Abstract
Abstract: An experience with electric shock can support two opposing kinds of behavioral effects: Stimuli that precede shock during training are subsequently avoided as predictors for punishment, whereas stimuli that follow shock during training are later on approached, as they predict relief. We show here, for the fruit fly Drosophila, that upon the loss of white-function, the balance between these two kinds of learning is distorted in favor of punishment learning: white1118 mutants show stronger punishment learning and weaker relief learning, as compared to wild type flies. Thus, white1118 mutants establish, overall, more “negative” memories for the shock experience. This only concerns the mnemonic effects of the shock; the immediate, reflexive responsiveness to shock remains unaltered. Also, learning about reward is apparently unaffected, both in adult and larval Drosophila. Prompted by the proposed function of the White protein as the transporter for biogenic amine precursors, we probed the brains of white1118 mutants for the amounts of biogenic amines (octopamine, tyramine, dopamine, and serotonin) by using high-perssure liquid chromatography coupled to mass spectrometry. Using this method, we found, however, no difference between white1118 and wild type files for any of the probed amines. In any event, analyses of how the white1118 mutation affects the balance between punishment and relief learning should provide a study case of how heritable distortions of such balance can come about. Finally, the effects of the white1118 mutation should be considered as a source of confound when using white as the “marker gene” in behavior-genetic analyses of any sort.
INTRODUCTION
The first mutant animal ever described as such was a white-eyed Drosophila fruit fly (Morgan et al., Citation1915), which consequently, was called white. Subsequent analyses revealed that the gene is located on the first chromosome and codes for a “half-size ATP-binding cassette transporter” (O'Hare et al., Citation1984). Heterodimers of the White protein with two other such transporters, Scarlet (Tearle et al., Citation1989) and Brown (Dreesen et al., Citation1988), respectively, pump tryptophan and guanine into cells. In Drosophila retinal pigment cells, these are precursors for the pigments (Sullivian & Sullivian, Citation1975), the lack of which makes the eyes appear unpigmented (i.e., white).
Given its historical primacy and conspicuous phenotype, the white gene has become one of the most widely used tools in Drosophila genetics. In particular, white1118, which is a null allele of the white gene resulting from the spontaneous deletion of a part of white (Hazelrigg et al., Citation1984), is employed as a “marker” to keep track of transgenic constructs (see Discussion). Given the extensive use of such transgenes in Drosophila research, the effects of alterations in white-function on behavior may be critical. These effects are manifold: Ectopic, ubiquitous overexpression of White induces male-to-male courtship (Zhang & Odenwald, Citation1995; Hing & Carlson, Citation1996; Nilsson et al., Citation2000; An et al., Citation2000), and loss of white-function (in the white1118 mutant) suppresses male-male aggression (Hoyer et al., Citation2008). Further, white1118 mutant flies are impaired in heat-reinforced place learning, whereas in associative odor-shock learning, they perform better than the wild type (Diegelmann et al., Citation2006). How does the white gene affect such a broad spectrum of behavioral phenotypes? We note that in neurons, tryptophan, one cargo of the White transporter, is converted to serotonin, a notorious modulator of behavior (e.g., circadian rhythmicity, sleep [Yuan et al., Citation2005, Citation2006], aggression [Dierick & Greenspan, Citation2007], and learning [Sitaraman et al., Citation2008]). Also, White's other cargo, guanine, is converted to 6H-tetrahydrobiopterin, a cofactor for the synthesis of serotonin, dopamine, and nitric oxide (NO) (reviewed by Koshimura et al., Citation2000). Dopamine, apart from signaling aversive reinforcement (Schwaerzel et al., Citation2003; Riemensperger et al., Citation2005; Schroll et al., Citation2006), affects arousal (Andretic et al., Citation2005) and decision making (Zhang et al., Citation2007). Last, but not least, NO is an atypical neurotransmitter in the synapses of the olfactory, visual, and mechanosensory systems, as well as at the neuromuscular junction (reviewed by Bicker, Citation2001). Thus, the roles of White in behavior may, conceivably, come about by its effects on serotonin, dopamine, and/ or NO signaling.
Here, following up on Diegelmann et al. (Citation2006), we analyzed how the loss of white-function in the white1118 mutant affects olfactory associative learning. We did so with respect to two opposing kinds of memory, which are established upon painful experience: In wild type flies, those odors that precede an electric shock are learned as predictors for punishment and are subsequently avoided (i.e., punishment learning), whereas those odors that follow shock are learned as signals for relief and are subsequently approached (i.e., relief learning) (Tanimoto et al., Citation2004; Yarali et al., 2008). In addition, we tested whether white1118 larvae are altered in associating an odor with a sugar reward. In order to offer an explanation for any potential behavioral alterations, we provide an analysis of the brain levels of biogenic amines (e.g., octopamine, tyramine, dopamine, and serotonin) by using high-pressure liquid chromatography coupled to mass spectrometry (HPLC-MS).
MATERIALS AND METHODS
Flies
Drosophila melanogaster were reared in mass culture at 25°C, at 60–70% relative humidity, under a 14-10-hour light-dark cycle. The Canton-Special wild type strain was used as a control for the White-null white1118 strain, which was back-crossed to this wild type strain for more than six generations to adjust genetic background (Hazelrigg et al., Citation1984; also see Diegelmann et al., Citation2006; Hoyer et al., Citation2008).
Adult Behavior
One day prior to experiments, 1–4-day-old flies were collected in fresh food vials and kept overnight at 18°C and 60–70% relative humidity. For sugar reward learning, flies were starved prior to experiments for 18–20 hours at 25°C and 60–70% relative humidity in vials equipped with moist tissue and a moist filter paper. The experimental setup was as described by Schwaerzel et al. (Citation2003). Flies were trained and tested in groups of 100–150; training took place under dim red light that did not allow the flies to see; the tests were done in complete darkness. As odorants, 90 µl of benzaldehyde (BA) or 340 µl 3-octanol (OCT) (both from Fluka, Steinheim, Germany) were applied in 1-cm-deep Teflon containers of 5 or 14 mm diameter, respectively.
