Abstract
Vascular smooth muscle cell proliferation has been known to be predominant in vascular remodeling of pulmonary hypertensive. The GATA family proteins, a group of zinc finger transcription factors, play an important role during cell proliferation. The aim of present study was to investigate the expression of GATA-6 gene in experimental pulmonary hypertensive rats and explore the effect of regulation of GATA-6 expression by simvastatin on pulmonary vascular remodeling. The male Sprague-Dawley rats model was established with receiving pneumonectomy and monocrotaline (MCT) administration. Right pulmonary artery remodeling in these animals was compared with untreated rats or rats receiving simvastatin. The level of GATA-6 mRNA and protein expression was detected by reverse transcriptase–polymerase chain reaction (RT-PCR) and Western blotting, respectively. Pneumonectomized, MCT-treated rats had significantly increased mean pulmonary arterial pressure (mPAP), RV/(LV + S) ratio (ratio of the right ventricular to left ventricular and septum weights), vascular occlusion scores (VOSs), and percent media wall thickness on day 35, all the indices were significantly decreased after simvastatin administration in these rats. The level of GATA-6 mRNA and protein were markedly decreased in these pneumonectomy and MCT-treated rats, and they were significantly up-regulated in these rats after receiving simvastatin. These results indicate that the development and progression of pulmonary hypertension is prevented by simvastatin by up-regulating GATA-6 expression in the lung tissue.
Hypertensive pulmonary vascular disease is characterized by pulmonary vascular remodeling, involving abnormal proliferation of vascular endothelial and smooth muscle cells and extracellular matrix deposition, leading to occlusion of pulmonary arterioles, pulmonary hypertension, right ventricular failure, and death. Pulmonary arterial endothelial cell injury are considered prerequisites for the induction of advanced vascular remodeling in pulmonary arterial hypertension [Citation[1], Citation[2], Citation[3], Citation[4]]. Migration, proliferation, and matrix synthesis of pulmonary artery smooth muscle cells play a key role in pulmonary vascular remodeling. Normal adult pulmonary arterial smooth muscle cells do not migrate, grow, or synthesize matrix once normal development is completed. Dysfunction of endothelium and elevated shear stress may result in smooth muscle cells proliferation, migration, and matrix synthesis. Increase in shear stress due to high flow modulates production of platelet-derived growth factor, transforming growth factor-β, nitric oxide, and transcription factors of the GATA family that regulate cell growth, migration, and matrix synthesis [Citation[5], Citation[6]].
The GATA family proteins are a group of zinc finger transcription factors that play an important role during mammalian organ morphogenesis, cell proliferation, and sex differentiation [Citation[7]]. These factors bind to a consensus DNA motif (A/T)GATA(A/G) in the promoters of target genes in a variety of tissues [Citation[7]]. Six GATA family members have been identified in vertebrates and can be divided into 2 groups based on their tissue distribution and homology: GATA-1, -2, and -3 and GATA-4, -5, and -6 [Citation[8]]. GATA-1, -2, and -3 genes are predominantly expressed in hematopoietic cells where they are involved in proliferation and differentiation of several cell lineages [Citation[9], Citation[10], Citation[11], Citation[12]]. GATA-4, -5, and -6 are expressed in heart, liver, lungs, and the gastrointestinal tract where they mediate tissue-specific gene expression, and GATA-6 is the only member of the GATA family expressed in vascular smooth muscle cells (VSMCs) [Citation[13], Citation[14], Citation[15], Citation[16], Citation[17]].
Analysis of vascular smooth muscle cells has demonstrated an important role of GATA-6 in regulating cell proliferation in response to mitogenic or mechanical stimulation. GATA-6 mRNA levels are down-regulated in proliferating vascular smooth muscle cells, suggesting that GATA-6 expression is linked to the cell cycle in these cells and plays as an important regulator of the VSMC phenotype [Citation[18], Citation[19], Citation[20]]. More specifically, forced expression of GATA-6 in vascular smooth muscle cells induces growth arrest through a mechanism involving enhanced expression of the cyclin-dependent kinase inhibitor p21 [Citation[20], Citation[21]]. In vivo, adenovirus- mediated gene transfer of GATA-6 in balloon-injured carotid arteries prevented vessel lesions associated with vascular smooth muscle cell phenotypic modulation [Citation[21]]. Interestingly, laminar shear stress was recently demonstrated to activate transcription factor GATA-6 [Citation[22]], which binds to a GATA consensus element located in the region of downstream gene promoter regulating these gene expression. A study in vitro reveals that GATA-6 regulates a set of genes associated with synthetic SMC functions and suggests that this transcriptional pathway may be independent from myocardin-induced SMC differentiation in VSMCs. An unbiased microarray screen of genes regulated by GATA-6 in VSMCs identifies that endothelin-1 and angiotensin 1a (AT1a) receptor genes are direct GATA-6 target genes [Citation[23]]. Based on these studies, we speculate that activity of transcription factor GATA-6 may be regulated by disturbed laminar shear stresses occurring in pulmonary hypertension.
