Abstract
Sydney Brenner’s choice of Caenorhabditis elegans as a model organism for understanding the nervous system has accelerated discoveries of gene function in neural circuit development and behavior. In this review, we discuss a striking example of synaptic remodeling in the C. elegans motor circuit in which DD class motor neurons effectively reverse polarity as presynaptic and postsynaptic domains at opposite ends of the DD neurite switch locations. Originally revealed by EM reconstruction conducted over 40 years ago, DD remodeling has since been investigated by live cell imaging methods that exploit the power of C. elegans genetics to reveal key effectors of synaptic plasticity. Although synapses are also extensively rewired in developing mammalian circuits, the underlying remodeling mechanisms are largely unknown. Here, we highlight the possibility that studies in C. elegans can reveal pathways that orchestrate synaptic remodeling in more complex organisms. Specifically, we describe (1) transcription factors that regulate DD remodeling, (2) the cellular and molecular cascades that drive synaptic remodeling and (3) examples of circuit modifications in vertebrate neurons that share some similarities with synaptic remodeling in C. elegans DD neurons.
Introduction
As a pioneer in the use of phage genetics to unravel the fundamental mechanisms of gene expression, Sydney Brenner possessed an insightful understanding of how mutant analysis could be exploited to tackle more complex questions in biology. With the overarching goal of understanding how genes build the brain, he chose Caenorhabditis elegans because its simple nervous system could be fully described and because its rapid, 3-day life cycle facilitates genetic analysis (Brenner, Citation1974). Its small size also mattered, not only for the practical advantage of culturing large numbers of animals for mutant screens but also because Brenner understood that it would be necessary to use electron microscopy (EM) to define the ‘wiring diagram’ (Brenner, Citation1973). In an early step toward this goal, Brenner et al. published a description of serial section EM reconstruction of the adult ventral nerve cord (White, Southgate, Thomson, & Brenner, Citation1976). An accompanying analysis of the ventral cord cell lineage by John Sulston suggested that eight motor neuron classes were generated in two developmental periods (Sulston, Citation1976; Sulston & Horvitz, Citation1977), initially DA, DB and DD motor neurons in the embryo and then VA, VB, VC, VD and AS classes from a second wave of cell divisions late in the first larval stage. This finding was intriguing because it suggested that the motor circuit of newly hatched larvae with only three motor neuron types (DA, DB and DD) should differ from that of the adult with its full complement of eight motor neuron classes (DA, DB, DD, VA, VB, VC, VD, AS; ).
Figure 1. Dorsal D motor neurons undergo synaptic remodeling during early development. (A) (Left) The newly hatched L1 larva contains 3 classes of ventral cord motor neurons: DA, DB, DD. (Right) Five additional postembryonic motor neuron classes (VA, VB, VC, VC, AS) are added to the ventral cord during the L1 to L2 larval transition. (B) During the first larval stage (L1), DD motor neurons (black) provide output to body muscles at ventral presynaptic boutons (purple) and receive input from cholinergic DA/DB neurons (gray) through ACR-12 nACh receptors at postsynaptic terminals (green) on the dorsal side. Arrowhead points to commissure. (C) (Top) DD motor neurons (black) remodel to place presynaptic boutons (purple) on the dorsal side, and relocate postsynaptic terminals (green) to the ventral side for cholinergic input from VA/VB motor neurons (gray). Arrowhead points to commissure. D. DD presynaptic boutons labeled with mCherry::RAB-3 and DD postsynaptic terminals marked with ACR-12::GFP before in early L1 (Left) and after (Right) remodeling at L4 stage. Asterisk labels cell bodies. Scale bar = 10 µm. Images adapted from (He et al., Citation2015). (E) DD neurons remodel over a 4-6-hour period that spans the transition from the L1 to L2 larval stages (yellow).
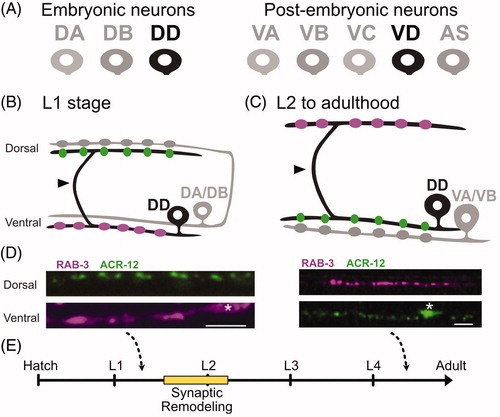
Caenorhabditis elegans development involves four successive larval stages (L1 to L4) before adulthood. EM reconstruction of an early L1 larva yielded the unanticipated finding that the functional polarity of DD motor neurons is reversed in comparison to the adult (White, Albertson, & Anness, Citation1978). In the newly hatched L1, each of the six DD motor neurons innervates ventral muscles and also extends a circumferential commissure to the dorsal nerve cord to receive synaptic inputs from DA and DB motor neurons (). In the adult, this stereotypical DD morphology is retained but synaptic output is switched to dorsal muscles and input is provided by VA and VB motor neurons in the ventral nerve cord (). Reconstruction of an L2 larva revealed DD neurons with adult-like connectivity. Thus, DD synaptic remodeling was likely to occur during the L1 to L2 transition, when post-embryonic motor neurons are generated (). Importantly, the second member of the D class, ventral D (VD) motor neuron, develops post-embryonically and synapses onto ventral muscles while receiving input on the dorsal side (White et al., Citation1976). In other words, VD neurons adopt the synaptic arrangement of early L1 DD neurons ( and ).
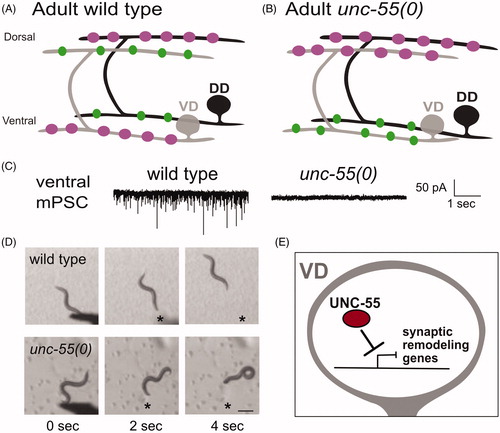
Figure 2. Ventral D (VD) motor neurons ectopically remodel in unc-55 mutants. (A) In adult wild-type worms, DD (black) presynaptic boutons (magenta) are located on the dorsal side and postsynaptic terminals (green) are positioned on the ventral side. VD (gray) presynaptic terminals are positioned on the ventral side (magenta) whereas postsynaptic terminals are located on the dorsal side. (B) In adult unc-55(0) mutants, both DD (black) and VD (gray) presynaptic boutons (magenta) are located on the dorsal side. Postsynaptic terminals (green) of both DD and VD neurons are positioned on the ventral side. (C) (Left) Wild-type worms show robust miniature Post Synaptic Currents (mPSCs) in ventral muscles whereas mPSCs are not detected in unc-55 mutants (Right). Adapted from (Petersen et al., Citation2011). (D) Head touch (asterisk) evokes backward movement in the wild type (top) but unc-55 mutants coil ventrally with head touch (asterisk) due to absence of inhibitory GABAergic input on the ventral side. Scale bar = 250 µm. Adapted from (Petersen et al., Citation2011). (E) The UNC-55/COUP-TF transcription factor functions in VD motor neurons to antagonize expression of synaptic remodeling genes.
To determine if the arrival of larval neurons was necessary for DD remodeling, White et al. (Citation1978) reconstructed the dorsal and ventral nerve cords of a lin-6 mutant that blocks post-embryonic cell division. This experiment showed that the relocation of DD presynaptic boutons to the dorsal cord is not impaired by the absence of postembryonic ventral cord motor neurons. However, input from DA and DB neurons, which is typically eliminated in the wild type, persists on the dorsal process of DD neurons in the lin-6 mutant background. These results suggest that post-embryonic neurons are not required for presynaptic DD remodeling, but may be necessary for further refinement of this circuit, (i.e. elimination of DA and DB inputs).
Remarkably, 20 years elapsed before the publication of a subsequent study of DD remodeling, a breakthrough paper from Yishi Jin’s lab reporting the first use of a novel GFP synaptic marker to monitor DD remodeling (Hallam & Jin, Citation1998). This live-cell imaging approach, which has since been widely adopted for studies of synaptic morphogenesis and plasticity (Hendi, Kurashina, & Mizumoto, Citation2019), depended on several key technological advances. These include (1) methods for generating transgenic strains (Mello, Kramer, Stinchcomb, & Ambros, Citation1991), (2) cloning of genes specifically expressed in GABAergic neurons (e.g. unc-25; Eastman, Horvitz, & Jin, Citation1999; Mclntire, Jorgensen, & Horvitz, Citation1993a; Mclntire, Jorgensen, Kaplan, & Horvitz, Citation1993b) and (3) the discovery that GFP could be used for visualizing neuronal morphology in vivo (Chalfie, Tu, Euskirchen, Ward, & Prasher, Citation1994) and for tagging the presynaptic protein, Synaptobrevin/SNB-1 (Jorgensen et al., Citation1995; M L Nonet, Citation1999). Hallam and Jin exploited these methods to produce a transgenic line that used the unc-25 promoter (Eastman et al., Citation1999; Michael L. Nonet, Saifee, Zhao, Rand, & Wei, Citation1998) for selective labeling of GABAergic presynaptic terminals with SNB-1::GFP. Direct observation confirmed the earlier prediction from EM reconstruction (White et al., Citation1978) that DD presynaptic domains relocate from the ventral nerve cord to dorsal DD process during the L1 to L2 transition (Hallam & Jin, Citation1998; ).
The additional key prediction, that the postsynaptic apparatus is also relocated during DD remodeling, was not confirmed by live-cell imaging until almost 40 years after the original EM reconstruction. As noted above, in the early L1, the dorsal arm of each DD neuron is postsynaptic to cholinergic DA and DB motor neurons (). After remodeling in the L2, DD inputs are switched to the ventral nerve cord where they are postsynaptic to cholinergic VA and VB motor neurons (; White et al., Citation1978). With the determination that the nicotinic acetylcholine receptor (nAChR) subunit, ACR-12, is required for cholinergic activation of ventral cord GABAergic neurons (Cinar, Keles, & Jin, Citation2005; Petrash, Philbrook, Haburcak, Barbagallo, & Francis, Citation2013), it was possible to monitor the relocation of the DD postsynaptic domain in live animals. ACR-12::GFP localizes as discrete puncta on the dendrites of both GABAergic motor neurons (DDs and VDs) in apposition to cholinergic presynaptic terminals (Cuentas-Condori et al., Citation2019; He et al., Citation2015; Petrash et al., Citation2013; Philbrook et al., Citation2018). In DD neurons, ACR-12::GFP is initially positioned on the dorsal neurite at the L1 stage and then relocates to the ventral cord as remodeling ensues (He et al., Citation2015; Howell, White, & Hobert, Citation2015; ). Thus, as originally deduced from EM reconstruction in 1978, presynaptic and postsynaptic complexes are repositioned to opposite ends of the DD neuron and effectively switch locations during early larval development (White et al., Citation1978; ).
