Abstract
Background
Cyclic peptide nanotubes (cPNTs) formed from the spontaneous beta-sheet stacking of peptide rings may serve as a safe and effective oral delivery vehicle/adjuvant for DNA vaccines.
Aim
In this study, we sought to determine if a DNA vaccine expressing the VP2 protein of goose parvovirus, adjuvanted with cPNTs, may elicit virus-specific antibody response through oral vaccination.
Material and methods
Forty 20-day-old Muscovy ducks were randomly assigned to two groups of 20 ducks each and vaccinated. Ducks were orally vaccinated (Day 0) and boosted (Day 1 and Day 2) or were mock-vaccinated with saline as the negative control. For immunohistochemical staining, the primary antibody used comprised a rabbit anti-GPV antibody, and the secondary antibody was a goat anti-rabbit antibody. Goat-anti-mouse-IgG was used as a tertiary antibody. IgG and IgA antibody titers in serum were analyzed by the GPV virus-coated ELISA. For IgA antibody analysis, intestine lavage was harvested too.
Results
A DNA vaccine, coated with cPNTs, can induce a significant antibody response in ducklings. Immunohistochemical staining of tissues from vaccinated ducklings showed that VP2 proteins can be detected in the intestines and livers for up to six weeks, confirming the antigen expression by the DNA vaccine. Antibody analysis found that this vaccine formulation was very efficient at inducing IgA antibodies in the serum and the intestinal tract.
Conclusion
A DNA vaccine adjuvanted with cPNTs can effectively express the antigen and can significantly induce an antibody response against goose parvovirus through oral vaccination.
1. Introduction
Cyclo-peptide nanotube (cPNT) was used for many application purposes in recent years, such as anti-virus material (Horne et al. Citation2005; Montero et al. Citation2011), antibacterial effect (Fernandez-Lopez et al. Citation2001), drug delivery (Chen et al. Citation2016), and gene delivery vector (Lee et al., Citation2015; Li et al. Citation2016). Since the cPNT shows many advanced features, including hollow tubular structure, high surface area, high aspect ratio, long retention period in blood, and low toxicity (Blanco et al. Citation2015), cPNT is a good material for in vivo gene delivery (Hsieh et al. Citation2012; Chapman et al. Citation2012).
The self-assembling properties make the cPNT good for the pharmaceutical industry’s mass production purposes (Hsieh and Liaw Citation2019). It is well developed to be a drug delivery system. It provides outstanding efficacy to deliver therapeutic or antigen genes into the lung, spleen, liver, and reticuloendothelial systems (RES). This shows a good potential to carry antigens and induce immune responses as a vaccine (Caldorera-Moore et al. Citation2010; Kulkarni and Feng Citation2013; Blanco et al. Citation2015).
In previous studies, cPNT was well studied in mammalian animals. However, the gene delivery and immune stimulation characteristics of cPNT are not clear in birds. Here, two aspect-ration PNT materials were designed to carry the duck parvovirus VP2 DNA vaccine and studied immune response in ducks.
Goose parvovirus (GPV) infects waterfowl, ducklings, and gosling, causing Derzu’s disease with high morbidity and mortality (Chen et al. Citation2015). It shows 90–100% death in birds under 10 days of age. The clinical symptoms include depression, growth retardation, and different pathological lesions: necrotizing enterocolitis, cardiac necrosis, fibrinous pericarditis, meningitis, hepatitis, and catarrhal enteritis associated with watery diarrhea. This disease causes a significant economic loss in the waterfowl production industry in European and Asia (Ju et al. Citation2011). Although this viral disease mainly attacks young birds, adult birds can also be infected without severe clinical signs (Woźniakowski et al. Citation2012).
There are two open reading frames (ORFs) in the GPV genome, the 5′ end ORFs code for non-structural proteins, and the 3′ end ORFs code for viral structural proteins. In the three viral structural proteins, VP2 shows as an immunodominant region. The VP2-induced antibody can neutralize GPV infection (Chu et al., Citation2001). In the previous study, we already established a vaccine candidate composed of a recombinant rVP2 protein and CpG ODN adjuvant. After intramuscular (IM) vaccination, the specific antibody, lymphocyte proliferation, CD4+/CD8+ cell population, and the amounts of induced cytokine mRNA were analyzed (Lee et al., Citation2010). The results demonstrated the adapted immune response induced by the rVP2 protein can protect ducklings against GPV infection (Lee et al. Citation2016).