The 3-hydroxymethyl-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, statins, improve cardiovascular outcomes independent of their effects on cholesterol reduction [Citation[24]]. The immunosuppressive and anti-inflammatory properties of statins [Citation[25]] may contribute to the improved survival of patients with atherosclerosis [Citation[24], Citation[26]]. Statins can suppress endothelial and vascular smooth muscle cell proliferative and inflammatory responses to injury [Citation[27], Citation[28]]. These effects involve inhibition of isoprenylation of Rho and Rac family GTPases that couple growth factor receptors to the intracellular mitogen-activated protein/extracellular signal-regulated kinase (MAP/ERK) kinase signaling pathways and induction of the cell cycle inhibitor p27Kip1 [Citation[27], Citation[28]]. Statins also improve endothelium-dependent relaxation through mechanisms that involve induction of endothelial nitric oxide synthase (eNOS) and nitric oxide production [Citation[29], Citation[30]]. Several studies demonstrate that simvastatin, a member of the statin family, attenuates pulmonary vascular remodeling in rat neointimal and hypoxic models of pulmonary arterial hypertension (PAH) [Citation[31], Citation[32], Citation[33]]. Interestingly, simvastatin is recently demonstrated in vitro to induce the expression of smooth muscle α-actin (SM-a-actin) and major histocompatibility complex (SM-MHC), highly specific markers of differentiated phenotype [Citation[34]]. So we speculated whether pleiotropic atheroprotective effects of HMG-CoA reductase inhibitors may be mediated by the expression of other members of GATA family that play an important role in cell proliferation.
In this study, we used an animal model of pulmonary hypertension by combination of monocrotaline (MCT) administration with pneumonectomy to investigate the efficacy of simvastatin and its mechanism of reversing established neointimal vascular occlusion and pulmonary hypertension. We showed that the expression of GATA-6 mRNA and protein was down-regulated in this pulmonary hypertension model, which was reversed by simvastatin administration.
MATERIALS AND METHODS
Animal Model
Pathogen-free male Sprague-Dawley rats weighing between 300 and 400 g (12 weeks old) were used for this study. All animals were obtained from Sichuan University Animal Centre (Sichuan, Chendu, China) and received humane care. On day 0, rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate (400 mg/kg) and injected subcutaneously atropine sulfate (50 μg/kg) in the right hindlimb. Left pneumonectomy was performed as previously described [Citation[35]]. On day 7, rats were injected subcutaneously in the nape with monocrotaline (MCT) (60 mg/kg; Sigma, USA). MCT was dissolved in 0.5 N HCl and adjusted to pH 7.4 with 0.5 N NaOH solution. Rats were housed with a 12/12-hour light/dark cycle and given water and standard rat chow ad libitum.
Treatment Groups
Pneumonectomized rats were randomized to receive simvastatin or vehicle by daily gavage, or neither simvastatin nor vehicle. Six groups were studied: Rats in group PMV1−35 (n = 12) received a vehicle from days 1 to 35. Group PMV21−35(n = 12) received a vehicle from days 21 to 35. Group PMS1−35 (n = 12) received simvastatin (2 mg/kg per day) [Citation[31]] from days 1 to 35. Group PMS21−35(n = 12) received simvastatin (2 mg/kg per day) from days 21 to 35. Groups PM1−21 (n = 10) and PM1−35 (n = 12) received neither simvastatin nor vehicle, and were sacrificed on day 21 or 35 (post pneumonectomy) to provide reference point for disease progression in this model. Ten additional rats were studied as a control group without any intervention. On day 35 after pneumonectomy, rats were sacrificed and organs harvested for the following analysis.