By exploiting these and other synaptic markers, several laboratories have sought to elucidate the underlying molecular mechanisms that drive remodeling of the presynaptic and postsynaptic compartments in D-class motor neurons. Some of these findings were recently reviewed (Jin & Qi, Citation2018; Kurup & Jin, Citation2016). Here, we seek to integrate these studies in a historical narrative that highlights Sydney Brenner’s role in launching this field of research and also incorporate new findings on the mechanism of synaptic disassembly. This review will discuss (1) Transcriptional pathways that regulate synaptic remodeling, (2) Downstream targets that mediate cell biological mechanisms for synaptic remodeling and the role of activity in these pathways, (3) The relevance of these findings to understanding synaptic remodeling in the mammalian nervous system.
Synaptic remodeling of D-type motor neurons is transcriptionally regulated
The stereotypical occurrence of DD remodeling during a specific developmental period (i.e. L1 stage larvae) points to regulation by a genetic program (White et al., Citation1978). This idea was substantiated by the finding that the heterochronic protein, LIN-14, controls the timing of DD remodeling. The presynaptic marker, SNB-1::GFP, is precociously relocated to the dorsal nerve cord in lin-14 mutants, suggesting that LIN-14 normally prevents the premature activation of the presynaptic remodeling program (Hallam & Jin, Citation1998; ).
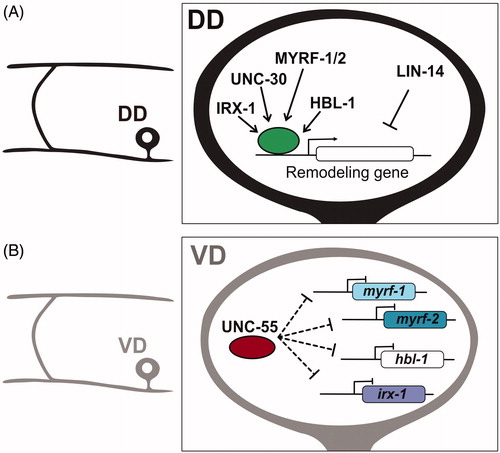
Figure 3. Transcriptional regulation of synaptic remodeling in D-type GABAergic motor neurons. (A) (Left) Morphology of DD motor neuron. (Right) Transcription factors IRX-1/Iroquois MYRF-1/MYRF-2 and HBL-1/Hunchback promote expression of DD remodeling genes, whereas LIN-14 antagonizes remodeling genes. (B) (Left) Morphology of VD motor neuron. (Right) The transcription factor UNC-55/COUP-TFII inhibits expression of IRX-1/Iroquois, HBL-1/Hunchback and MYRF-1/MYRF-2 in VD neurons to prevent ectopic synaptic remodeling.
UNC-55/COUP-TF
Although LIN-14 was known to be nuclear-localized in 1998 (Hallam & Jin, Citation1998; Ruvkun & Giusto, Citation1989) and later confirmed to function as a transcription factor (Hristova, Birse, Hong, & Ambros, Citation2005), direct evidence of transcriptional control of the DD synaptic remodeling program first emerged from studies of the unc-55 locus. As is the case for the majority of ‘uncoordinated’ or ‘unc’ loci, the original unc-55 mutant alleles were isolated by Sydney Brenner (Brenner, Citation1974). unc-55 mutants display a characteristic movement defect of strong ventral coiling during backward locomotion (). EM reconstruction by Leon Nawrocki, a postdoc with John White, revealed that VD class neurons adopt the DD pattern of dorsal synaptic output (Hardy, Citation1990; ), a conclusion confirmed by immunostaining results (Walthall & Plunkett, Citation1995). The absence of inhibitory GABA synapses on the ventral side () results in excess ventral cholinergic excitation and the consequent coiling phenotype (; Miller-Fleming et al., Citation2016; Thompson-Peer, Bai, Hu, & Kaplan, Citation2012; Walthall & Plunkett, Citation1995).
Molecular cloning revealed that unc-55 encodes a member of the conserved COUP-TF family of transcription factors that is selectively expressed in VD but not DD motor neurons (Zhou & Walthall, Citation1998). As a likely transcriptional repressor, UNC-55 was proposed to function in VD neurons to block ectopic activation of the DD synaptic remodeling program (Shan, Kim, Li, & Walthall, Citation2005; ). This idea is consistent with several lines of evidence: (1) In unc-55 mutants, VD synapses are initially established with ventral muscles and then relocated to the dorsal nerve cord in a developmental sequence that mimics native DD remodeling (Petersen et al., Citation2011; Thompson-Peer et al., Citation2012); (2) Forced expression of UNC-55 in DD neurons antagonizes synaptic remodeling (Shan et al., Citation2005); (3) UNC-55 controls expression of downstream effectors of the DD remodeling program including transcription factors IRX-1/Iroquois (Petersen et al., Citation2011), MYRF1/2/Myelin Regulatory Factor (Meng et al., Citation2017; Yu et al., Citation2017) and HBL-1/Hunchback (Thompson-Peer et al., Citation2012), the DEG/ENaC protein, UNC-8 (Miller-Fleming et al., Citation2016), the Ig-domain protein OIG-1 (He et al., Citation2015; Howell et al., Citation2015) and regulators of cAMP homeostasis (Yu et al., Citation2017).
IRX-1/Iroquois
The IRX-1/Iroquois transcription factor was initially identified by a gene expression profiling strategy that detected unc-55-regulated transcripts in VD neurons. A subsequent RNAi screen determined that IRX-1 is required for synaptic remodeling (Miller-Fleming et al., Citation2020; Petersen et al., Citation2011). IRX-1 is a member of the conserved Iroquois family of homeodomain transcription factors that includes mammalian homologs that specify neuronal identity in the developing nervous system (Cavodeassi, Modolell, & Gómez-Skarmeta, Citation2001; Houweling et al., Citation2001). In C. elegans, IRX-1/Iroquois is expressed in DD motor neurons but is normally turned off by UNC-55 in the VD class (). Ectopic expression of IRX-1/Iroquois in unc-55 mutant VD neurons triggers the relocation of ventral VD presynaptic boutons to the dorsal nerve cord (Petersen et al., Citation2011) and also the reciprocal removal of dorsal postsynaptic nACh receptors for reassembly on the ventral side (He et al., Citation2015). In addition, forced expression of IRX-1/Iroquois in otherwise wild-type VD neurons is sufficient to induce the overall synaptic remodeling program (Petersen et al., Citation2011). Thus, IRX-1/Iroquois appears to orchestrate native DD synaptic remodeling by regulating expression of key downstream effectors that control both presynaptic and postsynaptic plasticity (see below; He et al., Citation2015; Petersen et al., Citation2011). Although cell-specific RNAi of irx-1 in DD neurons delays but does not block DD remodeling, the incomplete penetrance of this effect could be due to partial IRX-1 knockdown by RNAi (Petersen et al., Citation2011).
HBL-1/Hunchback
An independent approach revealed that UNC-55 also negatively regulates expression of the HBL-1/Hunchback transcription factor in VD neurons (). HBL-1 promotes the translocation of ventral presynaptic components to the dorsal side in unc-55 mutant VDs and also drives synaptic remodeling in wild-type DD neurons (Thompson-Peer et al., Citation2012). HBL-1 has not been tested, however, for a potential role in remodeling the DD postsynaptic apparatus (e.g. ACR-12, ). In addition, forced expression of HBL-1 in VD neurons is not sufficient to induce remodeling which suggests that HBL-1 could function downstream of IRX-1. Notably, the micro RNA, mir-84, antagonizes HBL-1 expression and this function is required to block the HBL-1-dependent precocious removal of DD presynaptic terminals in the early L1 larva in mir-84 mutants (Thompson-Peer et al., Citation2012).
MYRF1/2/Myelin Regulatory Factor
A forward genetic screen revealed that members of Myelin Regulatory Factor (MYRF) family of transcription factors mediate the translocation of ventral DD presynaptic domains to the dorsal nerve cord (; J. Meng et al., Citation2017). MYRF transcription factors are highly conserved and notably regulate myelination in mammals (Bujalka et al., Citation2013). The C. elegans paralogs, MYRF-1 and MYRF-2, are expressed in DD neurons and function together in a heteromeric complex to regulate DD remodeling (J. Meng et al., Citation2017). Interestingly, MYRF transcription factors localize to the ER where an N-terminal fragment is released by proteolytic cleavage for translocation to the nucleus to function as a transcription factor (Bujalka et al., Citation2013; J. Meng et al., Citation2017). MYRF-1 and MYRF-2 display a similar cell biological mechanism but the signal that activates the pathway in DD neurons is not known. Remodeling is blocked in a genetic background that selectively eliminates MYRF-1 function in DD neurons which suggests that MYRF-1/2 function is essential for DD remodeling (J. Meng et al., Citation2017). It is still an open question whether MYRF-1/2 function is required for the removal of dorsal postsynaptic ACR-12 complexes in DD neurons and their reassembly on the ventral side. In the future it will be important to delineate the specific roles of MYRF-1/2 versus that of IRX-1/Iroquois in DD remodeling.
UNC-30/PITX
Finally, DD remodeling is also disrupted by mutations that disable the UNC-30/PITX transcription factor (; Howell et al., Citation2015). In wild-type L1 larvae prior to remodeling, DD presynaptic markers localize to the ventral side whereas postsynaptic components are limited to the dorsal nerve cord (Hallam & Jin, Citation1998; He et al., Citation2015; ). In unc-30 mutants, however, fluorescently labeled DD presynaptic and postsynaptic markers are observed in both ventral and dorsal nerve cords of early L1 larvae (Howell et al., Citation2015). This effect of apparently precocious remodeling in unc-30 mutants could arise in part from the dysregulation of LIN-14 and OIG-1, both of which antagonize DD remodeling (Hallam & Jin, Citation1998; He et al., Citation2015; Howell et al., Citation2015). Alternatively, the abnormal synaptic organization of unc-30 mutant DD neurons could result from the fundamental role of UNC-30 in the differentiation of DD and VD neurons (Jin, Hoskins, & Horvitz, Citation1994; Mclntire et al., Citation1993b). In any case, the role of UNC-30 in DD remodeling is likely complex because UNC-30 is also necessary for IRX-1 expression which promotes remodeling (Petersen et al., Citation2011).
