Abstract
Hormonal responses were assessed in men with prostate cancer (T2-4, Nx, Mx) who were randomized to receive either a single injection of goserelin 3.6 mg or leuprolide 3.75 mg. Testosterone increased over the first week, with a significantly higher mean rate of change of total testosterone (day 3) and free testosterone (days 3 and 7) with leuprolide. Following the initial rise in luteinizing hormone (LH), the rate of decrease in LH levels was significantly greater with goserelin by day 28. There are significant differences in endocrine response to goserelin and leuprolide in the 4 weeks following administration.
INTRODUCTION
In recent years, luteinizing hormone (LH)-releasing hormone (LHRH) agonists are widely used to treat locally advanced and metastatic prostate cancer. Two LHRH agonists, namely goserelin and leuprolide, are approved for use in Japan; however, these drugs have different formulations and delivery systems. In Japan, goserelin is available as a 1-month (3.6 mg) or 3-month (10.8 mg) subcutaneous depot injection, while leuprolide is available as a 1-month (3.75 mg) or a 3-month (11.25 mg) microcapsule formulation administered by subcutaneous or intramuscular (IM) injection. Interestingly, the approved dose of leuprolide is 7.5 mg/month in the USA, suggesting there may be differences in the clinical efficacy of leuprolide between these two populations. Evidence from randomized controlled trials supports the use of goserelin and leuprolide as monotherapy for advanced prostate cancer [Kaisary et al. [Citation1991]; The Leuprolide Study Group [Citation1984]; Vogelzang et al. [Citation1995]] and as the LHRH agonist component of combined androgen blockade [Crawford et al. [Citation1989]; Denis et al. [Citation1993]]. However, goserelin is the only LHRH agonist shown to improve overall survival when used as an adjuvant to radiotherapy in locally advanced disease [Bolla et al. [Citation2002]; Pilepich et al. [Citation2005]] and to radical prostatectomy in patients with node-positive disease [Messing et al. [Citation1999]].
LHRH agonists act by inhibiting pituitary LH secretion resulting in a fall in testosterone over a 4-week period to a level seen in patients having undergone castration [Kaisary et al. [Citation1991]]. However, LHRH agonists initially induce a transient surge in serum LH levels, resulting in a temporary increase in testosterone which may produce a tumor-flare reaction in a minority of patients [Labrie et al. [Citation1984]; Labrie et al. [Citation1987]]. Although the endocrine response to LHRH agonists is well established, there have been no direct comparisons of the pharmacology of goserelin and leuprolide over the first 4 weeks of treatment.
This study was conducted to compare serum concentrations of total and free testosterone, LH, and prostate-specific antigen (PSA) over an initial treatment period in patients with prostate cancer who had received a single injection of either goserelin 3.6 mg or leuprolide 3.75 mg.
MATERIALS AND METHODS
This was an open-label study, conducted at the Nara Medical University and Nara Prefectural Nara Hospital. Patients were randomized to receive a single injection of either goserelin 3.6 mg (injected subcutaneously into the anterior abdominal wall below the navel line) or leuprolide 3.75 mg (injected subcutaneously into the upper arm). The institutional Review Board at Nara Medical University approved the study and written informed consent was obtained from all patients.
Men with histologically confirmed prostate cancer (T2-4, Nx, Mx) were enrolled into the study provided they were ≥ 20 years of age, had received no previous treatment for prostate cancer, and had a performance status of 0–3 [Japanese Urological Association and Japanese Society of Pathology [Citation2001]]. Key exclusion criteria were: baseline serum testosterone ≤ 50 ng/dl; life expectancy ≤ 1 month; previous history of hypersensitivity to LHRH agonist preparations; severe liver, renal, or bone marrow complications; or active multiple primary cancers. Any concomitant medications or therapeutic or surgical procedures known to affect androgenic hormone concentrations were not permitted.
Serum concentrations of total and free testosterone, LH, and PSA were measured at baseline and at 3, 7, 14, 21, and 28 days after the injection of LHRH agonist. The baseline PSA measurement was performed at least 1 week after prostate biopsy, transrectal ultrasound examination, and digital rectal examination. Prostate cancer symptoms and performance status were recorded at baseline and day 28. Tumor flare was defined as a transient increase in serum testosterone concentration from baseline prior to treatment, followed by a worsening of clinical symptoms.