Hemodynamic Studies and Tissue Preparation
On day 35, rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate. Mean pulmonary arterial pressures (mPAPs) were measured as previously described [Citation[36], Citation[37]]. After exsanguination, the right lung, right ventricle, left ventricle, and septum were collected for histology [Citation[36], Citation[37]]. Tissues were fixed in 10% neutral-buffered formalin, paraffin embedded, and sectioned. After EVG (elastin-van Gieson) staining, lung sections were examined histologically for evidence of pulmonary vascular disease. The severity of pulmonary vascular neointimal formation was assessed in 50 panacinar arteries from each animal. The severity of neointimal formation was expressed as the vascular occlusion score (VOS), which was scored according to the criteria of Okada and coworkers [Citation[35]]. Briefly, the absence of neointimal formation or luminal occlusion equals grade 0; the presence of neointimal formation causing less than 50% luminal occlusion equals grade 1; the presence of neointimal formation causing greater than 50% luminal occlusion equals grade 2. An average score for 50 vessels (bounded by 0 and 2) was calculated for each animal. Samples of right lung were immediately placed in liquid nitrogen for extraction of total RNA.
Pulmonary Artery Morphometry
Analysis of each section was carried out in a blinded fashion. To assess the degree of medial thickening of muscular pulmonary arteries, images of 30 to 50 vessels were recorded in subsets of animals at × 400. Each artery was classified by the structure of the accompanying airway as terminal bronchiole, respiratory bronchiole, alveolar duct, or alveolar wall. The software Image-Pro plus, version 4.5.0.29, was used to measure mean arterial diameter (between external elastic laminae) and media thickness (between internal and external elastic laminae) in complete muscular arteries that accompanied terminal and respiratory bronchioles. The percent medial wall thickness (%WT) was expressed as %WT = (media thickness × 2)/external diameter × 100 [Citation[38]].
GATA-6 Gene Expression Analysis
RNA Isolate and RT-PCR
Total RNA from rat lung was isolated using Trnzol (Tiantgen, China). Reverse transcriptase–polymerase chain reaction (RT-PCR) was used to amplify portions of the rat GATA-6 gene (GATA-6; GenBank accession number NM019185) and β-actin (β-actin; GenBank accession number NM031144) from rat lung. The primers used were
GATA-6, forward: 5′-CCCAGCGCAGACCTGTTGGAGGACC
GATA-6, reverse: 5′-TGTGACAGTTGGCACAGGACAG
β−Actin, forward: 5′-GACCCAGATCATGTTTGAGACC
β−Actin, reverse: 5′-GCAGTAATCTCCTCCTGCATCC
Western Blot Analysis
Whole-cell lysates were prepared and Western blot analyses were performed to detect GATA-6 protein expression. In brief, 100 mg of lung tissue was homogenated with 1 ml of tissue protein lysis buffer (MT-CelLytics; Biocolors, China). Equivalent amounts of protein (100 μg) were separated by 10% sodium dedecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) minigels for approximately 100 minutes at 200 V to separate the region between 50 and 60 kDa and were electroblotted onto polyvinylidene difluoride transfer membrane (Roche, Germany). Blocked membranes were incubated with polyclonal antibody GATA-6 (Santa Cruz; 1:750) in Tris-buffered saline supplemented with 0.05% of Tween-20 (TTBS) plus 1% nonfat milk overnight at 4°C. The GATA-6 antibody recognizes the first 20 amino acids at the C-terminus of recombinant human GATA-6. No blocking peptides are available for these antibodies. Following extensive washing with TTBS, membranes were incubated with a 1:1000 dilution of horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibodies (Santa Cruz; 1:1000) in TTBS plus 5% milk for 1 hour at room temperature. After extensive washing with TTBS, protein-antibody complexes were visualized by the enhanced chemiluminescene detection system (Pierce, USA). Membranes were reprobed for actin (Santa Cruz; 1:2000).
Statistical Analysis
Data were expressed as means ± SD. One-way analysis of variance (ANOVA) was used to compare means between groups and the Newman-Keuls method for post hoc multiple comparisons assuming unequal variances using SPSS13.0 software. A P value of <.05 was taken to indicate that conventional statistical significance had been achieved.
RESULTS
Simvastatin Prevented Development of Pulmonary Arterial Hypertension
Rats in group PM1−21 gradually developed severe pulmonary arterial hypertension (PAH) by day 21 (). Four out of 12 rats in group PM1−35 died by day 35 and the remainder demonstrated progression to severe PAH ().