To summarize, at least six transcription factors function together to specify both the timing and progression of synaptic remodeling in C. elegans GABAergic neurons. In this developmentally regulated program, some transcription factors promote synaptic remodeling (IRX-1/Iroquois HBL-1/Hunchback, MYRF-1/2/Myelin Regulatory Factor; Meng et al., Citation2017; Petersen et al., Citation2011; Thompson-Peer et al., Citation2012), others function to prevent it (LIN-14, UNC-55/COUP-TF; ; Hallam & Jin, Citation1998; Walthall & Plunkett, Citation1995) and at least one (UNC-30/PITX) exerts the dual role of both promoting and antagonizing remodeling (Howell et al., Citation2015; Petersen et al., Citation2011). The surprising intricacy of this gene regulatory network in the C. elegans nervous system with its 302 neurons (White, Southgate, Thomson, & Brenner, Citation1986) points to the potentially daunting challenge of dissecting transcriptional control of synaptic remodeling in more complex nervous systems that could involve a larger number of additional transcription factors with specialized functions deriving from the evolutionary amplification of transcription factor families (Babu, Luscombe, Aravind, Gerstein, & Teichmann, Citation2004). Ultimately, a detailed understanding of the relationship between transcription factors and their targets (Petersen et al., Citation2011; Yu et al., Citation2017) will be key to determining how genetic programs orchestrate synaptic remodeling.
Cellular and molecular mechanisms that regulate DD remodeling
Neuronal activity promotes DD synaptic remodeling
Although several genetic programs regulate synaptic remodeling in D-type motor neurons (), additional evidence suggests that neuronal activity also promotes remodeling (). For example, remodeling of the DD presynaptic domains is delayed by mutations that disable neurotransmitter release (e.g. unc-13/Munc13 and unc-18/Mun18) and accelerated by mutations that increase neurotransmission (e.g. tom-1/Tomosyn and slo-1/BK potassium channel; Thompson-Peer et al., Citation2012). Similarly, synaptic remodeling in the mammalian visual system is retarded but not blocked by sensory deprivation (i.e. absence of light) thus suggesting that both genetically encoded programs and neural activity promote remodeling in this circuit. (Kang et al., Citation2013). Indeed, in C. elegans, DD expression of HBL-1/Hunchback, a transcription factor that promotes remodeling (), is diminished in unc-13 and unc-18 mutants, which points to an activity-dependent mechanism for elevating HBL-1/Hunchback gene expression (). A downstream role for HBL-1/Hunchback is also suggested by the finding that a hbl-1 mutant blocks the acceleration of DD presynaptic remodeling by tom-1 and slo-1 mutants (Thompson-Peer et al., Citation2012). Additionally, optogenetic activation of L1 larval DD neurons drives precocious presynaptic remodeling (Miller-Fleming et al., Citation2016). Although the activity-dependent mechanisms that promote HBL-1 expression are unknown, other downstream effectors of synaptic remodeling that depend on neuronal activity are beginning to emerge ().
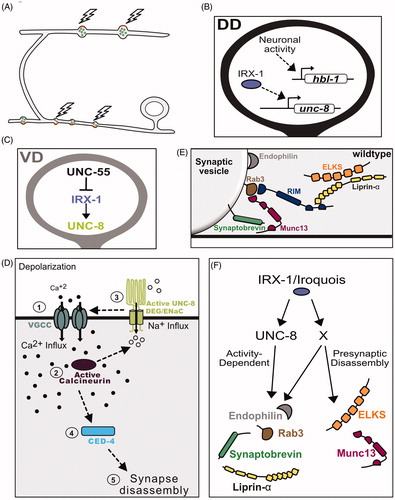
Figure 4. Neuronal activity promotes synaptic remodeling. (A) In DD neurons, neuronal activity promotes the disassembly of ventral presynaptic boutons as well as the formation of new terminals on the dorsal side. (B) Neuronal activity promotes expression of the pro-remodeling transcription factor HBL-1/Hunchback in DD neurons. The homeodomain transcription factor IRX-1/Iroquois/factor drives expression of the DEG/ENaC channel subunit UNC-8. (C) The transcriptional repressor UNC-55/COUP-TFII functions in VD neurons to block expression of the pro-remodeling transcription factor IRX-1/Iroquois and its downstream target UNC-8. (D) Proposed activity-dependent mechanism for synaptic remodeling (1) With depolarization, Voltage-gated Calcium-channels (VGCC) import Ca++ to (2) activate the Ca++-dependent phosphatase Calcineurin/CaN (3) CaN functions upstream of ENaC/UNC-8, which mediates Na++ import, leading to further membrane depolarization and activation of VGCC. These signaling events trigger a positive feedback loop that elevates intracellular Ca++ for activation (4) of a cell-death pathway involving CED-4 and potentially additional downstream effectors (5) of presynaptic disassembly. (E) Presynaptic proteins Endophilin/UNC-57, Rab3, RIM/UNC-10, Munc13/UNC-13, Synaptobrevin/SNB-1, ELKS and Liprin-alpha/SYD-2 regulate synaptic vesicle fusion and neurotransmitter release. (F) The transcription factor IRX-1/Iroquois drives presynaptic disassembly by promoting expression of UNC-8/ENaC expression and an unknown target (X) to remove Endophilin, Rab3, Synaptobrevin and Liprin-α in an activity-dependent mechanism. IRX-1 also drives the elimination of active zone proteins ELKS and Munc13 that are not disassembled by the UNC-8 pathway.
The DEG/ENaC cation channel protein, UNC-8, promotes synaptic disassembly in an activity-dependent mechanism
A role for neural activity in GABA neuron synaptic remodeling is also supported by experiments showing that DEG/ENaC cation channel subunit, UNC-8, triggers presynaptic disassembly in a mechanism that depends on intracellular Ca++ (Miller-Fleming et al., Citation2016). UNC-8 is expressed in DD neurons where it promotes remodeling but is turned off by UNC-55/COUP-TF in VD neurons to prevent ectopic removal of ventral VD presynaptic domains. UNC-55 regulation is likely indirect in this case as UNC-55 negatively regulates IRX-1 which in turn promotes UNC-8 expression (Miller-Fleming et al., Citation2020; ).
A reconstituted UNC-8 channel preferentially gates Na++ in Xenopus oocytes (Wang et al., Citation2013) which suggests that the native UNC-8 DEG/ENaC channel could depolarize the GABA neurons in which it is expressed. In turn, this effect is predicted to enhance Ca++ import by presynaptic Voltage Gated Calcium Channels (VGCC), an idea supported by the additional findings that UNC-2/VGCC is required for UNC-8-dependent presynaptic disassembly (; Miller-Fleming et al., Citation2016). This model parallels an earlier finding in which a Drosophila Pickpocket/DEG/ENaC channel is synaptically localized in motor neurons where it elevates intracellular Ca++ to promote neurotransmitter release (Orr et al., Citation2017; Younger, Mu, Tong, Pym, & Davis, Citation2013). The radically different outcome of synaptic destruction that arises from UNC-8 function in C. elegans depends on the serine/threonine phosphatase CalcineurinA/TAX-6 and its regulatory subunit CalcineurinB/CNB-1. Calcineurin/CaN is activated by intracellular Ca++ and genetic results suggest that it functions upstream of UNC-8. Thus, UNC-8/DEG/ENaC, UNC-2/VGCC and CaN/TAX-6 may constitute a positive feedback loop to amplify Ca++ influx (). In turn, elevated Ca++ might activate synaptically localized apoptotic components that have been previously shown to drive synapse removal and that likely function in the UNC-8 pathway (; L. Meng et al., Citation2015; Miller-Fleming et al., Citation2016). Intriguingly, CaN is known to antagonize postsynaptic function by dephosphorylating AMPA receptors to promote their endocytic removal (Lee, Kameyama, Huganir, & Bear, Citation1998; Sanderson et al., Citation2012; Winder, Mansuy, Osman, Moallem, & Kandel, Citation1998). It will be interesting to determine if presynaptic disassembly in remodeling GABA neurons could also depend on other CaN/TAX-6 targets that have yet to be identified at remodeling presynaptic terminals.
Notably, the unc-8 locus was initially detected by a dominant allele, unc-8(d), isolated in Sydney Brenner’s original screen for uncoordinated mutants (Brenner, Citation1974). The unc-8(d) mutant DEG/ENaC channel is constitutively active and results in the degeneration of ventral cord DA/DB cholinergic neurons that normally drive movement (Shreffler & Wolinsky, Citation1997; Wang et al., Citation2013). unc-8 is abundantly expressed in DA/DB motor neurons but at a substantially lower levels in DDs which could account for the selective degeneration of DA/DB neurons in unc-8(d) mutants (Miller-Fleming et al., Citation2020; Wang et al., Citation2013). Interestingly, UNC-8 does not activate synaptic remodeling in DA/DB motor neurons but is sufficient to induce presynaptic disassembly in both DD and VD class GABAergic neurons (Miller-Fleming et al., Citation2016). The differential effects of UNC-8 on motor neuron function and synaptic remodeling point to potential neuron-specific regulation of UNC-8 channel activity and intracellular localization.
Parallel-acting pathways dismantle the presynaptic apparatus in remodeling GABAergic neurons
Although mutations or pharmacologic treatments that inactivate UNC-8 channel function retard synaptic remodeling, disassembly of the presynaptic apparatus is not completely blocked thus suggesting that additional parallel acting pathways are likely involved (Miller-Fleming et al., Citation2016). Recent work has shown that the transcription factor IRX-1/Iroquois promotes UNC-8 expression in DD neurons (). Genetic evidence suggests that IRX-1/Iroquois must also regulate other downstream effectors to orchestrate presynaptic disassembly. Of particular interest is the finding that the UNC-8 pathway selectively removes a subset of presynaptic markers (e.g. SNB-1/V-SNARE, RAB-3/RAB3) but does not target key effectors of synaptic vesicle fusion and release, UNC-13/Munc13 and ELKS-1/ELKS (), both of which are removed by the additional IRX-1/Iroquois regulated pathway (; Miller-Fleming et al., Citation2020). This finding is significant because it suggests that the mechanism of synaptic destruction in this case selectively targets specific components and thus likely involves molecularly distinct pathways that operate in tandem to disassemble the presynaptic apparatus. Experiments that identify downstream targets of IRX-1/Iroquois should be helpful for unraveling the mechanism of this effect. Because expression of IRX-1/Iroquois is controlled by UNC-55, previous studies that have identified UNC-55-regulated genes could also point to proteins that work downstream of IRX-1/Iroquois (Petersen et al., Citation2011; Yu et al., Citation2017).