The dispersion of the mean percentage change from baseline in serum concentrations of total and free testosterone, LH, and PSA for goserelin and leuprolide was compared using the Folded F test to determine whether the difference in the formulation and administration of these agents would influence the pharmacodynamics. Patient characteristics and baseline serum concentrations of total and free testosterone, LH, and PSA were compared between the groups using the Mann-Whitney U test and Fisher's exact test. Differences in the mean percentage change from baseline in serum concentrations of total and free testosterone, LH, and PSA were analyzed at each time point using the Mann-Whitney U test. A P-value of < 0.05 was considered statistically significant.
RESULTS
Between September 2003 and August 2004, 22 patients were randomized to receive either goserelin 3.6 mg (n = 11) or leuprolide 3.75 mg (n = 11). The two groups were comparable with regard to age, disease characterisitics, and performance status at baseline. In addition, there was no statistical difference between the groups in baseline concentrations of total and free testosterone, LH, and PSA ().
TABLE 1. Patient Characteristics at Baseline
The dispersion of the rate of change of total testosterone concentration was significantly greater with leuprolide than with goserelin at day 21 (P = 0.0235; Folded F test). In both groups, there was a transient increase in total testosterone concentrations relative to baseline over the first 7 days of treatment. The mean rate of increase of total testosterone concentration at day 3 was significantly greater in the leuprolide group compared with the goserelin group. After 28 days, total testosterone decreased to within a range observed following castration (< 50.0 ng/dl) in all patients except for one in the leuprolide group.
A transient elevation in free testosterone relative to baseline was also observed in both groups over the first 7 days. The mean rate of change of free testosterone was significantly greater in the leuprolide group compared with the goserelin group at both days 3 and 7. The two groups showed a similar decline in free testosterone after day 7 (b). There were no significant differences between the two groups for the mean rate of increase of LH concentration over the first 7 days of treatment (c). However, at day 28, the mean rate of decrease of LH concentration was significantly greater in the goserelin group compared with the leuprolide group.
FIGURE 1 Mean rates of change in concentrations, following a single injection of either goserelin 3.6 mg or leuprolide 3.75 mg. (a) Total testosterone, (b) Free testosterone, (c) Luteinizing hormone (LH), (d) Prostate-specific antigen (PSA).
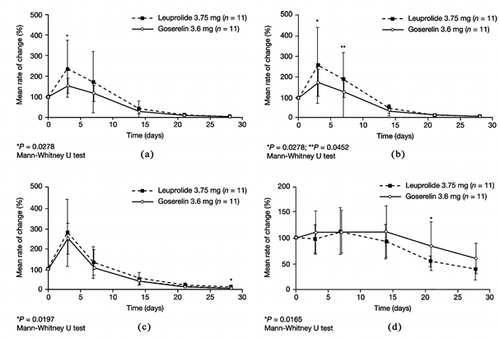
Prostate-specific antigen (PSA) began to decrease in both groups from day 14 onwards (d). At day 21, compared with goserelin, leuprolide was associated with a significantly smaller dispersion in the rate of decrease of PSA (d). Only three patients experienced mild tumor flare (two in the goserelin group and one in the leuprolide group). No patients withdrew from the study.
DISCUSSION
This, preliminary investigation is the first to assess the endocrine response over the initial 4 weeks following a single injection of either goserelin 3.6 mg and leuprolide 3.75 mg in patients with prostate cancer. As expected, there was a transient elevation in total and free testosterone concentrations in both groups during the first week of treatment. Interestingly, the mean rate of change was significantly greater with leuprolide compared with goserelin for total testosterone concentration on day 3 (P = 0.0278) and free testosterone concentration on days 3 (P = 0.0278) and 7 (P = 0.0452). This suggests that, of the two LHRH agonists, leuprolide may cause the greatest initial surge of testosterone. However, the incidence of tumor-flare and the initial rises in LH were comparable between the two treatment groups and, therefore, the clinical significance of this difference in testosterone is uncertain.