FIGURE 1 Simvastatin prevents the development and progression of pulmonary arterial hypertension in pneumonectomized, MCT-treated rats. (A) Mean pulmonary arterial pressures (mPAPs) in group PM1−21 (pneumonectomized, MCT-treated rats that were sacrificed on day 21; n = 10), Group PM1−35 (pneumonectomized, MCT-treated rats that were sacrificed on day 35; n = 8), and control (normal rats; n = 10). Bars are means ± SD. *P <.001 for groups PM1−21 and PM1−35 versus control; # P <.001 for group PM1−21 versus group PM1−35. (B) Mean pulmonary arterial pressures (mPAPs) in group PM1−35 as stated in A, group PMV1−35 (MCT-treated, pneumonectomized rats that received vehicle from days 1 to 35; n = 9), group PMS1−35 (MCT-treated, pneumonectomized rats that received simvastatin [2 mg/kg per day] from days 1 to 35; n = 12), group PMV21−35 (MCT-treated, pneumonectomized rats that received vehicle from days 21 to 35; n = 8), group PMS21−35 (MCT-treated, pneumonectomized rats that received simvastatin [2 mg/kg per day] from days 21 to 35; n = 10). Bars are means ± SD. § P <.001 for group PMS1−35 versus group PMV1−35; ** P <.001 for group PMS21−35 versus group PMV21−35; *** P <.001 for Group PMS1−35 versus group PMS21−35.
![FIGURE 1 Simvastatin prevents the development and progression of pulmonary arterial hypertension in pneumonectomized, MCT-treated rats. (A) Mean pulmonary arterial pressures (mPAPs) in group PM1−21 (pneumonectomized, MCT-treated rats that were sacrificed on day 21; n = 10), Group PM1−35 (pneumonectomized, MCT-treated rats that were sacrificed on day 35; n = 8), and control (normal rats; n = 10). Bars are means ± SD. *P <.001 for groups PM1−21 and PM1−35 versus control; # P <.001 for group PM1−21 versus group PM1−35. (B) Mean pulmonary arterial pressures (mPAPs) in group PM1−35 as stated in A, group PMV1−35 (MCT-treated, pneumonectomized rats that received vehicle from days 1 to 35; n = 9), group PMS1−35 (MCT-treated, pneumonectomized rats that received simvastatin [2 mg/kg per day] from days 1 to 35; n = 12), group PMV21−35 (MCT-treated, pneumonectomized rats that received vehicle from days 21 to 35; n = 8), group PMS21−35 (MCT-treated, pneumonectomized rats that received simvastatin [2 mg/kg per day] from days 21 to 35; n = 10). Bars are means ± SD. § P <.001 for group PMS1−35 versus group PMV1−35; ** P <.001 for group PMS21−35 versus group PMV21−35; *** P <.001 for Group PMS1−35 versus group PMS21−35.](/cms/asset/6bc520ff-1aea-46cb-ae8a-4a344c10d808/ielu_a_373851_uf0001_b.gif)
All simvastatin-treated rats in group PMS1−35 survived the entire course of the study (35 days). Two of 12 rats in group PMS21−35 died by day 35. Simvastatin delayed or partly reversed PAH and decreased mPAP in group PMS1−35 or PMS21−35, compared with the vehicle-treated rats in group PMV1−35 or PMV21−35 on day 35, respectively ().
Right Ventricular Hypertrophy
By day 35, pulmonary hypertensive rats demonstrated significant right ventricular hypertrophy. The ratio of the right ventricular to left ventricular and septum weights, RV/(LV + S), of pulmonary hypertensive rats significantly increased in comparison with normal rats ().
FIGURE 2 Simvastatin prevents the development and progression of right ventricular hypertrophy in pneumonectomized, MCT-treated rats. (A) Ratios of right vertricular weight to left ventricular and interventricular septal weights [RV/(LV + S)] are shown among the 3 groups of rats as stated in . Bars are means ± SD. * P <.001 for groups PM1−21 and PM1−35 versus control; # P <.001 for group PM1−35 versus group PM1−21. (B) Ratios of RV/(LV + S) are shown for the 5 groups of rats as stated in . Bars are means ± SD. § P <.001 for group PMS1−35 versus group PMV1−35; ** P <.001 for group PMS21−35 versus group PMV21−35; *** P <.001 for group PMS21−35 versus group PM1−35.