For electric shock–reinforced learning (A), flies received 6 training cycles. Each cycle started by loading the flies into the experimental setup (0 minutes). From 4 minutes on, a control odor was presented for 15 seconds. From 7.5 minutes on, electric shock was applied as 4 pulses of 100 V; each pulse was 1.2 seconds long and was followed by the next with an onset-to-onset interval of 5 seconds. In different groups, a to-be-learned odor was presented at different times relative to this shock; thus, the interval between the to-be-learned odor and the shock (i.e., the interstimulus interval; ISI) was varied between groups. Negative ISIs indicate first-odor-then-shock presentation, whereas positive ISIs indicate first-shock-then-odor presentation. At 12 minutes, flies were transferred out of the setup into food vials, where they stayed for 16 minutes until the next training cycle started. At the end of the sixth training cycle, after the usual 16-minute break, flies were loaded back into the setup. After a 5-minute accommodation period, they were transferred to a T-maze, where they could choose between the two odors that they had encountered during training. After 2 minutes, the arms of the maze were closed and flies on each side were counted. A preference index (PREF) was calculated, as shown in Equation 1:1
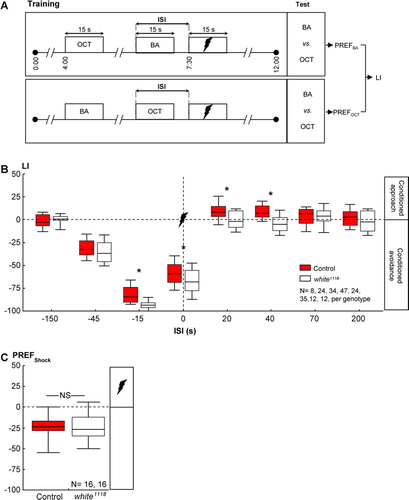
Figure 1 Memory of shock was, overall, more “negative” for white1118 mutants. (A) Adult flies were trained with two odors and pulses of electric shock. Between the groups, we varied the interval between the as-yet-to-be-learned odor and the shock (interstimulus interval; ISI). Negative ISIs indicate odor-then-shock presentation; positive values reflect shock-then-odor presentation. For each ISI, two subgroups were trained reciprocally, that is, with switched roles for the odors 3-octanol (OCT) and benzaldehyde (BA). After training, each reciprocal group was allowed to choose between the two odors; based on their odor preferences (PREFs), we calculated a learning index (LI). Positive LIs indicate conditioned approach, and negative values mean conditioned avoidance. (B) For wild-type control flies, the “sign” of conditioned behavior depended on the ISI: If, during training, the odor had shortly preceded or overlapped with shock (ISI = −45, −15, or 0 s), control flies later on avoided it. If, during training, the odor had closely followed shock (ISI = 20 or 40 s), control flies later approached it. If the two events were too far apart in time (−150, 70, or 200 s), flies showed no signs of conditioned behavior. Concerning the white1118 mutants, scores overall were shifted “southward,” that is, toward stronger conditioned avoidance. Sample sizes for the very long ISIs are lower because Tanimoto et al. (Citation2004) and Yarali et al. (Citation2008) showed that for such very long ISIs, the learning indices are zero in the wild type. In other words, expecting any kind of nonzero score for ISIs longer than 1 minute between odor and shock seems unlikely, in any genotype, such that differences between genotypes are unlikely, too. Therefore, a lack of difference for the long ISIs, although based on a small sample size, likely is real. * P < 0.05/8, while comparing between genotypes (i.e., Bonferroni correction; see Methods for details). Box plots represent the median as the midline; 25 and 75% as the box boundaries and 10 and 90% as the whiskers. (C) Control and white1118 mutant flies avoided shock indistinguishably well. NS, P > 0.05. Box plots are as in (B).
In this equation, # indicates the number of flies found in the respective maze arm. For each ISI, two subgroups of flies were trained and tested in parallel (A): For one of these, OCT was the control odor and BA was to be learned; the second group was trained reciprocally (i.e., the roles of these two odors were switched). A learning index (LI) was calculated, based on the PREF values from the two reciprocal measurements, as shown in Equation 2:2
Subscripts of PREF (BA or OCT) indicate the learned odor in the respective subgroups of flies. Positive LIs indicate conditioned approach to the learned odor, whereas negative values reflect conditioned avoidance.
To test for the immediate, reflexive shock response, flies were transferred to the choice point of a T-maze 5 minutes after being loaded into the setup. Then, 10 seconds later, one of the maze arms was electrified with four 1.2-second-long pulses of 100-V shock with 5-second interpulse intervals. Then, 10 seconds after the onset of the last pulse, the arms of the maze were closed and flies on each side were counted. A preference index for the electrified arm (PREFShock) was calculated, as shown in Equation 3:3
Again, # indicates the number of flies found in the respective maze arm, and negative PREFShock values indicate avoidance of the shock.
Sugar reward learning required a different set of training parameters to yield substantial learning scores; specifically, it used two training cycles (A). Each cycle started by loading the flies into the setup (0 minutes). Next, 1 minute later, flies were transferred to a tube lined with a filter paper soaked the previous day with 2 ml of 2-M sucrose solution and dried overnight. This tube was scented with the as-yet-to-be-learned odor. After 45 seconds, the odor was removed, and 15 seconds later, flies were taken out of the tube. After a 1-minute waiting period, flies were transferred into another tube lined with a filter paper, which was soaked with distilled water the previous day and also dried overnight. This second tube was scented with a control odor. After 45 seconds, this odor was removed, and 15 seconds later, flies were taken out of the tube. The next training cycle then started immediately. For half of the cases, training trials started with the as-yet-to-be-learned odor and sugar; in the other half, the control odor was given precedence. Once the training was completed, after a 3-minute waiting period, flies were transferred to the choice point of a T-maze between the two odors. After 2 minutes, the arms of the maze were closed, flies on each side were counted, and a PREF was calculated, according to Equation 1. As detailed above, two groups were trained reciprocally (A) and a learning index (LI) was calculated based on their PREF values, according to Equation 2.