GABA signaling promotes DD remodeling
Because GABA signaling can regulate neuronal plasticity in vivo (Afroz, Parato, Shen, & Smith, Citation2016; Deidda et al., Citation2015; Hensch et al., Citation1998; Wu et al., Citation2012), several groups have explored the possibility that DD synaptic remodeling in C. elegans is also regulated by GABA. Across species, GABA synthesis depends on the Glutamic Acid Decarboxylase (GAD) enzyme which is encoded by the unc-25 gene in C. elegans (Jin, Jorgensen, Hartwieg, & Horvitz, Citation1999). An early study determined that the density of dorsal GABAergic synapses, which are formed after DD remodeling, was not different between wild-type and unc-25 mutants in young adults (Jin et al., Citation1999) thus suggesting that GABA signaling is dispensable for DD remodeling. However, new findings from additional studies of this circuit, challenge this conclusion and suggest that GABA signaling regulates the progression of the DD remodeling program: (1) Optogenetic activation of DD neurons accelerates both presynaptic disassembly and dorsal reassembly of DD presynaptic domains synapses (Miller-Fleming, Citation2016; Miller-Fleming et al., Citation2016). (2) Mutants that disrupt GABA synthesis (GAD/unc-25) or vesicular uptake (VGAT/unc-47) delay removal of ventral GABAergic synapses (Miller-Fleming, Citation2016; Thompson-Peer et al., Citation2012). (3) GABA signaling accelerates DD remodeling in tom-1 mutants in which neurotransmitter release is elevated (Miller-Fleming, Citation2016; Thompson-Peer et al., Citation2012). (4) Disruption of GABA signaling during early development slows DD presynaptic remodeling (Han, Bellemer, & Koelle, Citation2015). Finally, (5) metabotropic GABA receptors (e.g. gbb-1 and gbb-2) and the GABA re-uptake transporter, SNF-11, promote elimination of ventral synapses in remodeling GABAergic neurons (Miller-Fleming, Citation2016). The original finding that the number of dorsally placed DD synapses in unc-25 mutants is indistinguishable from wild type at the adult stage (Jin et al., Citation1999) suggests that additional parallel acting pathways are likely responsible for completing the assembly of DD synaptic boutons when GABA signaling is impaired during early larval development. An important question for the future is to determine if delayed DD remodeling, as occurs in mutants with disrupted GABA signaling, also perturbs overall function of the mature motor circuit. This possibility seems plausible given that multiple new postembryonic motor neurons are incorporated into the ventral cord during the period in which DD remodeling transpires (Sulston, Citation1976; White et al., Citation1978; ).
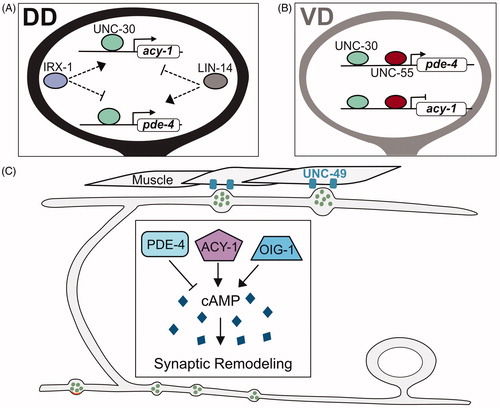
cAMP levels regulate presynaptic remodeling in GABAergic neurons
Genomic experiments (ChIP-Seq) to reveal targets of UNC-30/PITX and UNC-55/COUP-TF, transcription factors that regulate GABA neuron synaptic remodeling, detected key effectors of cAMP metabolism, notably PDE-4 (phosphodiesterase) and ACY-1 (adenylate cyclase; Yu et al., Citation2017). Genetic experiments that derive from these findings are consistent with the hypothesis that cAMP signaling promotes DD presynaptic remodeling (). For example, VD motor neurons remodel ectopically in unc-55 mutants () and this phenotype is correlated with reduced expression of PDE-4. This finding suggests that UNC-55 normally activates PDE-4 expression to limit cAMP levels and thus prevent VD remodeling (). Conversely, IRX-1/Iroquois, antagonizes PDE-4 expression (Yu et al., Citation2017), an effect consistent with the role of IRX-1/Iroquois in promoting DD remodeling (; Petersen et al., Citation2011). Direct measurements with an in vivo FRET assay confirmed that cAMP levels are correlated with DD synaptic remodeling. The regulation of cAMP levels is apparently complex as other antagonists of the synaptic remodeling pathway, LIN-14 and OIG-1 (see below), also limit cAMP levels potentially by promoting PDE-4 expression (). cAMP likely functions in combination with additional pathways because genetic mutants predicted to alter cAMP levels (e.g. pde-4) exert modest effects on synaptic remodeling (Yu et al., Citation2017). For example, the proposed role of UNC-8 in elevating intracellular Ca++ to promote presynaptic disassembly (Miller-Fleming et al., Citation2016) might also boost cAMP levels since adenylate cyclase activity is Ca++ dependent (Halls & Cooper, Citation2011; Koch et al., Citation2011). The downstream cell biological effects of cAMP are similarly uncharacterized but could potentially alter microtubule dynamics which is known to depend on cAMP signaling (Ghosh-Roy, Wu, Goncharov, Jin, & Chisholm, Citation2010) and to promote DD synaptic remodeling (see below; Kurup, Yan, Goncharov, & Jin, Citation2015).
Figure 5. cAMP promotes synaptic remodeling. (A) Transcriptional control of biosynthetic (acy-1/adenylate cyclase) and metabolic (pde-4/phosphodiesterase) regulators of cAMP in DD neurons by IRX-1/Iroquois, UNC-30/PITX and LIN-14. (B) In VD neurons, UNC-30/PITX and UNC-55/COUP-TF promote expression of pde-4/phosphodiesterase and antagonize expression of acy-1/adenylate cyclase to prevent cAMP levels from exceeding a critical threshold that triggers presynaptic remodeling. (C) cAMP promotes the elimination of ventral presynaptic vesicles (green) and the localization of dorsal synaptic vesicles (green) adjacent to clusters of the postsynaptic UNC-49 GABAergic receptors (blue) in dorsal muscles in remodeling DD neurons. cAMP levels are reduced by PDE-4/phosphodiesterase and elevated by the ACY-1/adenylate cyclase and OIG-1/One-Ig-domain transmembrane protein.
Presynaptic remodeling depends on microtubule dynamics
DD neurons adopt a ‘unipolar’ morphology in which a single neurite maintains axonal and dendritic compartments in separate locations (). Initially, in the early L1, the axonal neurotransmitter release machinery is restricted to a ventral region of the DD neurite proximal to the DD cell soma whereas the dendritic compartment is distally positioned in the dorsal segment of the DD neurite. With remodeling, these presynaptic (ventral) and postsynaptic (dorsal) domains exchange locations (; White et al., Citation1978). Interestingly, despite the switch in DD signaling polarity, microtubule (MT) orientation is not altered by remodeling. However, MT dynamics is elevated during this period and is required for DD remodeling (; Kurup et al., Citation2015).
Figure 6. Cellular regulators of synaptic remodeling. (A) Anterograde transport of synaptic vesicles by motor proteins Kinesin1/UNC-116 and Kinesin3/UNC-104 on microtubules (blue) to the anterior distal tip of the dorsal DD neurite is opposed by the retrograde motor complex of Dynein/DHC-1 and Dynactin/DNC-4 that relocates synaptic vesicles to the posterior DD neurite (blue). (B) Experiments with photoconverted Dendra2::RAB-3 demonstrated that RAB-3 from old synaptic terminals (magenta) can be relocated to new dorsal synapses in remodeling DD neurons. (C) Kinesin1/UNC-116 transports synaptic vesicles along microtubules (blue) in the DD commissure. (D) Stable microtubules (blue), intermediate filaments (brown) and the kinase TTBK-3 antagonize synaptic remodeling in tba-1(gf);dlk-1 double mutants (see text). (E) Cell-death pathway components, (EGL-1, CED-4, CED-3) associate with presynaptic mitochondria (yellow) to drive elimination of ventral synaptic terminals. (F) DLK-1 signaling promotes microtubule (blue) dynamics for synaptic remodeling.
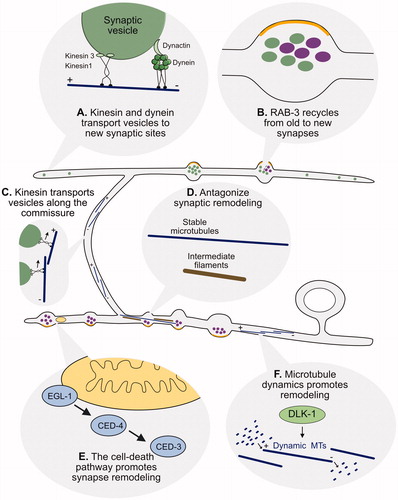
Most MTs in DD neurons adopt the ‘plus-end out’ orientation both before and after remodeling (; Kurup et al., Citation2015). ‘Plus-end’ refers to the MT end to which more α and β tubulin dimers are added during MT growth and removed during MT shrinkage (Baas & Lin, Citation2011). The resultant ‘dynamic instability’ of MTs is characteristically elevated during cell biological events (e.g. cell division) in which the MT cytoskeleton is actively reorganized (Gardner, Zanic, & Howard, Citation2013). Several lines of evidence indicate that DD remodeling depends on MT dynamics. First, genetic mutations that stabilize MTs block DD remodeling and this effect can be partially relieved by treatment with the MT depolymerizing drug, nocodazole (Kurup et al., Citation2015). Second, factors that regulate the transition from MT growth to shrinkage (i.e. ‘catastrophe’) such as the conserved kinase, DLK-1, and MT associated proteins, Kinesin-3/KLP-7 and Spastin/SPAS-1, promote DD remodeling (Kurup et al., Citation2015). Third, the MT stabilizing role of intermediate filaments antagonizes remodeling (; Kurup, Li, Goncharov, & Jin, Citation2018). A role for DLK-1 in synaptic remodeling is notable because DLK-1 also promotes axon regeneration in a cell biological mechanism that drives MT growth (Ghosh-Roy, Goncharov, Jin, & Chisholm, Citation2012). Additional unknown factors are likely required for activating MT dynamics in DD neurons, however, because a genetic ablation of DLK-1 activity results in only a slight delay in synaptic remodeling (Kurup et al., Citation2015).
The plus-end motors, UNC-116/Kinesin1 and UNC-104/Kinesin3 function together to deliver presynaptic components to the dorsal neurite during remodeling (; Kurup, Yan, Kono, & Jin, Citation2017; Park et al., Citation2011). Although MT dynamics is required for this kinesin-dependent function, the mechanistic basis for the effect is unknown (Kurup et al., Citation2015). Intriguingly, an optogenetic experiment suggests that at least some of the cargo delivered by UNC-104/Kinesin1 to the dorsal side may include recycled components of the presynaptic apparatus. In this study, the synaptic vesicle protein, RAB-3, was tagged with Dendra, a photoconvertible GFP, to confirm its translocation from disassembled ventral DD synapses to nascent dorsal synapses during remodeling (; Park et al., Citation2011). In the future, it will be interesting to determine if additional presynaptic components are also recycled for reassembly at new DD synapses and to delineate the cell biological mechanism of potential endocytic events that are likely involved. The essential role for motor-dependent trafficking in DD remodeling is underscored by the finding that the cyclin-dependent kinase, CDK-5, functions upstream of UNC-104 and promotes remodeling (Park et al., Citation2011). The molecular mechanism of CDK-5-dependent activation of UNC-104 is unknown.