![FIGURE 2 Simvastatin prevents the development and progression of right ventricular hypertrophy in pneumonectomized, MCT-treated rats. (A) Ratios of right vertricular weight to left ventricular and interventricular septal weights [RV/(LV + S)] are shown among the 3 groups of rats as stated in Figure 1A. Bars are means ± SD. * P <.001 for groups PM1−21 and PM1−35 versus control; # P <.001 for group PM1−35 versus group PM1−21. (B) Ratios of RV/(LV + S) are shown for the 5 groups of rats as stated in Figure 1B. Bars are means ± SD. § P <.001 for group PMS1−35 versus group PMV1−35; ** P <.001 for group PMS21−35 versus group PMV21−35; *** P <.001 for group PMS21−35 versus group PM1−35.](/cms/asset/6e7d886e-af86-459c-aa79-7d7a2639d482/ielu_a_373851_uf0002_b.gif)
Simvastatin prevented progression and partly reversed established right ventricular hypertrophy of diseased rats on day 35, with groups PMS1−35 and PMS21−35 showing lower RV/(LV + S) ratio () compared with groups PMV1−35 and PMV21−35, respectively ().
Simvastatin Decreased Medial Hypertrophy
Prominent medial wall hypertrophy was evident in muscular pulmonary arteries from diseased rats ( to ). The percent medial wall thickness (%WT) of rats in groups PM1−21 and PM1−35 was significantly higher than that of respective control animals ().
FIGURE 3 Simvastatin prevents the development of small pulmonary artery hypertrophy in pneumonectomized, MCT-treated rats. (A) Control; (B) PMV1−35; (C) PMV21−35; (D) PM1−35; (E) PMS1−35; (F) PMS21−35. Elastin–van Gieson staining (magnification, × 200) images show reduced small pulmonary artery hypertrophy in Groups PMS1−35 and PMS21−35.
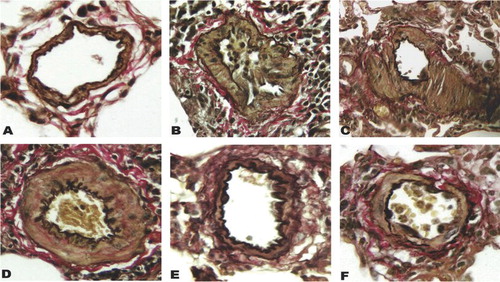
FIGURE 4 Reduced percent medial wall thickness of small pulmonary artery hypertrophy in pneumonectomized, MCT-treated rats by simvastatin. Small pulmonary artery (50 to 150 μm) hypertrophy measured by (2 × medial thickness/external diameter) × 100. (A) Percent medial wall thickness is shown among the 3 groups of rats as stated in . Bars are means ± SD. * P <.001 for Groups PM1−21 and PM1−35 versus control; # P <.001 for group PM1−35 versus group PM1−21. (B) Percent medial wall thickness is shown among the 5 groups of rats as stated in . Bars are means ± SD. Simvastatin alleviated arterial medial hypertrophy in pneumonectomized, monocrotaline-treated rats (§ P <.001 for group PMS1−35 versus group PMV1−35; ▵ P <.001 for group PMS21−35 versus group PMV21−35). Group PMS1−35 had significantly lower percent medial wall thickness than group PMS21−35 (*** P <.05).
In contrast, rats treated with simvastatin (groups PMS1−35 and PMS21−35) demonstrated decreased medial hypertrophy at different degree (), whereas rats treated with vehicle (groups PMV21−35 and PMV1−35) demonstrated significant medial hypertrophy ().
Histopathology
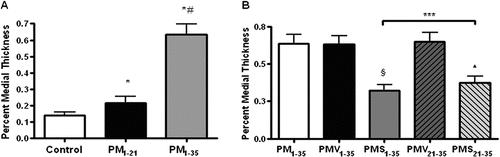
Representative morphologies of small pulmonary arteries in normal rats and rats in groups PM1−35, PMV1−35, PMV21−35, PMS1−35, and PMS21−35 are shown to be stained for elastin-van Gieson to reveal the inner elastin lamina ( to ). A quantitative analysis of luminal obstruction on 50 consecutive small pulmonary arteries from each rat was performed (). The distribution of the vascular lesions, and an average vascular occlusion score (between 0 and 2), are presented ( to ). The pneumonectomized, MCT-treated rats resulted in severe changes of neointimal proliferation and vascular occlusion ( versus A). The vascular occlusion score (VOS) in groups PMV1−35 and PMV21−35 was similar to an average VOS for our laboratory's historical data on group PM1−35 rats ( and versus B). This suggests that the treatment of vehicle does not alter the histopathology of this disease model. Compared with groups PMV1−35 and PMV21−35, groups PMS1−35 and PMS21−35 had a lower VOS, respectively ().