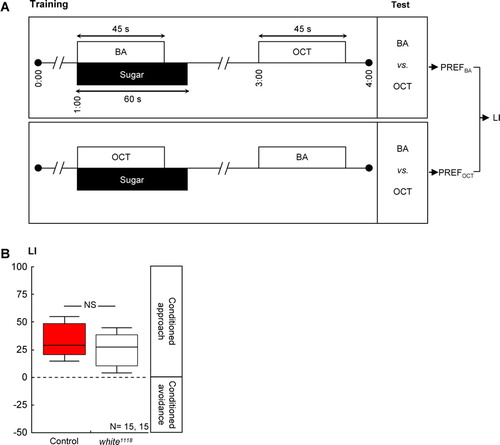
Figure 2 Loss of white-function did not affect olfactory reward learning in adult Drosophila. (A) Adult flies were successively exposed to an as-yet-to-be-learned odor in the presence of sugar and to a control odor without any sugar. Two subgroups were trained reciprocally, that is, with switched roles for the odors 3-octanol (OCT) and benzaldehyde (BA). Both subgroups were then given the choice between the two odors; a learning index (LI) was calculated based on their odor preferences (PREFs). Positive values indicate a conditioned approach toward the learned odor. (B) Control flies and white1118 mutants performed equally well in such reward learning. Details are as in C.
Larval Behavior
Larval learning experiments followed the mass assay described in Neuser et al. (Citation2005). Larvae, aged 5 days after egg laying, were assayed in groups of 30, under a fume hood at 24–28°C, in regular daylight. One day before the experiments, Petri dishes (Sarstedt, Nümbrecht, Germany), each with an 85-mm inner diameter, were filled with 1% agarose (electrophoresis grade; Roth, Karlsruhe, Germany), allowed to solidify, then covered with their lids and left untreated until the following day. As the sugar reward, 2 M of fructose (FRU, purity: 99%; Sigma, Steinheim, Germany) was added to the agarose 10 minutes after boiling. During the experiments, the regular lids of the Petri dishes were replaced by lids perforated in the center by ∼60 1-mm holes to improve aeration. The odor, n-amylacetate (AM; Merck, Darmstadt, Germany), was diluted 1:1600 in paraffin oil (Merck, Darmstadt, Germany) and applied in custom-made Teflon containers placed in each Petri dish on opposite sides, 7 mm from the edges; these containers were of a 5-mm inner diameter and closed with a lid with seven 0.5-mm holes.
To start training, 30 larvae were collected from food medium, briefly washed in tap water to, then as a group, be transferred into a Petri dish filled with sugar-added agarose, and with two containers filled with AM (A). Larvae were left to crawl in this Petri dish for 5 minutes and were then transferred into another Petri dish filled with agarose only and with two empty containers. Also, in this Petri dish larvae remained for 5 minutes. We repeated this training cycle three times, each time using fresh Petri dishes. At the end of training, we placed the larvae in the middle of a fresh Petri dish, filled with only agarose, and with one container of AM on one side and one empty container on the other side (sidedness was alternated for every other set of larvae). After 3 minutes, the number of animals on each side was counted. For each group of larvae thus trained (i.e., “AM+/Empty,” as in this example; note that in half of the cases, training was in reversed order, i.e., “Empty/AM+”), another group of larvae was trained reciprocally as “Empty+/AM” (or, in half of the cases as “AM/Empty+”; A). An LI was then calculated, as detailed above for adult learning.
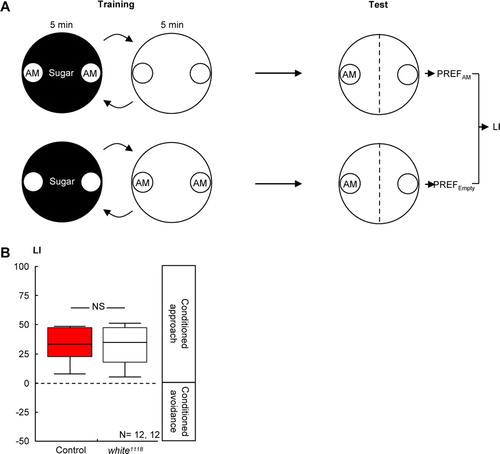
Figure 3 Loss of white-function did not affect olfactory reward learning in larval Drosophila. (A) Larvae were successively exposed to the odor n-amylacetate (AM), in the presence of sugar and to a no-odor situation (Empty) without any sugar. Another group of larvae was trained reciprocally. Both groups were then tested for their response to AM; a learning index (LI) was calculated based on their AM preferences (PREF). Positive LI values indicate appetitive learning. (B) Control larvae and white1118 mutant larvae performed equally well in such reward learning. Details are as in C.
Quantification of Biogenic Amine Amounts
We quantified the amounts of octopamine, tyramine, dopamine, and serotonin in the brains of adult fruit flies by using HPLC coupled to a tandem mass spectrometer (HPLC-MS/MS). For the non-specialist reader, we will first explain the principle of HPLC-MS/MS and the quantification method to put the present method into context of other previously used methods (see Discussion). Then, we will present the technical particulars.
Principle of Method
Extracts of fruit fly brain homogenate were loaded onto a liquid chromatography column that contained silica particles coated with C18 hydrocarbon chains. Biogenic amines, along with other organic molecules, were retained by this column material. By increasing the proportion of the organic solvent in the aqueous mobile phase, molecules were gradually separated and eluted before they entered the MS. Retention times of the molecules on the column depended largely on their lipophilicity (i.e., polar, hydrophilic compounds eluted early, while hydrophobic molecules elute late). Hence, molecules of interest reached the MS at different and characteristic retention times (RTs). As the molecules entered the MS, they became ionized through protonation (i.e., became positively charged). Molecule ions characterized by their specific mass-per-charge (m/z) ratios were physically separated by this first MS. Next, selected molecular ions were broken by collision-induced dissociation (CID) into a series of compound-specific fragments, which were then physically separated by a second MS that also recorded the ion intensities of the derived fragments. In the multireaction monitoring mode (MRM), even fragments from several molecules coeluting from the HPLC column (i.e., molecules with the same RT) can be sorted and analyzed within some hundreds of milliseconds. Hence, molecules were specifically identified and quantified according to their RT, the m/z value of the molecular ion, and the m/z value(s) of one or several fragment ions. In pilot experiments, all these values (e.g., RT, CID-energy, and m/z values) could be obtained by analyzing authentic reference compounds. Moreover, the technique allowed the use of internal standards labeled with stable isotopes that were added to the tissue prior to extraction. These standards displayed the same physical-chemical properties as the target molecules and only differed by their mass. Hence, compound losses occurring during sample preparation and processing were proportional for standard and target molecules. To quantify e.g., the amount of serotonin, a known amount of deuterated serotonin ([D4]serotonin) was added to the brain homogenate. Labeled and endogenous serotonin then were simultaneously extracted and purified by HPLC. The endogenous “light” serotonin and the heavier [D4]serotonin could be separated by the MS, according to their different m/z values, and the intensities of the ions could be determined. The ratio of the ion intensities should be equal to the ratio of the amounts initially present in the sample and, hence, the amount of endogenous serotonin in the unextracted sample could be calculated. To validate the method, for example with respect to serotonin, we initially prepared a series of samples; each sample contained 5 ng of [D4]serotonin and a certain known amount of unlabeled, light serotonin, varying between 5 and 1,000 pg. The amount of serotonin in each sample was then determined, as described above. A plot of the measured amount against the known actual amount results in a linear function; for serotonin, such a plot is shown in A-A′ (for the other amines, see the Supplemental Figs.). When isotopically labeled heavy standards are used, the slope of the linear fit is usually one, as in the case of octopamine (Supplemental A-A′). Sometimes, however, the ionization and fragmentation efficiencies differ between the isotopically labeled standard and the unlabeled, light molecule, resulting in a slope that is different from one; in such cases, a correction factor is employed (e.g., as in the case of serotonin [A- A′], tyramine [Supplemental A-A′], and dopamine [Supplemental A- 3A′]).
