree in duodenal, appendix, and rectal biopsies. YasumatsuCitation14 studied the application of celestine blue as a substitute nuclear stain in routine cytology specimens. Yasumatsu remarked that when the usual iron alum celestine blue stain was used, there was a tendency to slightly stain the cytoplasm. He added nitric acid to the stain to around pH 1 and suggested this resulted in no cytoplasmic staining. It is possible that adding acid to the celestine blue solution might reduce the incidence of acidic mucin staining.
Conclusion
This study is the first to offer evidence that blue-stained mucin with a low alum, high pH hematoxylin solution corresponds with carboxylated mucins as shown by the AB–PAS stain in the gastrointestinal tract. Iron alum celestine blue was also found to stain the mucin of a proportion of rectal biopsies and appendix as well as the carboxylated mucin of one duodenal biopsy.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Henwood A. Microscopic quality control of hematoxylin and eosin – know your histology. Dako Connect. 2010;14:115–20. Available from: http://www.dako.com/au/28829_2010_conn14_qc_of_hematoxylin_and_eosin_henwood.pdf
- Chan JK. The wonderful colors of the hematoyxlin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22:12–32.10.1177/1066896913517939
- Graham ETA. Quick-mixed aluminum hematoxylin stain. Biotech Histochem. 1991;66:279–81.10.3109/10520299109109988
- Catalano RA, Lillie RD. Iron alum celestin blue B substitution for hematoxylin in fat stain. Stain Technol. 1973;48:354.10.3109/10520297309116658
- Henwood T, Llewellyn B, Montgomery I, et al. Celestine blue and hemalum for staining nuclei. Biotech Histochem. 2007;82:167–8.
- Mowry RW. Special value of methods that colour both acidic and vicinal hydroxyl groups in the histochemical study of mucins. With revised directions for the colloidal iron stain, the use of Alcian blue 8GX and their combination with the periodic acid-Schiff reaction. Ann NY Acad Sci. 1963;106:402–23.
- Verfuerth R, Luna LG. Counterstains for hematoxylins. Histologic. 1975;1:61–177.
- Llewellyn BD. Nuclear staining with alum hematoxylin. Biotech Histochem. 2009;84:159–77.10.1080/10520290903052899
- Epstein JI. Diagnostic criteria of limited adenocarcinoma of the prostate on needle biopsy. Hum Pathol. 1995;26:223–9.10.1016/0046-8177(95)90041-1
- Chandrasoma PT, Der R, Dalton P, Kobayashi G, Ma Y, Peters J, et al. Distribution and significance of epithelial types in columnar-lined esophagus. Am J Surg Pathol. 2001;25:1188–93.10.1097/00000478-200109000-00010
- Panarelli NC, Yantiss RK. Do ancillary studies aid detection and classification of barrett esophagus? Am J Surg Pathol. (early view). 2016. doi:10.1097/PAS.0000000000000654.
- Titford M. The long history of hematoxylin. Biotech Histochem. 2005;80:73–8.10.1080/10520290500138372
- Hayashi I, Tsuda H, Shimoda T. Reappraisal of orthodox histochemistry for the diagnosis of minimal deviation adenocarcinoma of the cervix. Am J Surg Pathol. 2000;24:559–62.10.1097/00000478-200004000-00010
- Yasumatsu H. Stain using celestine blue B as substitute nuclear stain in routine cytologic examinations. Acta Cytolog. 1976;21:173–4.