In this study, we sought to determine if a GPV VP2 DNA vaccine adjuvanted with cPNTs may elicit a virus-specific antibody response through oral vaccination. cPNTs of two different aspect ratios, one long (L-cPNT) and one short (S-cPNT) in length, were tested. After oral immunization of ducklings with formulated vaccines, VP2 expression in tissues and the production of virus-specific antibody were verified.
2. Materials and methods
2.1. Vaccine preparation
cPNTs were prepared as described previously (Lee et al., Citation2015). Briefly, for L-cPNTs preparation, 5 mg of cyclo-(D-Trp-Tyr) (Bachem, Bubendorf, Switzerland) was mixed with 15 mL of 50% alcohol in a glass beaker and sonicated by an ultrasonic mixer for 5 min at room temperature. The mixture was placed in a 37 °C incubator overnight for the alcohol solution to evaporate. Dried, powdered forms of nanotubes formed at the bottom of the beaker were scraped off and stored at 4 °C. For S-cPNTs preparation, 1.5 mg of cyclo-(D-Trp-Tyr) were mixed with 1 mL of sterilized pure water in a 2-mL glass vial, completely dispersed using an ultrasonic mixer, and shaken at room temperature for 10 min. The supernatant was removed and stored at 4 °C. For L/S-cPNTs preparation, equal proportions of prepared L-cPNTs and S-cPNTs were mixed.
To construct DNA plasmids expressing VP2 of GPV, the VP2 gene (2,200 bp) was cloned into the plasmid vector pTCY as described previously (Lee et al., Citation2010). The resulting plasmid was kept in Escherichia coli (E. coli) DH5α for long-term storage. The alkaline lysis method was used for plasmid extraction, and purified plasmids were further diluted in sterile phosphate-buffered saline (PBS) to a final concentration of 2 μg/μL for storage at −20 °C.
Finally, to coat the DNA plasmids, 1.5 mg of dry cPNTs was added to 1 mL of a solution containing 50 or 100 μg of plasmids for gentle mixing overnight at 25 °C.
2.2. Cell co-culture cytotoxic observation
The BHK-21 cells were seeded into six-well culture plate (1.5 × 105 cells/well) with growth medium (MEM, Gibco Co. LTD., Waltham, MA, USA, with 10% FBS, Invitrogen, Waltham, MA, USA) at 37 °C. After overnight pre-culture, the cPNTs were added into the culture well (100 μg/well), respectively. The cells were cocultured with cPNTs for 48 h and imaged under the optical microscope.
2.3. Animals
Pekin and Muscovy ducks, depending upon availability, were obtained from the Yilan branch of the Livestock Research Institute, Council of Agriculture, Executive Yuan, Taiwan. The ducks were bred and housed in an aseptic environment to protect them from opportunistic infections. All efforts were made to minimize animal suffering, and at the experimental endpoint, ducks were humanely sacrificed through carbon dioxide asphyxiation. All experimental procedures were conducted in accordance with the Ethical Rules and Laws of the National Pingtung University of Science and Technology and were approved (NPUST-107-017) by the Institute Animal Care and Use Committee (IACUC) of the university.
2.4. DNA vaccine dose-finding experiment
To determine the appropriate dosage of DNA plasmids for the vaccine, nine-day-old Peking ducks were randomly assigned to five groups of five and were orally vaccinated (day 0) and boosted (day 1 and day 2) with the following five formulations of vaccine: (1) 50 μg/mL VP2 DNA + 1.5 mg/mL S-PNT, (2) 100 μg/mL VP2 DNA + 1.5 mg/mL S-PNT, (3) 50 μg/mL VP2 DNA + 1.5 mg/mL L-PNT, (4) 100 μg/mL VP2 DNA + 1.5 mg/mL L-PNT, and (5) saline as negative control. Serum samples were collected before the first vaccination and every four weeks until week 12 after primary immunization. Serum antibody titers were analyzed by enzyme-linked immunosorbent assay (ELISA).