Previous studies have also shown that castration levels of testosterone are achieved at different rates with different LHRH agonists [Heyns et al. [Citation2003]; Kuhn et al. [Citation1997]]. In addition, these studies indicated that inter-patient variance is greater for patients receiving leuprolide; this is supported in the present study. Individual LHRH agonists may have different pharmacodynamic profiles depending on their formulation and dose, although larger studies are needed to clarify the clinical significance of this. Suppression of PSA was evident in both groups from day 14 onwards. The mean rate of decrease in PSA was significantly greater for leuprolide compared with goserelin, particularly at day 21 (P = 0.0165), but there was no statistically significant difference between the two groups at day 28. The duration of the present study (4 weeks) was too short to observe maximum suppression of PSA; the endpoint for PSA normalization is usually at least 12 weeks after treatment initiation [Fernandez del Moral et al. [Citation1996]]. In addition, the higher baseline PSA level observed in the group receiving leuprolide may imply a higher potential for reduction suggesting further investigation is required to clarify these findings.
The variation in dispersion of the rate of change of total testosterone and decreasing PSA between goserelin and leuprolide suggests that the pharmacodynamics of these agents may be different. The difference in the formulation of these two agents may affect the pharmacokinetic profile and the results observed during this study are consistent although not statistically significant.
These data indicate that there are significant differences in the endocrine response to goserelin 3.6 mg and leuprolide 3.75 mg over the first 4 weeks after administration. In particular, leuprolide appears to be associated with a greater initial testosterone surge than goserelin, although the magnitude of the initial LH surge and low incidence of tumor-flare reactions was similar in both groups.
Notes
The publisher regrets that the article entitled Endocrine Response to a Single Injection of Goserelin 3.6 mg or Leuprolide 3.75 mg in Men with Prostrate Cancer by N. Tanaka, K. Fujimoto, Y. Hirao, K. Shimizu, S. Tsujimoto, and S. Samma in Archives of Andrology, Volume 53, Issue 2, pp 85–88 was published without amending several errors. The following is the correct version of the article in its entirety.
REFERENCES
- Bolla M, Collette L, , et al. (2002) Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360:103–108.
- Crawford ED, Eisenberger MA, , et al. (1989) A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321:419–424.
- Denis LJ, Carneiro de Moura JL, , et al. (members of the EORTC GU Group and EORTC Data Center) (1993) Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). Urology 42:119–130.
- Fernandez del Moral P, Dijkman GA, , et al. (1996) Three-month depot of goserelin acetate: clinical efficacy and endocrine profile. Dutch south east cooperative urological group. Urology 48:894–900.
- Heyns CF, Simonin MP, , et al. (2003) Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int 92:226–231.
- Japanese Urological Association and Japanese Society of Pathology (2001) General rules for clinical and pathological studies on prostate cancer. Kanehara, Tokyo, pp 72–89.
- Kaisary AV, Tyrrell CJ, , et al. (1991) Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 67:502–508.
- Kuhn JM, Abourachid H, , et al. (1997) A randomized comparison of the clinical and hormonal effects of two GnRH agonists in patients with prostate cancer. Eur Urol 32:397–403.
- Labrie F, Dupont A, , et al. (1984) Simultaneous administration of pure antiandrogens, a combination necessary for the use of luteinizing hormone-releasing hormone agonists in the treatment of prostate cancer. Proc Natl Acad Sci U S A 81:3861–3863.
- Labrie F, Dupont A, , et al. (1987) Flutamide eliminates the risk of disease flare in prostatic cancer patients treated with a luteinizing hormone-releasing hormone agonist. J Urol 138:804–806.
- Messing EM, Manola J, , et al. (1999) Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 341:1781–1788.
- Pilepich MV, Winter K, , et al. (2005) Androgen suppression adjuvant to definitive radiotherapy in carcinomas of the prostate - long term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys 61:1285–1290.
- The Leuprolide Study Group (1984) Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med 311:1281–1286.
- Vogelzang NJ, Chodak GW, , et al. (1995) Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Urology 46:220–226.