Chemicals
[D3]octopamine and [D4]serotonin were from Medical Isotopes (Pelham, USA); [D2]tyramine and [D3]dopamine were obtained using acid catalyzed isotope exchange between dopamine/ tyramine and deuterated water (Pajak & Kanska, Citation2006). Unlabeled octopamine, tyramine, dopamine and serotonin were purchased as hydrochloride salts from Sigma-Aldrich (Munich, Germany).
Sample Preparation
Each sample contained 5 female and 5 male brains (2–3 days old) from either white1118 mutant or Canton Special wild-type flies. Brains were dissected in ice-cold Ringer's solution and directly placed into 50 µl of ice-cold 50-mM citrate-acetate buffer (pH 4.5), which in addition contained 5 ng of each internal standard. Once 10 brains were collected (which took ∼30 min), they were homogenized in this solution on ice with a Teflon pestle. After centrifugation at 14,000 rpm for 5 minutes at room temperature, 10 µl of the supernatant was analyzed by HPLC-MS/MS.
HPLC-MS/MS Conditions
An Agilent 1200 HPLC system (Agilent Technologies, Waldbronn, Germany) coupled to a Waters Micromass Quattro Premier triple-quadrupole mass spectrometer (Milford, Massachusetts, USA), was used. Liquid chromatography was performed by using an Agilent Eclipse XDB-C18 column (150 mm×4.6 mm, 5-µm particle size; Agilent Technologies, Waldbronn, Germany). The column was eluted with a linear mobile-phase gradient (0.6 ml/min flow rate), starting from water containing 0.1% formic acid at 0 minutes to an acetonitrile:water:formic acid mixture (50:50:0.1, v/ v/ v) at 10 min.
For MS, ionization was achieved by using electrospray in the positive ionization mode (ESI+) with a capillary voltage of 2.5 kV. The temperature of the source block was set at 120°C, and nitrogen was used as desolvation and cone gas with a flow of 800 l/h at 350°C and 50 L/h, respectively. In order to establish the appropriate conditions for the individual compounds and their respective deuterated analogs, standard solutions were directly infused into the mass spectrometer and the cone voltage was adjusted to maximize the intensity of the protonated molecular species. CID of each compound was performed by using argon as collision gas with a flow rate of 0.3 ml/min and a pressure of 3.0×10−3 mBar; collision energy (eV) was adjusted to optimize the signal for the most abundant fragment ions, which were subsequently used for MRM analysis with a dwell time of 100 ms for each reaction. The MRM transitions and conditions for the measurement are given in .
Table 1 Multireaction monitoring mode transitions and conditions for the measurement of biogenic amines.
Statistics
All data were analyzed by using nonparametric statistics and are reported as box plots, showing the median as the midline and 10, 90, and 25%, 75% quantiles as whiskers and box boundaries, respectively. For comparing values of each group to zero, we used one-sample sign tests. To compare values between two groups, we used a Mann-Whitney U-test. When multiple tests were performed within a single experiment, we adjusted the experiment-wide error rate to 5% by Bonferroni correction; that is, we divided the critical P < 0.05 by the number of tests. For example, if 8 such comparisons were made, we report the P-level as P < 0.05/8. To compare more than two groups with each other, we used Kruskal-Wallis tests. Sample sizes are mentioned within the figures. All statistical analyses were performed on a PC, using Statistica (Statsoft, Tulsa, Oklahoma, USA).
RESULTS
white-Function and Olfactory Associative Learning
Regarding wild-type control flies, conditioned behavior depended on the relative timing of odor and shock (red displays in B: Kruskal-Wallis test; control flies: H = 168.96, df = 7; P < 0.05): If, during training, the odor had been presented either long before (B: one-sample sign test; control: ISI = −150 seconds; P > 0.05/8) or long after shock (B: one-sample sign tests; control: ISI = 70 and 200 seconds; P > 0.05/8 each), flies did not show any conditioned behavior. If the odor had shortly preceded or overlapped with shock during training, it was avoided in the test (i.e., punishment learning) (B: one-sample sign tests; control: ISI = −45, −15, and 0 seconds; P < 0.05/8 each). Contrarily, if the odor had shortly followed shock during training, wild-type flies later on approached it (i.e., relief learning) (B: one-sample sign tests; control: ISI = 20 and 40 seconds; P < 0.05/8 each). These results conform to the previous reports of Tanimoto et al. (Citation2004) and Yarali et al. (2008).
Next, we compared white1118 mutants’ learning to the the wild type. For very long ISIs, which did not support learning in the wild type to begin with, we found no difference between the two genotypes (B: U-tests: ISI = −150 seconds: U= 28.00; ISI = 70 seconds: U = 70.00; ISI = 200 seconds: U = 58.00; P > 0.05/8 each). In contrast, using short ISIs, which did support learning in the wild-type flies, the loss of white-function did have an effect: Namely, regardless of the sequence of the odor and the shock during training, the learning scores of the white1118 mutants were shifted “southward” that is, toward stronger conditioned avoidance (B: U-tests: ISI = −15 seconds: U = 183.00; ISI = 0 seconds: U = 745.00; ISI = 20 seconds: U = 157.00; ISI = 40 seconds: U = 226.00; P < 0.05/8 each; note, however, that for the −45-second ISI, U = 239.00; P = 0.32). Thus, the “take-home message” from the shock episode, overall, was more negative for the white1118 mutants than for wild-type flies.