2.5. Immunohistochemistry of GPV VP2 expression after oral vaccination
Forty 20-day-old Muscovy ducks were randomly assigned to two groups of 20 ducks each and vaccinated. For Group 1, ducks were orally vaccinated (Day 0) and boosted (Day 1 and Day 2) with 100 μg VP2 DNA + 1.5 mg L/S-PNT, and for Group 2, ducks were mock-vaccinated with saline as the negative control. On weeks 2 and 6, four ducks from each group were sacrificed for intestinal and liver tissue sampling. For positive control tissues, four ducks from the saline group were challenged on Week 4 with the GPV strain Tw1999 (at 105.0 EID50/mL (Chu et al., Citation2001), propagated in embryonic eggs (Lee et al. Citation2016)). For immunohistochemical (IHC) staining of tissue samples, the following was carried out. The sections of paraffin-embedded tissues were dried at for 24 h in the oven. Xylene was used for deparaffinization and an ethanol gradient (100%–50%) was used for rehydration. Antigen retrieval was performed with 0.01 M citrate buffer solution (10 mM citrate acid, 0.05% tween-20, sterilized water, pH 6.0) and nonspecific protein-binding sites were blocked with 10% BSA. The slides were dripped with the primary antibody (1:250 dilution of a rabbit anti-GPV antibody produced by our laboratory) in a humid chamber at 4 °C overnight. To block endogenous enzymes, the slides were immersed in 0.3% H2O2 for 15 min. For secondary antibodies, the slides were then incubated with goat anti-rabbit IgG HRP (1:1000) for 1 h at 37 °C. The slides were stained with a 3,3-diaminobenzidine (DAB) Chromogen/Substrate kit (ScyTek Laboratories, Inc., Logan, UT, USA), counterstained with Hematoxylin solution (Merck, Darmstadt, Germany), and blued with bluing reagent (Scotts Taq Water, Merck, Darmstadt, Germany). The slides were dehydrated in graded alcohol, made transparent with xylene, and mounted with Glycerol Gelatin (SIGMA-ALDRICH, Rockville, MD, USA) (Anis et al. Citation2013; Chen et al. Citation2009; Zhu et al. Citation2020; Shen et al. Citation2010).
2.6. Rabbit anti-GPV polyclonal antibody
To generate an anti-GPV antibody for detection purposes, the purified recombinant protein (GST-GPV, 248-516aa) (1 mg/mL) was mixed with adjuvant (Diluvac Forte; Intervet, Amsterdam, the Netherlands) in equal proportions as the immunizing antigen. Two New Zealand white rabbits (2–3 kg each, purchased from the Livestock Research Institute, Taiwan, R.O.C.) were immunized subcutaneously with 2 mL of the antigen preparation and boosted on the 14th and 21st days. Rabbits were sacrificed on the 28th day, and all blood was collected for serum separation.
2.7. Antibody analysis of vaccinated ducks
Twelve 9-day-old Muscovy ducks were randomly assigned to two groups of six ducks each and vaccinated. For Group 1, ducks were orally vaccinated (day 0) and boosted (day 14) with 100 μg VP2 DNA + 1.5 mg L/S-PNT, and for Group 2, ducks were mock-vaccinated with saline as the negative control. Serum samples were collected on days 0 and 14. On day 21, all ducks were sacrificed for serum, liver, and intestine sampling. Liver (0.5 g sample in 0.5 mL PBS) and intestinal (1 cm section wash or ground in 1 mL PBS) tissue samples were obtained and processed as tissue homogenates. The homogenates were then centrifuged at 10,000 × g for 20 min at 4 °C, and the resulting supernatants were collected for storage at −20 °C (Huang et al. Citation2014).
GPV-specific IgG antibody titer of collected sera was measured using an indirect enzyme-linked immunosorbent assay (ELISA). GPV viral particles suspended in coating buffer (15 mM Na2CO3, 35 mMNaHCO3, 3 mM NaN3, pH = 9.6, 10 μg/mL) were used to coat flat-bottomed 96-well microplates at 4 °C overnight. After washing with PBS containing 0.5% Tween-20 (PBS-T), the microplates were blocked using 1% BSA in PBS-T for 1 h at 37 °C. Thereafter, duck sera diluted at 1:50 with PBS were added, and the microplates were incubated for 1.5 h at 37 °C. After washing, goat anti-duck-IgG HRP (KPL, MD, USA) at 1:1000 dilution was added to each well, and the microplates were incubated for 1 h at 37 °C. The microplates were then washed, and 100 μL of TMB peroxidase substrate (KPL, MD, USA) was added and allowed to react for 5 min. Subsequently, 100 μl of TMB stop solution (KPL, MD, USA) was added to stop the reaction, following which the microplates were read at 450 nm using a multi-well plate reader (Anthos 2020, Cambridge, UK). S/P ratio = (sample − negative group)/(positive group −negative group).