FIGURE 5 Simvastatin suppresses the development of pulmonary arterial neointimal formation. The vascular occlusion score (VOS) was the average of 50 consecutive intra-acinar pulmonary arteries. (A) Normal rat intra-acinar artery without evidence of neointimal proliferation (grade 0). (B, C, and D) Grade 2 neointimal lesions (> 50% luminal occlusion) in groups PM1−35, PMV1−35, and PMV21−35. (E) Predominance of grade 2 lesson in group PMS21−35. (F) Grade 1 neointimal lesion (< 50% lumenal occlusion) in group PMS1−35. All samples were stained with EVG. The images are × 400 magnification. (G) Average grades of VOS among the 6 groups of rats as stated in and B. * P <.001 for group PM1−35 versus control; * P <.001 for group PMS1−35 versus group PMV1−35; * P <.001 for group PMS21−35 versus group PMV21−35; * P <.001 for group PMS1−35 versus group PMS21−35, respectively.
GATA-6 Gene Expression in the Lung Tissue
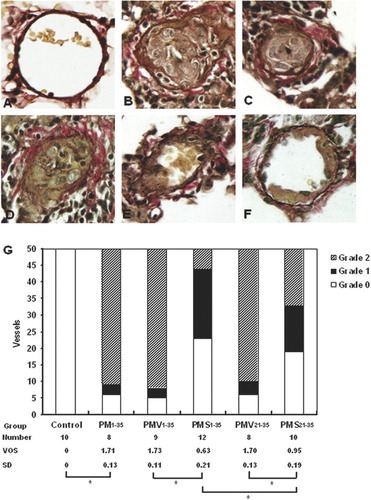
Changes in lung GATA-6 mRNA expression were assessed semiquantitatively by RT-PCR (). The expression of GATA-6 mRNA, normalized to β-actin expression, was significantly lower in diseased rats (groups PM1−21 and PM1−35) than in normal rats (). Simvastatin treatment increased the expression of GATA-6 mRNA in MCT-treated, pneumonectomized rats ().
FIGURE 6 Reduction in GATA-6 mRNA expression during the development of pulmonary hypertension in pneumonectomized, MCT-treated rats that was reversed by simvastatin. (A) RT-PCR analysis of GATA-6 and β-actin gene expression in representative rat lungs from groups stated in and . (B) Relative abundance of GATA-6/β-actin mRNA expression among the 3 groups of rats stated in . Mean and standard deviation of GATA-6 mRNA expression normalized to β-actin is shown (n = 3/group). * P <.001 for groups PM1−21 and PM1−35 versus control; # P <.01 for group PM1−35 versus group PM1−21. (C) Relative abundance of GATA-6/β-actin mRNA expression among the 5 groups of rats stated in . Mean and standard deviation of GATA-6 mRNA expression normalized to β-actin is shown (n = 3/group). Simvastatin restored GATA6 mRNA expression level in diseased rats (** P <.001 for group PMS1−35 versus group PMV1−35; §P <.001 for group PMS21−35 versus group PMV21−35). Group PMS1−35 had significantly higher GATA-6 mRNA expression level than Group PMS21−35 (*** P <.01).
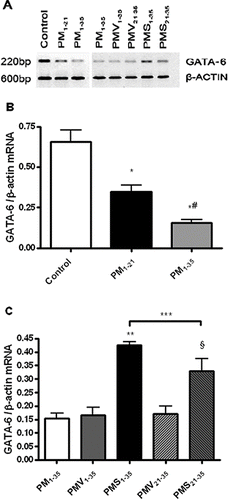
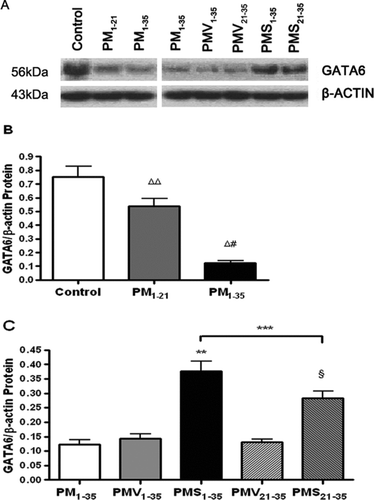
The lung expression of GATA-6 protein was assessed qualitatively by Western blotting (). A GATA-6 antiserum-immunoreactive band is detected at 56 kDa (). Compared with normal rats, diseased rats in groups PM1−21 and PM1−35 showed decreased expression of GATA-6 protein (). Simvastatin treatment significantly increased GATA-6 protein expression ().