The cell death pathway promotes remodeling of D-type motor neurons
Presynaptic Synaptobrevin/SNB-1 puncta are transiently localized to the axially projecting neurites of RME neurons in a remodeling event that mimics the sequential assembly and removal of DD presynaptic domains (L. Meng et al., Citation2015). The RME remodeling phenotype was exploited in a genetic screen that revealed that members of the canonical cell-death pathway are required to remove transient RME presynaptic terminals. Interestingly, the apoptotic pathway is also involved in the removal of ventral presynaptic terminals during DD remodeling (). Additional genetic and imaging experiments suggested that apoptotic components are delivered to the presynaptic domain in association with mitochondria (L. Meng et al., Citation2015) where CED-3/Caspase-mediated activation of the actin-severing protein, gelsolin, triggers presynaptic disassembly. Recent genetic results suggest that the apoptotic pathway may function in an activity-dependent mechanism of presynaptic disassembly that is triggered by transcriptionally regulated expression of the UNC-8/DEG/ENaC sodium channel subunit (; Miller-Fleming et al., Citation2016).
Regulation of postsynaptic remodeling in D-type motor neurons
Although both presynaptic and postsynaptic compartments are relocated in remodeling DD neurons (), little is known of the postsynaptic mechanism in part because a reliable markers for the postsynaptic apparatus, ACR-12 and LEV-10, were only recently identified (Cuentas-Condori et al., Citation2019; He, Cuentas-Condori, & Miller, Citation2019; Petrash et al., Citation2013). ACR-12 encodes an α-subunit of a heteromeric nicotinic acetylcholine receptor (nAChR) in GABAergic motor neurons that also contains UNC-29, UNC-38, UNC-63 and LEV-1 AChR subunits (Philbrook et al., Citation2018). GFP-marked ACR-12 and the auxiliary protein, LEV-10, localize to the postsynaptic compartments of DD and VD neurons in close apposition to presynaptic input from ventral cord cholinergic motor neurons (; Cuentas-Condori et al., Citation2019; He et al., Citation2019; Petrash et al., Citation2013; Philbrook et al., Citation2018). Initially, in early L1 larvae, ACR-12::GFP localizes to the dorsal DD neurite but then disappears as remodeling ensues and nascent ACR-12::GFP puncta emerge on the ventral side (; He et al., Citation2015).
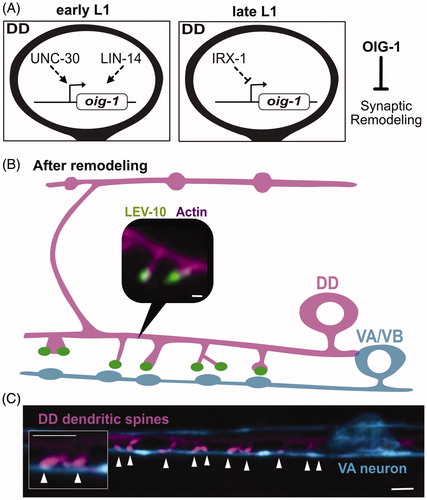
The Ig-domain protein, OIG-1, antagonizes DD synaptic remodeling
The translocation of dorsal ACR-12::GFP puncta to the ventral side is accelerated in oig-1 mutants (He et al., Citation2015; Howell et al., Citation2015). The oig-1 locus encodes a small protein of 137 amino acids with a single ImmunoGlobulin-(Ig) domain. OIG-1 is up-regulated by LIN-14 and the UNC-30/PITX transcription factor in early L1 larval DD neurons (Howell et al., Citation2015) but is turned off in the late L1 by IRX-1/Iroquois as the DD remodeling program is activated (; He et al., Citation2015). Thus, OIG-1 normally functions to antagonize DD remodeling and is repressed by the transcription factor IRX-1/Iroquois to prevent this effect. OIG-1 also appears to antagonize ectopic remodeling in VD neurons. OIG-1 is highly expressed in the VD neurons throughout development due to repression of IRX-1 by UNC-55/COUP-TF (He et al., Citation2015). OIG-1 expression in VD neurons may also depend on direct interaction of UNC-55 with the oig-1 promoter (Howell et al., Citation2015). In wild-type animals, ACR-12::GFP is exclusively localized to dorsal VD neurites but also appears on the ventral side in oig-1 mutants (He et al., Citation2015). Remarkably, genetic analysis indicates that OIG-1 also antagonizes remodeling of the presynaptic apparatus in both DD and VD neurons. For example, DD presynaptic markers (e.g. SNB-1::GFP) are precociously translocated to the ventral side in oig-1 mutants (He et al., Citation2015; Howell et al., Citation2015). Notably, OIG-1 is the only known downstream effector that regulates both presynaptic and postsynaptic remodeling.
Figure 7. Postsynaptic remodeling. (A) The One-Ig-domain protein, OIG-1, is upregulated by the PITX/UNC-30 transcription factor in early L1 larval DD neurons but turned off by Iroquois/IRX-1 during the late L1 to prevent OIG-1 from antagonizing synaptic remodeling. (B) Graphical representation of dendritic spines protruding from the ventral postsynaptic neurite of a DD neuron and contacting presynaptic terminals of cholinergic VA/VB neurons. Inset shows a fluorescent image of the actin marker, LifeAct::mCherry (magenta), and the postsynaptic protein, LEV-10 (green) at the spine tip. Scale bar = 200 nm. Adapted from (Cuentas-Condori et al., Citation2019) (C) Fluorescent image shows DD dendritic spines (magenta) projecting toward a presynaptic VA neuron (blue). Arrowheads denote sites of contact between postsynaptic spines and the VA process. Scale bar = 1 µm. Adapted from (Cuentas-Condori et al., Citation2019).
The OIG-1 mechanism of action is unclear. Although OIG-1 contains a canonical signal peptide and is secreted when over-expressed in transgenic animals (He et al., Citation2015; Howell et al., Citation2015), secretion is not required for its synaptic remodeling function (He et al., Citation2015); when expressed at native levels, the endogenous OIG-1 protein is not secreted and shows an intracellular location (He et al., Citation2019). An intracellular role is also consistent with the finding that OIG-1 expression in GABAergic neurons rescues the misplacement of ACR-12::GFP in oig-1 mutants whereas forced expression of OIG-1 in nearby cholinergic ventral cord motor neurons does not complement the oig-1 ectopic remodeling phenotype (He et al., Citation2015; Howell et al., Citation2015). The reported role of OIG-1 in limiting cAMP levels for presynaptic remodeling is similarly unknown (Yu et al., Citation2017).
Caenorhabditis elegans GABAergic neurons have functional dendritic spines
In addition to the replacement of presynaptic components with the ACR-12 postsynaptic receptor, the remodeling mechanism transforms the initially oblong DD axonal compartments into dendritic spines that protrude from the ventral DD neurite (; Cuentas-Condori et al., Citation2019; Philbrook et al., Citation2018). The possibility that DD neurons might display dendritic spines was first noted by John White et al. (Citation1978, Citation1976, Citation1986) in EM reconstructions of the ventral cord. This idea is notable because dendritic spines are specialized postsynaptic structures that detect neurotransmitter release from presynaptic neurons. In mammalian neurons, spine morphogenesis is dynamic and responsive to stimuli correlated with learning and memory (Hering, Sheng, & Medical, Citation2001).
Recent studies have confirmed that dendritic spines in C. elegans DD and VD GABAergic motor neurons display key hallmarks of mammalian spines as they (1) are structurally defined by a dynamic actin cytoskeleton, (2) localize postsynaptic proteins in apposition to excitatory presynaptic terminals (), (3) localize ER and ribosomes, (4) display Ca++ transients evoked by presynaptic activity and, (5) respond to activity-dependent signals that modulate spine density (Cuentas-Condori et al., Citation2019; Philbrook et al., Citation2018). Interestingly, postsynaptic spine formation and maintenance requires the trans-synaptic adhesion protein Neurexin/NRX-1, which functions in presynaptic cholinergic motor neurons. Surprisingly, this Neurexin/NRX-1-dependent effect does not require the canonical trans-synaptic interacting partner, the membrane protein neuroligin/NLG-1 and thus likely interacts with an alternative component that is currently unknown (Philbrook et al., Citation2018). The dramatic emergence of dendritic spines in remodeling DD neurons offers a unique opportunity to exploit the power of C. elegans genetics and live cell imaging to define evolutionarily ancient but shared mechanisms that drive spine morphogenesis.
Insights from synaptic remodeling in C. elegans
Sydney Brenner set out to understand how genes define the structure of the nervous system (Brenner, Citation1974). He selected C. elegans for this undertaking because he expected that its rapid life cycle would facilitate mutant analysis and its small size would allow the use of EM to define the wiring diagram. By design, his research strategy did not attempt to tackle directly the extraordinarily more difficult problem of unraveling how genes specify the brain. He chose instead to rely on the premise that all nervous systems are built with fundamental genetic programs and thus, that these secret plans could be divined much more easily in a nematode than in a more complex organism (Brenner, Citation1973).
As described above, DD motor neurons effectively reverse functional polarity with presynaptic (axonal) and postsynaptic (dendritic) compartments exchanging locations at opposite ends of each DD neuron (). Similar examples of polarity reversal are currently unknown in other organisms. Remarkably, however, for neurons in multiple species, the asymmetric features that distinguish dendritic vs axonal compartments can be reallocated in response to injury. Axotomy, for example, can result in the transformation of an existing dendrite into an axon both in cultured neurons (Dotti & Banker, Citation1987) and in a living organism (Whitington & Sink, Citation2004). In addition, synapses are extensively relocated in the developing mammalian nervous system (De Paola et al., Citation2006; Stettler, Yamahachi, Li, Denk, & Gilbert, Citation2006) and thus could depend on molecular pathways that also drive synaptic remodeling in DD neurons. Outlined below are additional examples of synaptic remodeling in more complex organisms for which studies of DD remodeling in C. elegans could be informative.
Remodeling of GABAergic neurons in the mammalian brain
In C. elegans, as in mammals, GABA-dependent inhibition determines circuit function (Lehmann, Steinecke, & Bolz, Citation2012; Pelkey et al., Citation2017; Schuske, Beg, & Jorgensen, Citation2004). Strong conservation of key molecular determinants of GABAergic function, including the GABA biosynthetic enzyme, GAD (UNC-25), vesicular GABA transporter, VGAT (UNC-47) and GABA ionotropic (UNC-49) and metabotropic (GBB-1/2) receptors highlight striking molecular similarities and suggest that developmental mechanisms that control GABA-dependent circuit refinement might also be conserved (Jin et al., Citation1999; Mclntire et al., Citation1993a; Citation1993b). GABAergic neurons constitute about 20–30% of the mammalian cortex, typically provide inhibitory input to glutamatergic neurons and are structurally and functionally diverse (Hendry, Schwark, & Jones, Citation1987; Pelkey et al., Citation2017; Sherwood et al., Citation2010). Similar to DD neurons, some mammalian GABAergic neurons receive excitatory input through dendritic spines and others innervate target cells through en-passant boutons (Kawaguchi, Karube, & Kubota, Citation2006; Pelkey et al., Citation2017). GABAergic interneurons can also be extensively refined during postnatal development. Of particular interest, the elimination of perisomatic inputs from GABAergic basket cells to glutamatergic pyramidal neurons depends on a mechanism that requires GABA signaling (Sullivan et al., Citation2018; Wu et al., Citation2012). The parallel role of GABA in promoting the removal of presynaptic termini in developing DD neurons in C. elegans could be indicative of shared cell biological pathways for synaptic remodeling (Miller-Fleming, Citation2016).