GPV-specific IgA antibody titer of collected sera, intestinal wash, and the liver homogenate was also measured using indirect ELISA with a similar protocol as described above, except with the addition of a tertiary antibody. Duck sera were diluted at 1:200 with PBS and mouse anti-duck-IgA1 heavy chain (BIO-RAD, California, USA) secondary antibody was diluted at 1:2000 for use. A tertiary antibody, goat-anti-mouse-IgG HRP (Croyez Bioscience, Taipei City, Taiwan), was diluted at 1:2000 for use and color development.
2.8. Statistical analysis
Statistical analysis was performed using SAS (version 9.0; Cary, NC, USA) general linear models. Ducan’s multiple range test was used for comparison between groups, with different superscript letters (a,b,c) indicating a significant difference at p < 0.05.
3. Results
3.1. cPNTs of long and short lengths were synthesized and combined with GPV VP2 DNA vaccine
The two aspect-ration PNT were synthesized similar to the previously published study (Lee et al., Citation2015), and the morphology was demonstrated in Supplementary Figure 1. The long-type PNT (L-PNT) was synthesized in an organic solvent and dried into powder (Supplementary Figure 1B). The short type PNT (S-PNT) was made in pure water and ready for DNA binding (Supplementary Figure 1D).
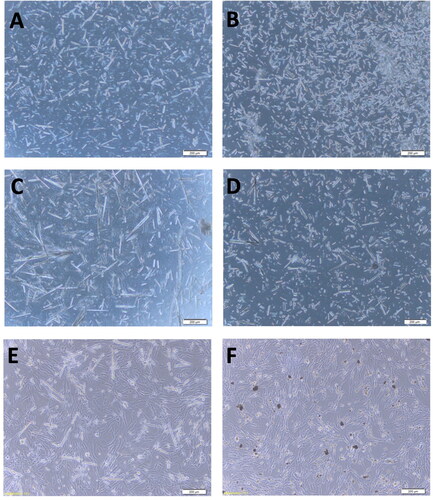
The two types of cPNT were added to the BHK-21 cell culture for the cytotoxic effect observation. The L-PNT shows a long needle-like shape with 100–200 μm length, and the S-PNT shows a short and cluster morphology with 1 μm length. Both types of PNTs didn’t induce any observable cytopathic effect on the BHK-21 cells under the microscope (). The cell attachment and morphology were similar to the untreated cell control.
Figure 1. The PNT morphology under a 100× microscope. (A) L-PNT before DNA binding; (B) S-PNT before DNA binding; (C) L-PNT binding with DNA vaccine; (D) S-PNT binding with DNA vaccine; (E) L-PNT co-culture with BHK-21 cells, for 48 hr; (F) S-PNT co-culture with BHK-21 cells, for 48 hr. The scale bar shows 200 µm.
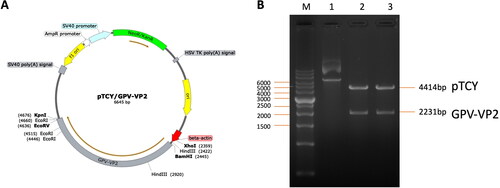
The GPV-VP2 gene fragment was constructed into the pTCY expression vector (). This expression vector had been confirmed the gene expressing and immune induction efficacy in the mouse, pigs, and birds (Wu et al. Citation2019). The beta-actin promoter sequence was used to drive the GPV-VP2 antigen gene sequence (2200 bp) (Supplementary Figure 2) and provided consistent antigen protein expression. Compared to the CMV promoter, other popular used antigen driving promoters in DNA vaccines, this beta-actin driving system can stably express the antigen gene without interference.
Figure 2. The pTCY/GPV-VP2 DNA vaccine construction map. (A) The GPV VP2 sequence (2200 bp) was cloned into the multi-cloning site after the beta-actin promoter (labeled red-arrow). The unique restriction cutting sites were labeled respectively. (B) The DNA construct was confirmed by KpnI and BamHI restriction enzyme digestion gel electrophoresis. The labeled 1, 2, and 3 were none-treated, double digested colony 1 and 2 plasmid DNA, respectively.