Is this effect specific for shock-related memories, or is it that the white1118 mutants regard the shock experience itself as more aversive? That is, is the effectiveness of shock as reinforcer, or its capacity to release avoidance behavior, altered? We found that wild-type control flies and white1118 mutants avoided shock to the same extent (C: U-test: U = 123.5; P > 0.05; one-sample sign test for the pooled data set: P < 0.05). Further, loss of white-function left olfactory discrimination ability, in principle, intact, as odor-reward learning remained unaffected: After odor-sugar training (A), learning scores did not differ between genotypes (B: U-test: U = 82.00; P > 0.05); when pooled, they reflected a conditioned approach (one-sample sign test for the pooled data set: P < 0.05). Also, white1118 mutant larvae were not different from the wild type with respect to odor-sugar learning (B: U-test: U = 71.00: P > 0.05).
No Effect of the Loss of white-function on Whole-Brain Amounts of Biogenic Amines
Next, we probed the white1118 mutants’ brains for abnormalities in the levels of the biogenic amines: octopamine, tyramine, dopamine, and serotonin. This was because the White protein provides neurons with the precursor for serotonin, as well as the precursor for a cofactor of serotonin and dopamine synthesis (see Introduction for details). Indeed, Sitaraman et al. (Citation2008) have recently reported lower whole-head levels of serotonin and dopamine in white1118 mutants, as compared to wild-type flies.
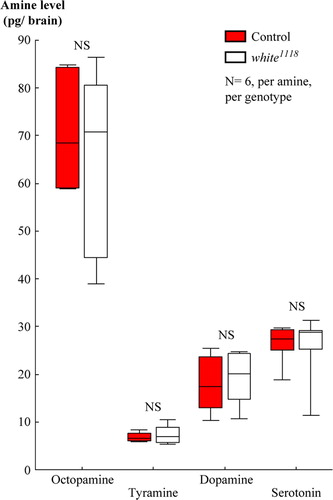
Using HPLC-MS, we did not find a difference between white1118 mutants and wild-type control flies in terms of the amounts of octopamine, tyramine, dopamine, or serotonin in brain homogenates (: U-tests: octopamine: U = 16.00, P = 0.75; tyramine: U = 17.00, P = 0.87; dopamine: U = 16.00, P = 0.75; serotonin: U = 16.00, P = 0.75). As they stand, these data thus do not allow the effect of the loss of white-function on learning to be attributed to an abnormality in the brain amounts of biogenic amines.
Figure 4 Loss of white-function did not affect the adult brain amounts of biogenic amines. High-pressure liquid chromatography, coupled to tandem mass spectrometry, revealed no difference between wild-type controls and white1118 mutants in terms of the brain amounts of octopamine, tyramine, dopamine, or serotonin. From samples that each included 10 brains, we report amine levels as pg per single brain. NS, P > 0.05. Box plots are as in B.
DISCUSSION
In this paper, we report an effect of the loss of white-function on what fruit flies remember about a shock episode (B). Namely, white1118 mutants, as compared to wild-type flies, build stronger aversive memories about the painful onset of shock (a finding in accord with the results from Diegelmann et al., Citation2006) and build weaker appetitive memories about its relieving offset. In other words, white1118 mutants remember the shock episode as, overall, more “negative” than the wild-type flies. Importantly, the immediate aversiveness of shock remains unaltered for the white1118 mutants (C), arguing that it is, indeed, their memories of the shock episode, but not the shock itself, which appears more negative to them.
Keeping Balance, Losing It
As the case of the white1118 mutant shows, punishment and relief learning have common genetic determinants, keeping both processes in balance. This echoes Solomon and Corbit's (Citation1974) theory of “opponent processes,” which suggests that a painful stimulus, in addition to its primary effect, also induces a state of relief upon its offset; the balance between these two opponent states is suggested to govern behavior toward painful stimuli, as well as toward the stimuli associated with them. Distortion of the balance between these opponent processes in man are conceivably implicated in psychiatric conditions (anxiety: Vincent & Kukstas, Citation1998; addiction: Koob & Le Moal, Citation2008). Fruit flies seem to be an appropriate model to study the molecular and neuronal pivots of such balance, because comparable paradigms are available for assessing the behavioral consequences of both pain and relief. Importantly, the critical molecules may well be conserved from fly to man. Indeed, the human homolog of the white gene (i.e., hW, which has been mapped to chromosome 21q22.3) is implicated in mood and panic disorders (Straub et al., Citation1994; Croop et al., Citation1997; Nakamura et al., Citation1999).
white-Effect Related to Brain Levels of Biogenic Amines?
In an attempt to account for the molecular mechanism by which the white1118 mutation exerts its effect, we probed for the brain levels of the biogenic amines, octopamine, tyramine, dopamine, and serotonin. The amounts of these substances, in the present analysis, appeared indistinguishable between white1118 mutants and the wild type (). This contrasts to the finding of Sitaraman et al. (Citation2008), who report that white1118 mutants’ heads contain less serotonin and less dopamine than the heads of wild-type flies.
In , we compare the present data on amine amounts to those previously reported. Obviously, the reported values substantially vary between studies. As a general remark, one potential source of variability always is that, in some cases, mutations may cause phenotypes dependent on the genetic background (de Belle & Heisenberg, Citation1996). Second, the sample preparation differs between studies, in that homogenates from either whole heads or from only brains are assayed. This, indeed, can make a difference, even within a given study (Hardie & Hirsh, Citation2006; compare red triangles vs. red circles in ): Levels of, for example, dopamine are much higher in the head than in the brain, conceiveably because some dopamine is contained in the cuticle (Wright, Citation1987). Third, sample purification, detection, and quantification differ across studies. Most studies cited in coupled HPLC to an electrochemical detector (HPLC-ECD), with two exceptions: 1) the present study, for all amines, employed HPLC-MS and 2) for measuring dopamine in unpurified head extracts, Sitaraman et al. (Citation2008) used an enzyme immunoassay. Electrochemical detection has the drawback that oxidizable phenols and catechols in the sample, which comigrate through the HPLC column with biogenic amines, may accidentally yield ECD signals, potentially resulting in overestimations of amine levels. Therefore, methods relying on HPLC-ECD have to be carefully evaluated, especially when unpurified samples from nonstandard biological sources, potentially including unknown metabolites of the target trace-amount molecules, are analyzed. A similar caveat may be raised concerning immunoassays: Since antibodies rarely display absolute specificity, in particular, for small molecules, cross-reactivities with structurally related metabolites are often observed and may cause problems when unpurified samples are measured. In any event, both of these two methods do not employ isotopically labeled internal standards, which help to compensate for variable extraction efficiencies, chemical degradation (i.e., auto-oxidation), and losses during sample purification. Therefore, for trace analysis, in particular, of small molecules, coupled techniques in which the molecules of interest are first physically separated in a first dimension (i.e., by HPLC, gas chromatography or electrophoresis) and then are specifically detected and quantified by MS, arguably, seem preferable. MS/MS, as used in this study, adds two further dimensions of physical separation of molecules (i.e., the separation of the molecular ions in the first MS and the separation and quantification of specific fragment ions in the second MS). In addition, the ionization method and the collision energy employed further limit the type of molecules that can interfere with analysis, hence resulting in low background noise. Thus, apart from being highly specific, HPLC-MS/MS is also one of the most sensitive analytical methods available.