Activity-dependent removal of presynaptic domains in the mammalian visual circuit
In the developing mammalian visual circuit, retinal ganglion cells (RGCs) project to the thalamus to innervate geniculate neurons. Initially, presynaptic boutons are dispersed throughout each RGC axon to synapse with multiple geniculate targets. This florid pattern of connectivity is then refined in a mechanism that eliminates distal RGC boutons while clustering others at proximal locations (; Hong et al., Citation2014). Importantly, RGC axonal projections remain intact as boutons are relocated and are not retracted until a later, separate pruning step (Hong & Chen, Citation2011). Inputs to RGCs from rod Bipolar Cells (BCs) in the retina are also eliminated from stable axonal-dendritic contacts during development (; Morgan, Soto, Wong, & Kerschensteiner, Citation2011). Thus, synaptic remodeling within existing RGC and BC axons parallels refinement of the DD circuit in which presynaptic domains are repositioned to new locations within the DD neurite. As discussed above, at least one presynaptic protein, RAB-3, is recycled from old to new boutons in DD neurons (Park et al., Citation2011). It will be interesting to determine whether RGCs similarly recycle existing synaptic material from distal regions to the newly formed bouton clusters. This possibility seems plausible because a related phenomenon occurs in the mature mammalian nervous system in which presynaptic components can be actively exchanged between en-passant synapses (Tsuriel et al., Citation2006).
Figure 8. Presynaptic remodeling within intact axons in vertebrate circuits. (A) Initially, a Retinal Ganglion Cell (RGC) axon (black) innervates broadly multiple geniculate neurons in the thalamus (gray). Activity (lightning bolt) induces the relocation and clustering of RGC axons at proximal positions. (B) RGC dendrites (gray) receive input from Rod Bipolar cell axons (black). During early development, synaptic boutons (red) in Rod Bipolar Cells are eliminated while intact axonal trajectories are maintained. (C) Activity drives clustering of presynaptic boutons in the auditory circuit of barn owls. Normal (untrained) juveniles associate visual and auditory cues in the normal axonal region (gray box, arrowhead) where active synaptic boutons cluster (red). Prism-trained owls learn to associate auditory cues with an optically imposed object location. In this paradigm, active synaptic boutons (red) cluster in the adaptive region (gray box, arrowhead) whereas inactive boutons (black) remain in the normal region. After prisms are removed, active synaptic boutons (red) cluster at the normal region (gray box, arrowhead), whereas inactive boutons (black) remain in the adaptive zone.
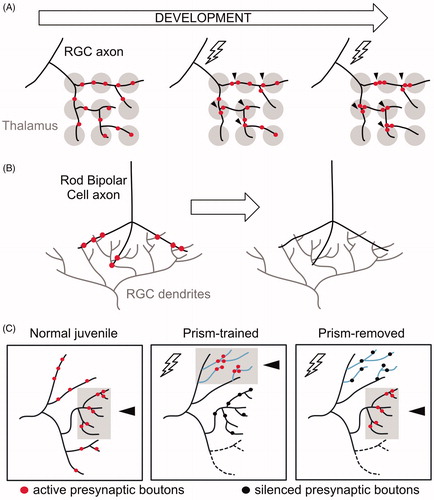
Synaptic remodeling in RGC axons is also activity-dependent as deprivation of sensory neuron input diminishes bouton clustering (Hong et al., Citation2014). Similarly, reduced synaptic activity impairs DD remodeling by delaying both formation of new synapses in the dorsal DD neurite (Thompson-Peer et al., Citation2012) as well as the elimination of old presynaptic domains on the ventral side (Miller-Fleming et al., Citation2016). The activity-dependent effect on DD presynaptic disassembly depends on the cell-autonomous roles of the UNC-8/DEG/ENaC cation channel and the serine-threonine phosphatase TAX-6/Calcineurin which function together to elevate intracellular Ca++ (Miller-Fleming et al., Citation2016). It will be interesting to determine if similar components direct synaptic remodeling in RGCs.
Altered behavior in the barn owl involves the reallocation of the presynaptic apparatus
Anatomical and functional studies of the barn owl auditory localization pathway provide additional examples of activity-dependent remodeling that involves the coincident assembly of synapses in new locations as others are removed (). Juvenile owls, fitted with prisms that distort the visual field, learn to associate auditory cues with the imposed new optical location (Knudsen & Knudsen, Citation1989). This phenomenon is correlated with the expansion of axonal arbors into receptive fields associated with the learned behavior (Debello, Feldman, & Knudsen, Citation2001). Clustering of presynaptic boutons in these new adaptive zones is also enhanced in comparison to the normal receptive field but the overall number of synapses in each region is not significantly different. Thus, this mechanism appears to have effectively reduced the separation between adjacent presynaptic domains by balancing nascent assembly with synaptic elimination in nearby regions (Debello et al., Citation2001; Mcbride, Rodriguez-Contreras, Trinh, Bailey, & Debello, Citation2008). Notably, synaptic remodeling in the C. elegans GABAergic circuit also involves the elimination of established synapses paired with assembly of presynaptic boutons in new locations (). Interestingly, prism-trained owls retain the capacity to associate auditory cues with the normal visual field after the prisms are removed. This finding suggests that the adaptive synaptic clusters, which are maintained in trained animals, are functionally silenced with the restoration of normal visual cues (Mcbride & Debello, Citation2015). We speculate that this example of synaptic silencing in the barn owl auditory circuit could be potentially accomplished as in the C. elegans GABAergic neurons by the selective disassembly of key components such as ELKS or Munc13 that are required for neurotransmitter release but are not needed for the maintenance of synaptic structure (; Liu et al., Citation2014; Miller-Fleming et al., Citation2020; Varoqueaux et al., Citation2002). In the case of the barn owl, this surgically precise mechanism could facilitate an adaptive response to temporal cues while also maintaining the long-term capacity to restore normal visual input (Mcbride & Debello, Citation2015).
Conclusions and future prospects
Sydney Brenner was a supreme optimist. After concluding that all of the major questions about gene expression had been answered, he set out to deduce how genes determine behavior (Brenner, Citation1973). To simplify the problem of acquiring this information, he selected a small organism with a limited number of neurons and facile genetics. Although EM reconstruction ultimately revealed the complete wiring diagram of the C. elegans nervous system and, genetic analysis detected hundreds of ‘unc’ mutants that altered movement (Brenner, Citation1974; White et al., Citation1986), Brenner’s original goal of deducing the genomic logic of behavior has yet to be realized. Nevertheless, the tools that he developed and his vision of how they could be employed, inspired an army of enthusiastic peers (Emmons, et al., Citation2015). Here we have featured their studies of synaptic plasticity, a dynamic facet of the nervous system that tunes circuit function. We have limited our focus to studies of a developmentally regulated remodeling event that alters the architecture of DD class GABAergic motor neurons in the C. elegans ventral nerve cord. Remarkably, DD presynaptic and postsynaptic domains swap locations at opposite ends of the DD neurite with no apparent alterations in external DD morphology during early development (; Hallam & Jin, Citation1998; White et al., Citation1978). The stereotypical timing of DD remodeling is indicative of a genetic program and multiple transcription factors that regulate DD rewiring have been identified (). In fact, two of these transcription factors, UNC-30/PITX and UNC-55/COUP-TF, were initially detected as movement-defective alleles in Brenner’s original genetic screen (Brenner, Citation1974; Howell et al., Citation2015; Shan et al., Citation2005). Although DD rewiring is subject to genetic control, the developmentally regulated translocation of the DD presynaptic domain is accelerated by neuron activity (Miller-Fleming et al., Citation2016; Thompson-Peer et al., Citation2012). Because the mechanism of this effect involves the voltage-gated calcium channel UNC-2/VGCC and the calcium-activated phosphatase, TAX-6/Calcineurin, elevated intracellular calcium is an attractive choice for a likely driver of presynaptic disassembly. Genetic experiments suggest that downstream calcium-dependent effects could target a canonical apoptotic pathway (Miller-Fleming et al., Citation2016). The reported involvement of the CED-3/caspase-activated actin-severing protein, gelsolin, points to a critical role for the actin cytoskeleton in presynaptic remodeling but the mechanism of this effect is unknown (L. Meng et al., Citation2015). Recent studies revealed the surprising finding that the activity-dependent remodeling pathway targets a subset of presynaptic components. At least two key regulators of neurotransmitter release, UNC-13/Munc13 and ELKS (Liu et al., Citation2014; Varoqueaux et al., Citation2002), are instead disassembled by a separate parallel-acting pathway regulated by the homeodomain transcription factor, IRX-1/Iroquois (; Miller-Fleming et al., Citation2020). The existence of distinct disassembly pathways is indicative of specific molecular interactions that selectively eliminate specific presynaptic components. RNA-Seq profiling experiments to identify IRX-1/Iroquois targets (Spencer et al., Citation2014; Taylor et al., Citation2019) could be useful for delineating the biochemical mechanism of these effects. This approach could also be useful for delineating the mechanism of postsynaptic remodeling, which is also regulated by IRX-1/Iroquois, but has not been extensively investigated. Of particular interest is the question of whether known regulators of presynaptic remodeling () are also involved in dismantling the postsynaptic apparatus (). Only one downstream effector is known to regulate remodeling of both presynaptic and postsynaptic DD compartments, the small single-Ig domain protein OIG-1, but its role is mysterious and requires further investigation (He et al., Citation2015, Citation2019; Howell et al., Citation2015). Finally, future studies should continue to exploit an additional key strength of C. elegans as an experimental organism that was also recognized by Sydney Brenner; worms are transparent and small enough to fit on a microscope slide. One of the major challenges of delineating mechanisms that regulate synaptic dynamics in mammals () is the relative inaccessibility of developing circuits to live cell imaging (Südhof, Citation2018). By using new methods of bright, cell-specific fluorescent labeling of native proteins, it should be possible to monitor the dynamics of synaptic destruction and reassembly in time-lapse imaging experiments that avoid potential artifacts arising from multicopy transgenic arrays (He et al., Citation2019; Hefel & Smolikove, Citation2019; Schwartz & Jorgensen, Citation2016).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Afroz, S., Parato, J., Shen, H., & Smith, S.S. (2016). Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABA A receptors on dendritic spines. eLife, 5, e15106. doi:10.7554/eLife.15106
- Baas, P.W., & Lin, S. (2011). Hooks and comets : The story of microtubule polarity orientation in the neuron. Developmental Neurobiology, 71(6), 403–418. doi:10.1002/dneu.20818
- Babu, M.M., Luscombe, N.M., Aravind, L., Gerstein, M., & Teichmann, S.A. (2004). Structure and evolution of transcriptional regulatory networks. Current Opinion in Structural Biology, 14(3), 283–291. doi:10.1016/j.sbi.2004.05.004
- Brenner, S. (1973). The genetics of behaviour. British Medical Bulletin, 29(3), 269–271. doi:10.1093/oxfordjournals.bmb.a071019
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics, 77 (1), 71–94.