3.2. Oral administration with 100 μg/dose DNA vaccine induced high IgG antibody titer
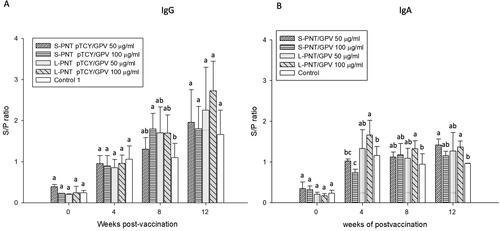
The preliminary oral administration trial was designed to observe the immunization characteristics of the DNA vaccine carried by two different PNT vectors. Two different dosages of DNA vaccine were bound with S-PNT and L-PNT, respectively, to make low (50 μg/dose) and high (100 μg/dose) dosage forms. There are four types of dosage forms, S-PNT/GPV 50 μg, S-PNT/GPV 100 μg, L-PNT/GPV 50 μg, and L-PNT/GPV 100 μg, which were administrated to 9-day-old duckling groups for oral administration for three days subsequently. The blood serum was harvested on days 0, 4, 8, and 12 weeks after vaccination, and the IgG and IgA antibody titer were analyzed by the GPV virus coated ELISA and the results are shown in . Both S-PNT/GPV and L-PNT/GPV induced IgG immune response. The 100 μg dosage form showed a significantly higher antibody titer compared to the control group in 8 weeks serum samples. Furthermore, the high IgG titer persisted until 12 weeks (). The animals for IgA antibody analysis were sacrificed, and the intestine lavage was harvested accordingly. Unlike the late IgG induction, the anti-GPV intestine washout fluid IgA was detected at 4 weeks and lasted to 12 weeks in the L-PNT/GPV 100 μg group (). The DNA vaccine carried by cPNT induced a notable IgA response in the duck.
Figure 3. Antibody titers of ducklings orally vaccinated with high or low dosage of DNA vaccine. Ducklings (n = 5) were orally vaccinated with vaccine formulations differing in DNA dosage and cPNT types. (A) Serum IgG and (B) intestinal IgA levels after vaccination were analyzed by ELISA. The titers were calculated as S/P ratios and presented as mean ± SD. Different superscript letters(a,b) indicate significant differences between treatment groups. Analyzed by ANOVA Duncan’s Multiple Range Test (α = 0.05).
3.3. Expression of GPV VP2 can be detected in intestine and liver tissues six weeks after vaccination
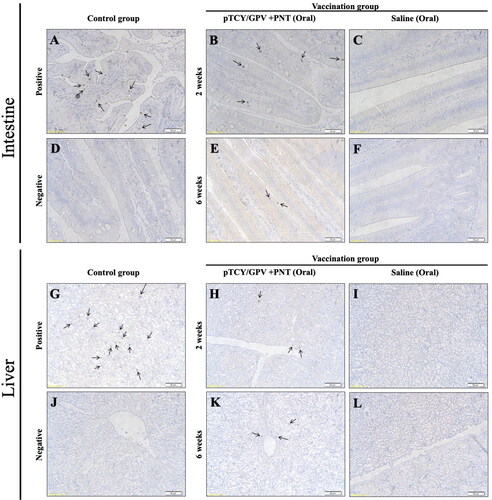
To analyze the antigen gene delivery and expression efficacy of the cPNT carried DNA vaccine, the S-PNT/GPV and L-PNT/GPV were mixed with a 1:1 ratio (L/S-PNT/GPV) and oral vaccinated to the ducklings. After 2 and 6 weeks of vaccination, the animals were sacrificed for the intestine and liver tissue sampling for immunohistochemistry. The GPV-VP2 antigen was probed by a rabbit anti-GPV polyclonal antibody, as mentioned before. The results showed that the cPNT carrying DNA vaccine GPV-VP2 antigen could be detected (labeled brown) in both the intestine () and the liver (). The amounts of signals were similar between post-two and six weeks of vaccinated animal samples. It was suggested that the oral cPNT GPV-VP2 antigen can steadily express for at least 6 weeks in ducks with oral vaccination. This is very important for saving labor power and the cost of domestic animal husbandry. In this experiment, the positive control group sample is from the virus-challenged animal and showed a significantly higher signal than the oral vaccinated group. This result shows the infection efficacy of natural GPV is still higher than that of the PNT-delivered DNA vaccine.
Figure 4. Immunohistochemistry of duck organs after vaccination. Ducklings were orally vaccinated and two organs, intestine (upper) and liver (lower) were isolated for IHC staining by using anti-VP2 antibody. The GPV-infected animal sample (A and G) and non-infected tissues (D and J) served as positive and negative controls, respectively. Tissues from the pTCY/GPV + PNT oral vaccinated (B, E, H, and K) and the saline mock vaccinated (C, F, I, and L) group ducklings are shown. Arrows indicate VP2-positive cells.