Figure 5 Meta-analysis of amine amounts. We compared various high-pressure liquid chromatography (HPLC)-based studies (color coded) in terms of the biogenic amine amounts they found in whole-head (triangles) or only-brain (circles) homogenates from wild-type control or white1118 mutant flies. We plotted mean values throughout in pg/brain or head to enable comparison between studies. Please note the different Y-axes for each amine.
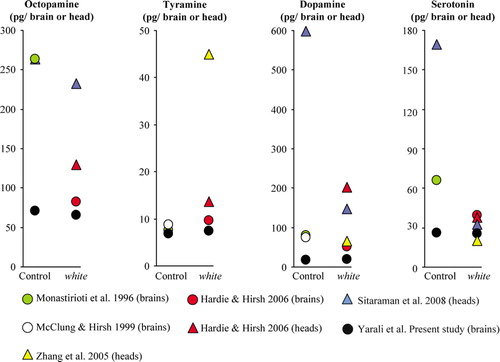
With such methodology, the current study did not detect a difference between white1118 mutant and wild-type brains in terms of biogenic amine levels. This contrasts to the finding of Sitaraman et al. (Citation2008), that wild-type heads contain more dopamine and more serotonin than white1118 mutant heads. We take serotonin as a case to discuss whether such a between-genotype difference could, in principle, have been detected by using the present method. As can be seen in , a number of independent reports, including the present one, agree upon the amount of serotonin per white mutant head and brain. As compared to this “consensus level” of serotonin in the white mutant, Sitaraman et al. (Citation2008) found 5–6-fold more serotonin in wild-type heads. Could our method have measured such a high serotonin amount? In A- A′, the dynamic range of the present measurement, with respect to serotonin, can be seen. To reveal this dynamic range, we analyzed, by HPLC-MS/MS, a series of samples, each containing 5 ng of labeled [D4]serotonin and known amounts of unlabeled serotonin, ranging from 5 to 1000 pg. We plotted, for each sample, the measured serotonin amount against the actual, known amount; within a range of more than two orders of magnitude, these two amounts corresponded well. Within this dynamic range, the total amount of serotonin in a homogenate of 10 brains, as found in this study (A- A′: black arrow) fell approximately in the middle, allowing to detect potential decreases, as well as increases, in serotonin levels. Specifically, it would, in principle, be possible to detect a 4-fold higher serotonin level than actually found in this study. This argument against a “ceiling effect” obviously is derived from measurements of serotonin over a solvent “background”; does it apply for the experimental measurements of serotonin as well (i.e., for measurements over the brain-homogenate “background”)? In other words, is the detection of serotonin within the brain homogenate possible with the same specificity as over the solvent background? We compared chromatograms obtained over a solvent background, on the one hand (B), with the measurements over a brain-homogenate background, on the other hand (B′); both measurements have a reasonably good signal-to-noise ratio, arguing that the present method can detect serotonin equally well over either background. These arguments also apply for octopamine, tyramine, and dopamine (see Supplemental Figs.).
Figure 6 Assessment of serotonin measurement. (A) Using high-pressure liquid chromatography coupled with tandem amss spectrometry (HPLC-MS/MS), we analyzed a series of samples, each containing 5 ng of [D4]serotonin and a known amount of unlabeled serotonin, ranging from 5 to 1000 pg. For each sample, we plot the measured amount of unlabeled serotonin against the actual, known amount. Mean ± standard deviations were obtained from three independent measurements. The black arrow marks the mean total amount of serotonin we found in a homogenate of 10 wild-type brains (i.e., we multiplied the single-brain value from by 10). (A′) Close-up on the lower range of (A). (B) Example HPLC-MS/MS chromatograms for unlabeled serotonin (top) and labeled [D4]serotonin (bottom) over a solvent “background.” As expected, their retention times were equal. (B′) Example HPLC-MS/MS chromatograms obtained by analyzing a homogenate of 10 wild-type brains, added with isotope-labeled serotonin (5 ng). Both unlabeled, endogenous serotonin (top) and labeled [D4]serotonin (bottom) are clearly detectable. As expected, their retention times were the same. Note that the signal-to-noise ratio for the measurements over the solvent background in (B) does not apparently differ from the measurements over the brain-homogenate background. On the top-right corner of (B) and (B′), the first line indicates the specific MRM transitions and the second line the maximal ion current in arbitrary units.