- Bujalka, H., Koenning, M., Jackson, S., Perreau, V.M., Pope, B., Hay, C.M., … Emery, B. (2013). MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biology, 11(8), e1001625. doi:10.1371/journal.pbio.1001625
- Cavodeassi, F., Modolell, J., & Gómez-Skarmeta, J.L. (2001). The Iroquois family of genes: From body building to neural patterning. Development (Cambridge, England), 128 (15), 2847–2855.
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W., & Prasher, D. (1994). Green fluorescent protein as a marker for gene expression. Science, 263(5148), 802–805. doi:10.1126/science.8303295
- Cinar, H., Keles, S., & Jin, Y. (2005). Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Current Biology : CB, 15 (4), 340–346. https://doi.org/10.1016/j. doi:10.1016/j.cub.2005.02.025
- Cuentas-Condori, A., Mulcahy, B., He, S., Palumbos, S., Zhen, M., & Miller, D.M. (2019). C. elegans neurons have functional dendritic spines. eLife, 8, e47918. doi:10.7554/eLife.47918
- De Paola, V., Holtmaat, A., Knott, G., Song, S., Wilbrecht, L., Caroni, P., & Svoboda, K. (2006). Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron, 49 (6), 861–875. doi:10.1016/j.neuron.2006.02.017
- Debello, W.M., Feldman, D.E., & Knudsen, E.I. (2001). Adaptive axonal remodeling in the midbrain auditory space map. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 21(9), 3161–3174. doi:10.1523/JNEUROSCI.21-09-03161.2001
- Deidda, G., Allegra, M., Cerri, C., Naskar, S., Bony, G., Zunino, G., … Cancedda, L. (2015). Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nature Neuroscience, 18(1), 87–96. doi:10.1038/nn.3890
- Dotti, C., & Banker, G. (1987). Experimentally induced alteration in the polarity of developin neurons. Nature, 330(6145), 254–256. doi:10.1038/330254a0
- Eastman, C., Horvitz, H.R., & Jin, Y. (1999). Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. The Journal of Neuroscience, 19(15), 6225–6234. doi:10.1523/JNEUROSCI.19-15-06225.1999
- Emmons, S.W. (2015). The beginning of connectomics: A commentary on White et al. (1986) ‘The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1666), 20140309. doi:10.1098/rstb.2014.0309
- Gardner, M.K., Zanic, M., & Howard, J. (2013). Microtubule catastrophe and rescue. Current Opinion in Cell Biology, 25(1), 14–19. doi:10.1016/j.ceb.2012.09.006
- Ghosh-Roy, A., Goncharov, A., Jin, Y., & Chisholm, A.D. (2012). Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Developmental Cell, 23(4), 716–728. doi:10.1016/j.devcel.2012.08.010
- Ghosh-Roy, A., Wu, Z., Goncharov, A., Jin, Y., & Chisholm, A.D. (2010). Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(9), 3175–3183. doi:10.1523/JNEUROSCI.5464-09.2010
- Hallam, S.J., & Jin, Y. (1998). lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature, 395(6697), 78–82. doi:10.1038/25757
- Halls, M.L., & Cooper, D.M.F. (2011). Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harbor Perspectives in Biology, 3(1), a004143. doi:10.1101/cshperspect.a004143
- Han, B., Bellemer, A., & Koelle, M.R. (2015). An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans. Genetics, 199 (4), 1159–1172. doi:10.1534/genetics.114.173963
- Hardy, P.A. (1990). Genetic aspects of nervous system development. Journal of Neurogenetics, 6(3), 115–131. doi:10.3109/01677069009107105
- He, S., Cuentas-Condori, A., & Miller, D.M. (2019). NATF (Native and Tissue-Specific Fluorescence): A strategy for brigth, tissue-specific GFP labeling of native proteins in Caenorhabditis elegans. Genetics, 212(2), 387–395. doi:10.1534/genetics.119.302063
- Hefel, A., & Smolikove, S. (2019). Tissue-specific split sfGFP system for streamlined expression of GFP tagged proteins in the Caenorhabditis elegans germline. G3 (Bethesda, Md.), 9(6), 1933–1943. doi:10.1534/g3.119.400162
- Hendi, A., Kurashina, M., & Mizumoto, K. (2019). Intrinsic and extrinsic mechanisms of synapse formation and specificity in C. elegans. Cellular and Molecular Life Sciences, 76(14), 2719–2738. doi:10.1007/s00018-019-03109-1
- Hendry, S.H.C., Schwark, H.D., & Jones, E.G. (1987). Numbers and proportions of GABA-immunoreactive different areas of monkey cerebral cortex neurons. Journal of Neuroscience, 7, 1503–1519.
- Hensch, T.K., Fagiolini, M., Mataga, N., Stryker, M.P., Baekkeskov, S., & Kash, S.F. (1998). Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science (New York, N.Y.), 282(5393), 1504–1509. doi:10.1126/science.282.5393.1504
- He, S., Philbrook, A., McWhirter, R., Gabel, C.V., Taub, D.G., Carter, M.H., … Miller, D.M. (2015). Transcriptional control of synaptic remodeling through regulated expression of an immunoglobulin superfamily protein. Current Biology : CB, 25(19), 2541–2548. doi:10.1016/j.cub.2015.08.022
- Hering, H., Sheng, M., & Medical, H.H. (2001). Dendritic spines : Structure, dynamics and regulaion. Nature Reviews Neuroscience, 2(12), 880–888. doi:10.1038/35104061
- Hong, Y.K., & Chen, C. (2011). Wiring and rewiring of the retinogeniculate synapse. Current Opinion in Neurobiology, 21(2), 228–237. doi:10.1016/j.conb.2011.02.007
- Hong, Y.K., Park, S.H., Litvina, E.Y., Morales, J., Sanes, J.R., & Chen, C. (2014). Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron, 84(2), 332–339. doi:10.1016/j.neuron.2014.08.059
- Houweling, A.C., Dildrop, R., Peters, T., Mummenhoff, J., Moorman, A.F., Rüther, U., & Christoffels, V.M. (2001). Gene and cluster-specific expression of the Iroquois family members during mouse development. Mechanisms of Development, 107 (1-2), 169–174. doi:10.1016/S0925-4773(01)00451-8
- Howell, K., White, J.G., & Hobert, O. (2015). Spatiotemporal control of a novel synaptic organizer molecule. Nature, 523(7558), 83–87. doi:10.1038/nature14545
- Hristova, M., Birse, D., Hong, Y., & Ambros, V. (2005). The Caenorhabditis elegans heterochronic regulator LIN-14 is a novel transcription factor that controls the developmental timing of transcription from the insulin/insulin-like growth factor gene ins-33 by direct DNA binding. Molecular and Cellular Biology, 25(24), 11059–11072. doi:10.1128/MCB.25.24.11059-11072.2005
- Jin, Y., Hoskins, R., & Horvitz, H.R. (1994). Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature, 372(6508), 780–783. doi:10.1038/372780a0
- Jin, Y., Jorgensen, E., Hartwieg, E., & Horvitz, H.R. (1999). The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 19(2), 539–548. doi:10.1523/JNEUROSCI.19-02-00539.1999
- Jin, Y., & Qi, Y.B. (2018). Building stereotypic connectivity: Mechanistic insights into structural plasticity from C. elegans. Current Opinion in Neurobiology, 48, 97–105. doi:10.1016/j.conb.2017.11.005
- Jorgensen, E.M., Hartwieg, E., Schuske, K., Nonet, M.L., Jin, Y., & Horvitz, H.R. (1995). Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature, 378(6553), 196–199. doi:10.1038/378196a0
- Kang, E., Durand, S., Leblanc, J.J., Hensch, T.K., Chen, C., & Fagiolini, M. (2013). Visual acuity development and plasticity in the absence of sensory experience. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(45), 17789–17796. doi:10.1523/JNEUROSCI.1500-13.2013
- Kawaguchi, Y., Karube, F., & Kubota, Y. (2006). Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cerebral Cortex (New York, N.Y. : 1991), 16(5), 696–711. doi:10.1093/cercor/bhj015
- Knudsen, E.I., & Knudsen, F. (1989). Vision calibrates sound localization in developing barn owls. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 9(9), 3306–3313. doi:10.1523/JNEUROSCI.09-09-03306.1989
- Koch, S.M., Dela Cruz, C.G., Hnasko, T.S., Edwards, R.H., Huberman, A.D., & Ullian, E.M. (2011). Pathway-specific genetic attenuation of glutamate release alters select features of competition-based visual circuit refinement. Neuron, 71(2), 235–242. doi:10.1016/j.neuron.2011.05.045
- Kurup, N., & Jin, Y. (2016). Neural circuit rewiring: Insights from DD synapse remodeling. Worm, 5(1), e1129486. doi:10.1080/21624054.2015.1129486
- Kurup, N., Li, Y., Goncharov, A., & Jin, Y. (2018). Intermediate filament accumulation can stabilize microtubules in Caenorhabditis elegans motor neurons. Proceedings of the National Academy of Sciences, 115(12), 3114–3119. doi:10.1073/pnas.1721930115
- Kurup, N., Yan, D., Goncharov, A., & Jin, Y. (2015). Dynamic microtubules drive circuit rewiring in the absence of neurite remodeling. Current Biology : CB, 25(12), 1594–1605. doi:10.1016/j.cub.2015.04.061
- Kurup, N., Yan, D., Kono, K., & Jin, Y. (2017). Differential regulation of polarized synaptic vesicle trafficking and synapse stability in neural circuit rewiring in Caenorhabditis elegans. PLOS Genetics, 13(6), e1006844. doi:10.1371/journal.pgen.1006844
- Lee, H.K., Kameyama, K., Huganir, R.L., & Bear, M.F. (1998). NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron, 21(5), 1151–1162. doi:10.1016/S0896-6273(00)80632-7
- Lehmann, K., Steinecke, A., & Bolz, J. (2012). GABA through the ages: Regulation of cortical function and plasticity by inhibitory interneurons. Neural Plasticity, 2012, 892784. doi:10.1155/2012/892784
- Liu, C., Bickford, L.S., Held, R.G., Nyitrai, H., Su, T.C., & Kaeser, P.S. (2014). The active zone protein family ELKS supports Ca2+ influx at nerve terminals of inhibitory hippocampal neurons. The Journal of neuroscience : The official journal of the Society for Neuroscience, 34(37), 12289–12303. doi:10.1523/JNEUROSCI.0999-14.2014
- Mcbride, T.J., & Debello, W.M. (2015). Input clustering in the normal and learned circuits of adult barn owls. Neurobiology of Learning and Memory, 121, 39–51. doi:10.1016/j.nlm.2015.01.011
- Mcbride, T.J., Rodriguez-Contreras, A., Trinh, A., Bailey, R., & Debello, W.M. (2008). Learning drives differential clustering of axodendritic contacts in the barn owl auditory system. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(27), 6960–6973. doi:10.1523/JNEUROSCI.1352-08.2008
- Mclntire, S.L., Jorgensen, E., & Horvitz, H.R. (1993a). Genes required for GABA function in Caenorhabditis elegans. Nature, 364(6435), 334–337. doi:10.1038/364334a0
- Mclntire, S.L., Jorgensen, E., Kaplan, J., & Horvitz, H.R. (1993b). The GABAergic nervous system of C. elegans. Nature, 364(6435), 337–414. doi:10.1038/364337a0
- Mello, C., Kramer, J., Stinchcomb, D., & Ambros, V. (1991). Efficient gene transfer in C. elegans: Extrahcormosomal maintenance and integration of transforming sequences. The Embo Journal, 10(12), 3959–3970. doi:10.1002/j.1460-2075.1991.tb04966.x
- Meng, J., Ma, X., Tao, H., Jin, X., Witvliet, D., Mitchell, J., … Qi, Y.B. (2017). Myrf ER-bound transcription factors drive C. elegans synaptic plasticity via cleavage-dependent nuclear translocation. Developmental Cell, 41(2), 180–194.e7. doi:10.1016/j.devcel.2017.03.022
- Meng, L., Mulcahy, B., Cook, S.J., Neubauer, M., Wan, A., Jin, Y., & Yan, D. (2015). The cell death pathway regulates synapse elimination through cleavage of gelsolin in Caenorhabditis elegans neurons. Cell Reports, 11(11), 1737–1748. doi:10.1016/j.celrep.2015.05.031
- Miller-Fleming, T.W. (2016). Molecular dissection of synaptic remodeling in GABAergic neurons.