3.4. IgA production can be detected in vaccinated ducklings
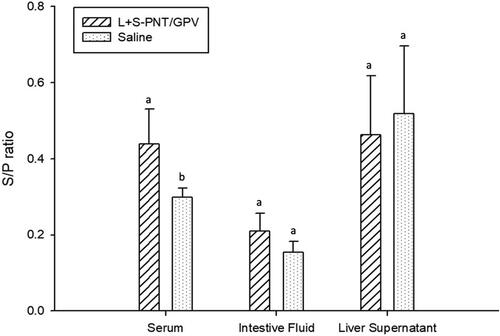
To determine the efficacy of the oral cPNT GPV-VP2 DNA vaccine by using the prime-boost immunization method, the 9-day-old ducklings were orally immunized on day 0 and boosted on day 14, and samples collected on days 0, 14, and 21. According to previous research, duck IgA could be detected in the intestine washout fluid sample or homogenized liver tissue (Huang et al. Citation2014; Mao et al. Citation2016; Ferreira et al. Citation2010). In this experiment, the serum IgG, serum IgA, intestine IgA, and liver IgA were isolated and analyzed by ELISA analysis. The results showed the serum IgG antibody was hardly distinguishable between the vaccination and control groups (). However, the serum IgA was significantly increased in the oral vaccination group in week 2 and week 3 samples (). This result was similar to the intestine washout fluid samples (). Despite the significant IgA induction in serum and intestine washout fluid, the IgA amounts in liver samples did not show a statistical difference between the vaccination and saline control group, due to the high background of liver samples (). According to the serum IgG response, we found that the prime and boost strategy may not be better than the continuous three days oral vaccination at the very beginning.
Figure 5. The IgG and IgA induction of the cPNT (L/S PNT) DNA vaccine administration. Two groups of 9-day-old ducklings (n = 20) were orally vaccinated by the cPNT GPV-VP2 (100 μg DNA/dose) on day 0 and boosted on day 14. The serum IgG (A) and IgA (B) at 0-, 2- and 3-weeks post-vaccination was analyzed by ELISA. The antibody titer results were calculated into S/P ratio and presented as mean ± SD and analyzed by ANOVA Duncan’s Multiple Range Test (α = 0.05). S/P ratio = (sample OD value − negative OD value)/(positive OD value − negative OD value).
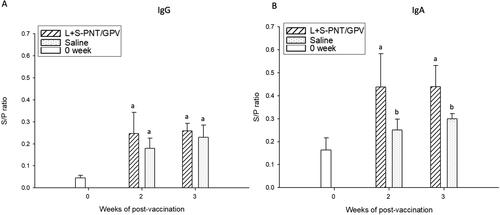
Figure 6. The IgA induction of the cPNT (L/S PNT) DNA vaccine administration in a different organ. Two groups of 9-day-old ducklings (n = 20) were orally vaccinated with the cPNT GPV-VP2 (100 μg DNA/dose) vaccine or saline on day 0 and boosted on day 14. The IgA titer at 0-, 2- and 3-weeks post-vaccination was analyzed by ELISA. The antibody titer results were calculated into S/P ratio and presented as mean ± SD and analyzed by ANOVA Duncan’s Multiple Range Test (α = 0.05). S/P ratio = (sample OD value − negative OD value)/(positive OD value − negative OD value).
4. Discussion
Our study demonstrated that oral vaccination with a DNA vaccine adjuvanted with cPNTs can induce a significant IgA antibody response in ducks. Expression of antigens by the DNA plasmid was found in the intestine and liver, analogous to a natural GPV infection where viral proteins are found in the intestine and liver, and are captured by the reticuloendothelial system. The appropriate location of gene expression may play a role in the effectiveness of the DNA vaccine.
The mucous immune response is very important and critical in many viral diseases, such as the porcine epidemic diarrhea virus (PEDV (Li et al. Citation2020) and porcine circovirus (PCV2) (Shi et al. Citation2020). In the oral-, nasal-cavity, or digestive tract, the amounts of virus primary attachment and infection could be largely inhibited by the mucous IgA antibodies. There are many different immunization strategies and routes developed for a mucosal vaccine, within them, the oral and intra-nasal are two kinds of more practical methods because it is easier and more widely for delivery (Souci et al. Citation2020). A previous study also showed that a nasal administrated DNA vaccine could successfully induce serum IgG and IgA in swine. Furthermore, the nanoparticle optimized intranasal formulation could enhance and prolong the IgA existing period in mucous (Li et al. Citation2020). In this study, there were two oral administration programs employed, respectively. The IgG antibody titer was significantly induced in the duckling serum by using three subsequent days of oral administration (). However, when we use a common immunization program, immunizing at day 0 and boosting after 2 weeks, only IgA was significantly induced in the animals (). These results revealed that the immunization program may affect the induction of immunization by using the oral cPNT carrying DNA vaccine formulation.