![Figure 6 Assessment of serotonin measurement. (A) Using high-pressure liquid chromatography coupled with tandem amss spectrometry (HPLC-MS/MS), we analyzed a series of samples, each containing 5 ng of [D4]serotonin and a known amount of unlabeled serotonin, ranging from 5 to 1000 pg. For each sample, we plot the measured amount of unlabeled serotonin against the actual, known amount. Mean ± standard deviations were obtained from three independent measurements. The black arrow marks the mean total amount of serotonin we found in a homogenate of 10 wild-type brains (i.e., we multiplied the single-brain value from Figure 4 by 10). (A′) Close-up on the lower range of (A). (B) Example HPLC-MS/MS chromatograms for unlabeled serotonin (top) and labeled [D4]serotonin (bottom) over a solvent “background.” As expected, their retention times were equal. (B′) Example HPLC-MS/MS chromatograms obtained by analyzing a homogenate of 10 wild-type brains, added with isotope-labeled serotonin (5 ng). Both unlabeled, endogenous serotonin (top) and labeled [D4]serotonin (bottom) are clearly detectable. As expected, their retention times were the same. Note that the signal-to-noise ratio for the measurements over the solvent background in (B) does not apparently differ from the measurements over the brain-homogenate background. On the top-right corner of (B) and (B′), the first line indicates the specific MRM transitions and the second line the maximal ion current in arbitrary units.](/cms/asset/715e5f3f-a585-416e-a72d-da4b89148937/ineg_a_344305_f0006_b.gif)
In turn, it may be that the sample treatment in the current report unwittingly led to a degradation of serotonin, such that overall serotonin levels were too low to allow for between-genotype differences to be detected. As shown in A-A′ (black arrow), a 5-fold decrease of serotonin levels would still be in the linear range of the current methodology. Thus, the assumption that the current report could not detect between-genotype differences in serotonin levels because of a “floor-effect” does not seem to be valid—unless one would assume that, for as-yet-to-be-identified reasons, the degradation of serotonin were to happen in wild-type, but not in the white1118 mutants. The same argument applies for the other amines as well (see Supplemental Figs.).
With all these reasonings in mind, including the principle caveats of interpreting lack-of-difference results, we note that the present study did not find an abnormality of biogenic amine levels in the brains of white1118 mutants and hence could not offer such variations to explain the effect of the white1118 mutation on shock-related learning. Obviously, this statement does not question the roles of amines for learning, as such roles have extensively been analyzed with genetic methods independent of white, as well as by pharmacological, intervention (fruit fly: Schwaerzel et al., Citation2003; Schroll et al., Citation2006; Sitaraman et al., Citation2008; honey bee: Hammer, Citation1993; Hammer & Menzel, Citation1998; Farooqui et al., Citation2003; Vergoz et al., Citation2007; cricket: Unoki et al., Citation2005, Citation2006). In other words, both the mentioned amines and white can matter for learning, but these effects, based on the present data, appear independent of each other.
A Role for NO Signaling?
Interestingly, guanine, which is transported into cells by the White-Brown heterodimer (Dreesen et al., Citation1988), is converted to 6H-tetrahydrobiopterin, which in turn, is a cofactor for NO synthesis (reviewed by Koshimura et al., Citation2000). Thus, effects of the white gene on NO signaling may explain its effects on learning. Indeed, NO may provide a retrograde signal at the output of the mushroom body Kenyon cells (Bicker & Hähnlein, Citation1995; Bicker et al., Citation1996), the suspected site of the odor-shock short-term memory trace (reviewed by Zars, Citation2000; Heisenberg, Citation2003; Gerber et al., Citation2004; Heisenberg & Gerber, Citation2008). Whether the effect of the white1118 mutation comes about via alterations in NO signaling remains to be tested.
Implications
Regardless of the underlying molecular mechanism, the behavioral effects of the white gene may, in general, concern Drosophila behavioral neurogeneticists. This is because a typical transgenic fly strain has a white1118 mutant genetic background and within the actual transgene carries a truncated so-called mini-white cDNA. This is done to ensure that a lack of insertion during the initial generation of the transgenic strain or loss of the transgene will reveal itself by white eye color (this is why white is called a “marker” gene). Thus, a confound in interpretation may arise when, for example, attempting to rescue a behavioral defect in a mutant X by transgenically expressing the cDNA of gene X, using the GAL4-UAS system: In this case, the experimental flies not only transgenically express the potentially rescuing gene, but they also bear both the GAL4 and the UAS transgenes and thus two copies of the mini-white cDNA. To the extent that the loss of white-function impairs the tested behavior, the experimental flies may, indeed, perform better than the controls, but conceivably not because of a rescue of gene X, but because two mini-white cDNAs rescue the white1118 mutant phenotype better than one mini-white does in the genetic control strains (which carry either only the GAL4 or only the UAS construct). Thus, it would seem wise to probe for effects of white before launching a neurogenetic behavior analysis of any sort.
CONCLUSIONS
To summarize, we report that punishment learning (as induced by shock onset) is enhanced and relief learning (as induced by shock offset) is diminished in white1118 mutants, as compared to the wild type; thus, the balance between punishment learning and relief learning in the white1118 mutant is distorted in favor of punishment learning. The molecular mechanisms of this distortion, in particular, regarding the role of serotonin, however, remain controversial.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft via the grants SFB 554/ A10 Arthropode Behaviour, SFB-TR 58/ A6 Fear, Anxiety and Anxiety Disorders, and a Heisenberg Fellowship (to B.G.), GK 1156 Synaptic and Behavioural Plasticity (to B.M.), a PhD fellowship from the Federal Excellence Initiative Grant Graduate School Life Sciences Würzburg (to T.S.), as well as by the Boehringer Ingelheim Fonds (PhD fellowship, to A.Y.). The authors are especially grateful to E. Münch for the generous support to A.Y. during the start-up phase of her PhD. The continuous support of the members of the Würzburg group, especially of M. Heisenberg, K. Oechsener, and H. Kaderschabek, is much appreciated, just as are the collegial discussions with T. Zars (University of Missouri–Columbia).