- Miller-Fleming, T.W., Cuentas-Condori, A., Palumbos, S., Manning, L., Richmond, J.R., & Miller, D.M. (2020). Transcriptional control of parallel-acting pathways that remove discrete presynaptic proteins in remodeling neurons. BioRxiv.
- Miller-Fleming, T.W., Petersen, S.C., Manning, L., Matthewman, C., Gornet, M., Beers, A., … Miller, D.M. (2016). The DEG/ENaC cation channel protein UNC-8 drives activity-dependent synapse removal in remodeling GABAergic neurons. eLife, 5, e14599. doi:10.7554/eLife.14599
- Morgan, J.L., Soto, F., Wong, R.O.L., & Kerschensteiner, D. (2011). Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron, 71(6), 1014–1021. doi:10.1016/j.neuron.2011.08.025
- Nonet, M.L. (1999). Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. Journal of Neuroscience Methods, 89(1), 33–40. doi:10.1016/S0165-0270(99)00031-X
- Nonet, M.L., Saifee, O., Zhao, H., Rand, J.B., & Wei, L. (1998). Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 18(1), 70–80. doi:10.1523/JNEUROSCI.18-01-00070.1998
- Orr, B.O., Gorczyca, D., Younger, M.A., Jan, L.Y., Jan, Y.N., & Davis, G.W. (2017). Composition and control of a Deg/ENaC channel during presynaptic homeostatic plasticity. Cell Reports, 20(8), 1855–1866. doi:10.1016/j.celrep.2017.07.074
- Park, M., Watanabe, S., Poon, V.Y.N., Ou, C.Y., Jorgensen, E.M., & Shen, K. (2011). CYY-1/Cyclin Y and CDK-5 differentially regulate synapse elimination and formation for rewiring neural circuits. Neuron, 70(4), 742–757. doi:10.1016/j.neuron.2011.04.002
- Pelkey, K.A., Chittajallu, R., Craig, M.T., Tricoire, L., Wester, J.C., & Mcbain, X.C.J. (2017). Hippocampal GABAergic inhibitory interneurons. Physiological Reviews, 97 (4), 1619–1747. doi:10.1152/physrev.00007.2017
- Petersen, S.C., Watson, J.D., Richmond, J.E., Sarov, M., Walthall, W.W., & Miller, D.M. (2011). A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(43), 15362–15375. doi:10.1523/JNEUROSCI.3181-11.2011
- Petrash, H.A., Philbrook, A., Haburcak, M., Barbagallo, B., & Francis, M.M. (2013). ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(13), 5524–5532. doi:10.1523/JNEUROSCI.4384-12.2013
- Philbrook, A., Ramachandran, S., Lambert, C.M., Oliver, D., Florman, J., Alkema, M.J., … Francis, M.M. (2018). Neurexin directs partner-specific synaptic connectivity in C. elegans. eLife, 7, e35692. doi:10.7554/eLife.35692
- Ruvkun, G., & Giusto, J. (1989). The Caenorhabditis elegans heterochronic gene lin-14 encodes a nuclear protein that forms a temporal developmental switch. Nature, 338(6213), 313–319. doi:10.1038/338313a0
- Sanderson, J.L., Gorski, J.A., Gibson, E.S., Lam, P., Freund, R.K., Chick, W.S., & Dell'Acqua, M.L. (2012). Akap150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(43), 15036–15052. doi:10.1523/JNEUROSCI.3326-12.2012
- Schuske, K., Beg, A.A., & Jorgensen, E.M. (2004). The GABA nervous system in C. elegans. Trends in Neurosciences, 27(7), 407–414. doi:10.1016/j.tins.2004.05.005
- Schwartz, M.L., & Jorgensen, E.M. (2016). SapTrap, a toolkit for high-throughput CRISPR/Cas9 gene modification in Caenorhabditis elegans. Genetics, 202(4), 1277–1288. doi:10.1534/genetics.115.184275
- Shan, G., Kim, K., Li, C., & Walthall, W.W. (2005). Convergent genetic programs regulate similarities and differences between related motor neuron classes in Caenorhabditis elegans. Developmental Biology, 280(2), 494–503. doi:10.1016/j.ydbio.2005.01.032
- Sherwood, C.C., Raghanti, M.A., Stimpson, C.D., Spocter, M.A., Uddin, M., Boddy, A.M., … Hof, P.R. (2010). Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proceedings. Biological Sciences, 277(1684), 1011–1020. doi:10.1098/rspb.2009.1831
- Shreffler, W., & Wolinsky, E. (1997). Genes controlling ion permeability in both motorneurons and muscle. Behavior Genetics, 27(3), 211–221. doi:10.1023/A:1025605929373
- Spencer, W.C., McWhirter, R., Miller, T., Strasbourger, P., Thompson, O., Hillier, L.W., … Miller, D.M. (2014). Isolation of specific neurons from C. elegans larvae for gene expression profiling. PLoS One., 9(11), e112102. doi:10.1371/journal.pone.0112102
- Stettler, D.D., Yamahachi, H., Li, W., Denk, W., & Gilbert, C.D. (2006). Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron, 49(6), 877–887. doi:10.1016/j.neuron.2006.02.018
- Südhof, T. (2018). Towards an understanding of synapse formation. Neuron, 100(2), 276–293. doi:10.1016/j.neuron.2018.09.040
- Sullivan, C.S., Gotthard, I., Wyatt, E.V., Bongu, S., Mohan, V., Weinberg, R.J., & Maness, P.F. (2018). Perineuronal net protein neurocan inhibits NCAM/EphA3 repellent signaling in GABAergic interneurons. Scientific Reports, 8(1), 1–15. doi:10.1038/s41598-018-24272-8
- Sulston, J.E. (1976). Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London, 275(938), 287–297.
- Sulston, J.E., & Horvitz, H.R. (1977). Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental Biology, 56 (1), 110–156. doi:10.1016/0012-1606(77)90158-0
- Taylor, S., Santpere, G., Reilly, M., Glenwinkel, L., Poff, A., McWhirther, R., … Miller, D.M. III, (2019). Expression profiling of the mature C. elegans nervous system by single-cell RNA-Sequencing. BioRxiv.
- Thompson-Peer, K.L., Bai, J., Hu, Z., & Kaplan, J. (2012). HBL-1 patterns synaptic remodeling in C. elegans. Neuron, 73(3), 453–465. doi:10.1016/j.neuron.2011.11.025
- Tsuriel, S., Geva, R., Zamorano, P., Dresbach, T., Boeckers, T., Gundelfinger, E.D., … Ziv, N.E. (2006). Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLoS Biology, 4(9), e271. doi:10.1371/journal.pbio.0040271
- Varoqueaux, F., Sigler, A., Rhee, J., Brose, N., Enk, C., Reim, K., & Rosenmund, C. (2002). Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proceedings of the National Academy of Sciences, 99(13), 9037–9042. doi:10.1073/pnas.122623799
- Walthall, W.W., & Plunkett, J.A. (1995). Genetic transformation of the synaptic pattern of a motoneuron class in Caenorhabditis elegans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 15(2), 1035–1043. doi:10.1523/JNEUROSCI.15-02-01035.1995
- Wang, Y., Matthewman, C., Han, L., Miller, T., Miller, D.M., & Bianchi, L. (2013). Neurotoxic unc-8 mutants encode constitutively active DEG/ENaC channels that are blocked by divalent cations. The Journal of General Physiology, 142(2), 157–169. doi:10.1085/jgp.201310974
- White, J.G., Albertson, D.G., & Anness, M. (1978). Connectivity changes in a class of motoneurone during the development of a nematode. Nature, 271 (5647), 764–766. doi:10.1038/271764a0
- White, J.G., Southgate, E., Thomson, J.N., & Brenner, S. (1976). The structure of the ventral nerve cord of Caenorhadbitis elegans. Philosophical Transactions of the Royal Society of London, 275:327–348.
- White, J.G., Southgate, E., Thomson, J.N., & Brenner, S. (1986). The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 314(1165), 1–340. doi:10.1098/rstb.1986.0056
- Whitington, P.M., & Sink, H. (2004). Development of a polar morphology by identified embryonic motoneurons. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience, 22 (1), 39–45. doi:10.1016/j.ijdevneu.2003.10.004
- Winder, D.G., Mansuy, I.M., Osman, M., Moallem, T.M., & Kandel, E.R. (1998). Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell, 92(1), 25–37. doi:10.1016/S0092-8674(00)80896-X
- Wu, X., Fu, Y., Knott, G., Lu, J., Di Cristo, G., & Huang, Z.J. (2012). GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(1), 331–343. doi:10.1523/JNEUROSCI.3189-11.2012
- Younger, M.A., Mu, M., Tong, A., Pym, E.C., & Davis, G.W. (2013). A Presynaptic ENaC Channel Drives Homeostatic Plasticity. Neuron, 79 (6), 1183–1196. doi:10.1016/j.neuron.2013.06.048
- Yu, B., Wang, X., Wei, S., Fu, T., Dzakah, E.E., Waqas, A., … Shan, G. (2017). Convergent Transcriptional Programs Regulate cAMP Levels in C. elegans GABAergic Motor Neurons. Developmental Cell, 43 (2), 212–215. doi:10.1016/j.devcel.2017.09.013
- Zhou, H., & Walthall, W. (1998). UNC-55, an Orphan Nuclear Hormone Receptor, Orchestrates Synaptic Specificity among Two Classes of Motor Neurons in Caenorhabditis elegans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 18(24), 10438–10444. doi:10.1523/JNEUROSCI.18-24-10438.1998