The reason for the above phenomenon needs to be further verified in other animals in the future. At present, it is impossible to confirm whether it is caused by the characteristics of the vaccine or the immune characteristics produced by oral vaccine antigens for birds. In some species, such as rats, rabbits, and chickens, up to 75% of the IgA produced within the intestinal wall may diffuse into the portal blood circulation and be carried to the liver. It is also a route by which pathogens bound to circulation IgA can be removed from the body.
The cPNT vector used in this study carries the DNA vaccine and is in a solid state, which may settle to the bottom of the container when added to water. Therefore, how to efficiently deliver vaccines will be a direction that can be further studied in the future, including the design of the delivery container, the estimation method of waterfowl drinking water, and the stability of the vaccine in the delivery container.
Regarding the analysis of IgG and IgA antibody levels, slightly higher background antibody levels were obvious in the negative control groups. While there are several potential causes for this observation, conclusions drawn from the results are based on statistically significant differences above the background level. In the design and use of poultry vaccines, in addition to vaccine safety and efficacy, it is also necessary to consider the method of immunization. Due to the rapid increase in the cost of injection manpower, the live virus vaccine is more favored by farmers than the dead virus injection dosage form. Therefore, if a nucleic acid oral dosage form vaccine is more convenient to administer and has no virulence regression problem, it will open up a new research direction for the future dosage form design of avian vaccines.
Supplemental Material
Download Zip (67.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anis Z, Morita T, Azuma K, Ito H, Ito T, Shimada A. 2013. Histopathological alterations in immune organs of chickens and ducks after experimental infection with virulent 9a5b newcastle disease virus. J Comp Pathol. 149(1):82–93.
- Blanco E, Shen H, Ferrari M. 2015. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 33(9):941–951.
- Caldorera-Moore M, Guimard N, Shi L, Roy K. 2010. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 7(4):479–495.
- Chapman R, Danial M, Koh ML, Jolliffe KA, Perrier S. 2012. Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem Soc Rev. 41(18):6023–6041.
- Chen H, Dou Y, Tang Y, Zhang Z, Zheng X, Niu X, Yang J, Yu X, Diao Y. 2015. Isolation and genomic characterization of a duck-origin GPV-related parvovirus from cherry valley ducklings in China. PLoS One. 10(10):e0140284.
- Chen J, Zhang B, Xia F, Xie Y, Jiang S, Su R, Lu Y, Wu W. 2016. Transmembrane delivery of anticancer drugs through self-assembly of cyclic peptide nanotubes. Nanoscale. 8(13):7127–7136.
- Chen S, Cheng A, Wang M, Zhu D, Luo Q, Liu F, Chen X. 2009. Immunohistochemical detection and localization of new type gosling viral enteritis virus in paraformaldehyde-fixed paraffin-embedded tissue. Vet Immunol Immunopathol. 130(3-4):226–235.
- Chu CY, Pan MJ, Cheng JT. 2001. Genetic variation of the nucleocapsid genes of waterfowl parvovirus. J Vet Med Sci. 63(11):1165–1170.
- Fernandez-Lopez S, Kim HS, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, et al. 2001. Antibacterial agents based on the cyclic D,L-alpha-peptide architecture. Nature. 412(6845):452–455.
- Ferreira HL, François Pirlot J, Kaspers B, Kothlow S, van den Berg T, Lambrecht B. 2010. Development of specific enzyme-linked immunosorbent assays to evaluate the duck immune response after experimental infection with H5N1 and H7N1 low pathogenic avian influenza viruses. Avian Dis Dig. 5(s1):e155–6–e156.
- Horne WS, Wiethoff CM, Cui C, Wilcoxen KM, Amorin M, Ghadiri MR, Nemerow GR. 2005. Antiviral cyclic D,L-α-peptides: targeting a general biochemical pathway in virus infections. Bioorg Med Chem. 13(17):5145–5153.
- Hsieh WH, Chang SF, Chen HM, Chen JH, Liaw J. 2012. Oral gene delivery with cyclo -(d -Trp-Tyr) peptide nanotubes. Mol Pharm. 9(5):1231–1249.
- Hsieh WH, Liaw J. 2019. Applications of cyclic peptide nanotubes (cPNTs). J Food Drug Anal. 27(1):32–47.