FIGURE 7 Decrease in GATA-6 protein expression during the development of pulmonary hypertension in pneumonectomized, MCT-treated rats that was restored by simvastatin. (A) Western blotting analysis of GATA-6 protein expression in representative rat lung protein extracts from each group as stated in and . (B) Relative abundance of GATA-6/β-actin protein expression among the three groups of rats stated in . Mean and standard deviation of GATA-6 protein expression normalized to β-actin is shown (n = 3/group). * P <.001 for group PM1−35 versus control; ** P <.01 for group PM1−21 versus control; # P <.01 for group PM1−35 versus group PM1−21. (C) Relative abundance of GATA-6/ β-actin protein expression among the 5 groups of rats stated in . Mean and standard deviation of GATA-6 protein expression normalized to β-actin is shown (n = 3/group). Simvastatin restored GATA-6 protein expression level in diseased rats (*** P <.001 for group PMV1−35 versus group PMS1−35; § P <.001 for group PMV21−35 versus group PMS21−35). Group PMS1−35 had significantly higher GATA-6 protein expression level than group PMS21−35 (**** P <.01).
DISCUSSION
Pulmonary arterial hypertension is characterized histopathologically by changes of abnormal proliferation of vascular endothelial and smooth muscle cells, occlusion of microvascular pulmonary arteries, and plexiform lesion formation in severe cases. Although the pathogenesis of most forms of pulmonary arterial hypertension is unknown, there have been many recent developments in pathogenesis involving inappropriate proliferation and constriction of vascular smooth muscle cells, and deficiencies of endogenous vasodilators such as prostacyclin and endothelial-derived nitric oxide [Citation[39]], especially pertaining to the molecular genetics and cell biology of idiopathic pulmonary arterial hypertension. The increase in pulmonary arterial pressure leads to right ventricular congestive failure and ultimately to death. Current treatment strategies for PAH include diuretics, anticoagulation, digoxin, and supplemental oxygen for congestive heart failure, and pulmonary vasodilating agents such as calcium channel antagonists and prostanoids, especially intravenous (IV) epoprostenol and endothelin-receptor antagonists. New therapies, especially theose directing at suppressing inappropriate neointimal proliferation in pulmonary arteries, are warranted [Citation[40], Citation[41], Citation[42], Citation[43]].
Statins, the HMG CoA reductase inhibitors, have had a dramatic impact on clinical outcomes in patients with coronary artery disease. Several studies have documented benefits unrelated to cholesterol lowering [Citation[44], Citation[45]], with multiple “pleiotropic” effects on vascular wall function that would be expected to attenuate vascular remodeling [Citation[46]]. These effects stem from their ability to reduce the production of the isoprenoid intermediates farnesyl and geranylgeranyl pyrophosphate, compounds that are distal to mevalonate in the cholesterol synthetic pathway. These lipophilic molecules are then covalently bound to Rho and other small G proteins in a post-translational modification that is essential for attachment of these important signaling proteins to cell membranes and regulators and for their activation of downstream effectors [Citation[47]].
In present study, we probed the hypothesis that statins could attenuate vascular remodeling and pulmonary hypertension in a rat model of neointimal pulmonary hypertension. Simvastatin administration produced a marked reduction in pulmonary artery pressure developed in animals with pneumonectomy and MCT administration. Simvastatin also significantly reduced the right ventricular hypertrophy, as indicated by the RV/(LV + S) weight ratio. Morphological analysis of the pulmonary vasculature of simvastatin-treated rats revealed considerable decrease in medial thickness of medium-sized arteries and neointimal formation. We also found that GATA-6 mRNA and protein expression dramatically decreased in pneumonectomized, MCT-treated rats that was restored by simvastatin administration.