Supplementary Material
Download GIF Image (124.8 KB)Supplementary Material
Download GIF Image (94.7 KB)Supplementary Material
Download GIF Image (103.5 KB)References
- An X., Armstrong J. D., Kaiser K., O'Dell K. M. The effects of ectopic white and transformer expression on Drosophila courtship behavior. J Neurogenet 2000; 14: 227–243
- Andretic R., van Swinderen B., Greenspan R. J. Dopaminergic modulation of arousal in Drosophila. Curr Biol 2005; 15: 1165–1175
- Bicker G. Sources and targets of nitric oxide signalling in insect nervous systems. Cell Tissue Res 2001; 303: 137–146
- Bicker G., Hähnlein I. NADPH-diaphorase expression in neurones and glial cells of the locust brain. Neuroreport 1995; 6: 325–328
- Bicker G., Schmachtenberg O., De Vente J. The nitric oxide/cyclic GMP messenger system in olfactory pathways of the locust brain. Eur J Neurosci 1996; 8: 2635–2643
- Croop J. M., Tiller G. E., Fletcher J. A., Lux M. L., Raab E., Goldenson D., Son D., Arciniegas S., Wu R. L. Isolation and characterization of a mammalian homolog of the Drosophila white gene. Gene 1997; 185: 77–85
- de Belle J. S., Heisenberg M. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm). Proc Natl Acad Sci USA 1996; 93: 9875–9880
- Diegelmann S., Zars M., Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem 2006; 13: 72–83
- Dierick H. A., Greenspan R. J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 2007; 39: 678–682
- Dreesen T. D., Johnson D. H., Henikoff S. The brown protein of Drosophila melanogaster is similar to the White protein and to components of active transport complexes. Mol Cell Biol 1988; 8: 5206–5215
- Farooqui T., Robinson K., Vaessin H., Smith B. H. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci 2003; 23: 5370–5380
- Gerber B., Tanimoto H., Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol 2004; 14: 737–744
- Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 1993; 366: 59–63
- Hammer M., Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem 1998; 5: 146–156
- Hardie S. L., Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high-performance liquid chromatography. J Neurosci Meth 2006; 153: 243–249
- Hazelrigg T., Levis R., Rubin G. M. Transformation of white locus DNA in Drosophila: dosage compensation, zeste interaction, and position effects. Cell 1984; 36: 469–481
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci 2003; 4: 266–275
- Heisenberg M., Gerber B. Behavioral analysis of learning and memory in Drosophila. Learning and Memory: A Comprehensive Reference, Vol. 1: Learning Theory and Behavior, R. Menzel, J. Byrne. Elsevier, Oxford 2008; 549–560
- Hing A. L., Carlson J. R. Male-male courtship behavior induced by ectopic expression of the Drosophila white gene: role of sensory function and age. J Neurobiol 1996; 30: 454–464
- Hoyer S. C., Eckart A., Herrel A., Zars T., Fischer S. A., Hardie S. L., et al. Octopamine in male aggression of Drosophila. Curr Biol 2008; 18: 159–167
- Koob G. F., Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008; 59: 29–53
- Koshimura K., Murakami Y., Tanaka J., Kato Y. The role of 6R-tetrahydrobiopterin in the nervous system. Prog Neurobiol 2000; 61: 415–438
- McClung C., Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol 1999; 9: 853–860
- Monastirioti, M., Linn, C. E., Jr, & White, K. 1996. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci, 16, 3900–3911.
- Morgan, T. H., Sturtevant, A. H., Muller, H. J., & Bridges, C. B. 1915. The Mechanism of Mendelian Heredity. New York: Holt Rinehart & Winston. Reprinted. Johnson Reprint Corporation, with an Introduction by Garland E. Allen, 1978.
- Nakamura M., Ueno S., Sano A., Tanabe H. Polymorphisms of the human homologue of the Drosophila white gene are associated with mood and panic disorders. Mol Psychiatry 1999; 4: 155–162
- Neuser K., Husse J., Stock P., Gerber B. Appetitive olfactory learning in Drosophila larvae: effects of repetition, reward strength, age, gender, assay type, and memory span. Anim Behav 2005; 69: 891–898
- Nilsson E. E., Asztalos Z., Lukacsovich T., Awano W., Usui-Aoki K., Yamamoto D. Fruitless is in the regulatory pathway by which ectopic mini-white and transformer induce bisexual courtship in Drosophila. J Neurogenet 2000; 13: 213–232
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol 1984; 180: 437–455
- Pajak M., Kanska M. Synthesis of isotopomers of dopamine labeled with deuterium or tritium. J Labelled Comp Radiopharm 2006; 49: 1061–1067
- Riemensperger T., Voller T., Stock P., Buchner E., Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 2005; 15: 1953–1960
- Schroll C., Riemensperger T., Bucher D., Ehmer J., Voller T., Erbguth K., et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 2006; 16: 1741–1747
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 2003; 23: 10495–10502
- Sitaraman D., Zars M., Laferriere H., Chen Y. C., Sable-Smith A., Kitamoto T., et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A 2008; 105: 5579–5584
- Solomon R. L., Corbit J. D. An opponent-process theory of acquired motivation. I. Temporal dynamics of affect. Psychol Rev 1974; 81: 119–145
- Straub R. E., Lehner T., Luo Y., Loth J. E., Shao W., Sharpe L., et al. A possible vulnerability locus for bipolar affective disorder on chromosome 21q22.3. Nat Genet 1994; 8: 291–296
- Sullivan D. T., Sullivan M.C. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem Genet 1975; 13: 603–613
- Tanimoto H., Heisenberg M., Gerber B. Event timing turns punishment to reward. Nature 2004; 430: 983
- Tearle R. G., Belote J. M., McKeown M., Baker B. S., Howells A. J. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics 1989; 122: 595–606
- Unoki S., Matsumoto Y., Mizunami M. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur J Neurosci 2005; 22: 1409–1416
- Unoki S., Matsumoto Y., Mizunami M. Roles of octopaminergic and dopaminergic neurons in mediating reward and punishment signals in insect visual learning. Eur J Neurosci 2006; 24: 2031–2038
- Vergoz V., Roussel E., Sandoz J. C., Giurfa M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE 2007; 2: 288
- Vincent J. D., Kukstas L. A. Opponent processes and anxiety: toward a neurophysiological formulation. Acta Psychiatr Scand Suppl 1998; 393: 50–55
- Wright T. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet 1987; 24: 127–222
- Yarali A., Niewalda T., Chen Y., Tanimoto H., Duerrnagel S., Gerber B. "Pain-relief’’ learning in fruit flies. Anim Behav 2008; 76: 1173–1185
- Yuan Q., Joiner W. J., Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol 2006; 16: 1051–1062
- Yuan Q., Lin F., Zheng X., Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005; 47: 115–127
- Zars T. Behavioural functions of the insect mushroom bodies. Curr Opin Neurobiol 2000; 10: 790–795
- Zhang Y., Friedman D., Wang Z., Woodruff E., Pan L., O'Donnell J., et al. Protein expression profiling of the Drosophila fragile X mutant brain reveals upregulation of monoamine synthesis. Cell 2005; 107: 591–603
- Zhang K., Guo J. Z., Peng Y., Xi W., Guo A. Dopamine-mushroom body circuit regulates saliency-based decision making in Drosophila. Science 2007; 316: 1901–1904
- Zhang S. D., Odenwald W. F. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc Nat Acad Sci U S A 1995; 92: 5525–5529