- Huang J, Jia R, Wang M, Shu B, Yu X, Zhu D, Chen S, Yin Z, Chen X, Cheng A. 2014. An attenuated Duck Plague Virus (DPV) vaccine induces both systemic and mucosal immune responses to protect ducks against virulent DPV infection. Clin Vaccine Immunol. 21(4):457–462.
- Ju H, Wei N, Wang Q, Wang C, Jing Z, Guo L, Liu D, Gao M, Ma B, Wang J, et al. 2011. Goose parvovirus structural proteins expressed by recombinant baculoviruses self-assemble into virus-like particles with strong immunogenicity in goose. Biochem Biophys Res Commun. 409(1):131–136.
- Kulkarni SA, Feng SS. 2013. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 30(10):2512–2522.
- Lee J-W, Lin Y-M, Liu C-H, Ke G-M, Chu C-Y. 2016. Passive and protective immunity in ducklings elicited by a parvovirus subunit vaccine with CpG adjuvant. Taiwan Vet J. 42(02):75–80.
- Lee J-W, Lin Y-M, Yen T-Y, Yang W-J, Chu C-Y. 2010. CpG oligodeoxynucleotides containing GACGTT motifs enhance the immune responses elicited by a goose parvovirus vaccine in ducks. Vaccine. 28(50):7956–7962.
- Lee YH, Chang SF, Liaw J. 2015. Anti-apoptotic gene delivery with cyclo-(D-Trp-Tyr) peptide nanotube via eye drop following corneal epithelial debridement. Pharmaceutics. 7(3):122–136.
- Li M, Ehlers M, Schlesiger S, Zellermann E, Knauer SK, Schmuck C. 2016. Incorporation of a non-natural arginine analogue into a cyclic peptide leads to formation of positively charged nanofibers capable of gene transfection. Angew Chem Int Ed Engl. 55(2):598–601.
- Li M, Wang Y, Sun Y, Cui H, Zhu SJ, Qiu HJ. 2020. Mucosal vaccines: strategies and challenges. Immunol Lett. 217:116–125.
- Mao S, Ou X, Zhu D, Chen S, Ma G, Wang M, Jia R, Liu M, Sun K, Yang Q, et al. 2016. Development and evaluation of indirect ELISAs for the detection of IgG, IgM and IgA1 against duck hepatitis A virus 1. J Virol Methods. 237:79–85.
- Montero A, Gastaminza P, Law M, Cheng G, Chisari FV, Ghadiri MR. 2011. Self-assembling peptide nanotubes with antiviral activity against hepatitis C virus. Chem Biol. 18(11):1453–1462.
- Shen FX, Ma GP, Cheng AC, Wang MS, Li CF, Sun KF, Chang H, Zhu DK, Jia RY, Chen XY, et al. 2010. Development and application of an indirect immunohistochemical method for the detection of duck plague virus vaccine antigens in paraffin sections and localization in the vaccinated duckling tissues. Poult Sci. 89(9):1915–1923.
- Shi F, Li Q, Zou Z, Wang Y, Hou X, Zhang Y, Song Q, Zhou S, Li H. 2020. The changes of immune-related molecules within the ileal mucosa of piglets infected with porcine circovirus type 2. J Vet Sci. 21(5):e78.
- Souci L, Jaunet H, Le Diguerher G, Guionnet J-M, Béven V, Paboeuf F, Montier T, Dory D. 2020. Intranasal inoculations of naked or PLGA-PEI nanovectored DNA vaccine induce systemic and mucosal antibodies in pigs: a feasibility study. Res Vet Sci. 132:194–201.
- Woźniakowski G, Samorek-Salamonowicz E, Kozdruń W. 2012. Quantitative analysis of waterfowl parvoviruses in geese and Muscovy ducks by real-time polymerase chain reaction: correlation between age, clinical symptoms and DNA copy number of waterfowl parvoviruses. vol 8.
- Wu HC, Lee JW, Lin JJ, Wang HY, Chu CY. 2019. A DNA priming and protein boosting immunization scheme to augment immune responses against parvovirus in ducks. J Appl Microbiol. 126(1):49–57.
- Zhu D, Chen H, Ou X, Liu M, Wang M, Zhao X, Jia R, Chen S, Sun K, Yang Q, et al. 2020. Comparison of immunohistochemistry and Ziehl-Neelsen staining for detecting the distribution of Mycobacterium avium subsp avium in naturally infected domestic Pekin ducks (Anas platyrhynchos domestica). Vet Med Sci. 6(2):242–247.