The exact mechanism of attenuation of neointimal pulmonary hypertension by simvastatin was not understood. Several actions of statins could account for our observations. Statins have been noted to reduce blood pressure in spontaneously hypertensive but not normotensive rats [Citation[38]]. Simvastatin treatment potently inhibits vascular remodeling by attenuates chronic hypoxic pulmonary hypertension and polycythemia in rats [Citation[33]], and reverses pulmonary arterial neointimal formation and PAH after toxic injury by down-regulating the inflammatory genes fos, jun, and tumor necrosis factor-α and up-regulating the cell cycle inhibitor p27Kip1, endothelial nitric oxide synthase, and bone morphogenetic protein receptor type 1a [Citation[31]]. Inhibition of HMG-CoA reductase by statins decreases isoprenoid intermediates such as farnesyl-PP and geranylgeranyl-PP, which leads to an inhibition of isoprenylation of small GTPases such as Ras, Rho, Rab, and Rap. Among the Rho GTPases are RhoA, Rac1, and Cdc42 [Citation[48]]. Statins have been shown to induce apoptosis of pulmonary vascular smooth muscle cells in serum-free medium [Citation[49]]. Both endothelin-1 [Citation[50]] and endothelin receptor [Citation[51]] transcriptions are reduced by statins, which would attenuate chronic hypoxic pulmonary hypertension. Simvastatin markedly decreased the platelet-derived growth factor–induced proliferation of vascular smooth muscle cells by preventing Rho GTPase-induced downregulation of p27kip1, an important negative regulator of cell proliferation [Citation[28]]. Simvastatin is also demonstrated to regulate the differentiation and the phenotype expression of VSMCs [Citation[34]]. In addition, statins have potent antioxidant effects [Citation[52]]. Given that oxidative stress may be an important mediator of chronic hypoxic pulmonary hypertension [Citation[38]] as well as acute hypoxic pulmonary vasoconstriction [Citation[53]], the antioxidant effect of simvastatin may also play a role in attenuation of pulmonary hypertension observed here.
In this study, we found that down-regulation of GATA-6 expression was concurrent with development and progression of pulmonary hypertension, right ventricular hypertrophy, arterial medial hypertrophy, and neointimal formation in the neointimal pulmonary hypertensive model. GATA-6 gene expression has been detected in the pericardial mesoderm, embryonic heart tube, and primitive gut during early embryonic development [Citation[54]]. However, during development, GATA-6 becomes the only member of the family expressed in vascular smooth muscle cells [Citation[15], Citation[55]] and its expression is down-regulated in proliferating vascular smooth muscle cells [Citation[18]]. These data suggest that down-regulation of GATA-6 expression is closely associated with pulmonary arterial smooth muscle cells proliferation and that the level of GATA-6 expression may be an indicator of severity of hypertensive pulmonary vascular disease.
Some recent reports show that simvastatin up-regulates GATA-3 expression in dendritic cells and up-regulates the binding activity of GATA-6 to SM-MHC GATA site, whereas mutating the GATA-6 binding site abolished this activation [Citation[34], Citation[56]]. In the present study, we also found that down-regulation of GATA-6 expression was reversed in pulmonary hypertensive rats treated with simvastatin. However, the precise mechanism that statins regulate expression of GATA family members is unknown. Further studies will be performed to investigate the mechanism of GATA-6 expression by regulated by simastatin and examine GATA-6 expression in the lung tissue of human patients with pulmonary hypertension.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported in part by a grant from the National Natural Science Foundation of China (30300145).
REFERENCES
- Budhiraja R, Tuder R M, Hassoun P M. Endothelial dysfunction in pulmonary hypertension. Circulation 2004; 109: 159–165
- Botney M D. Role of hemodynamics in pulmonary vascular remodeling: implications for primary pulmonary hypertension. Am J Respir Crit Care Med. 1999; 159: 361–364
- van Albada M E, Schoemaker R G, Kemna M S, et al. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J 2005; 26: 487–493
- Stenmark K R, Mecham R P. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 1997; 59: 89–144
- Sokabe T, Yamamoto K, Ohura N, et al. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2004; 287: H2027–H2034
- Davies P F. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995; 75: 519–560
- Orkin S H. GATA-binding transcription factors in hematopoietic cells. Blood 1992; 80: 575–581
- Molkentin J D. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000; 275: 38949–38952
- Ting C N, Olson M C, Barton K P, et al. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 1996; 384: 474–478
- Tsai F Y, Keller G, Kuo F C, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 1994; 371: 221–226
- De Maria R, Zeuner A, Eramo A, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 1999; 401: 489–493
- Orkin S H, Shivdasani R A, Fujiwara Y, et al. Transcription factor GATA-1 in megakaryocyte development. Stem Cells. 1998; 16(Suppl 2)79–83
- Patient R K, McGhee J D. The GATA family (vertebrates and invertebrates). Curr Opin Genet Dev 2002; 12: 416–422
- Yang H, Lu M M, Zhang L, et al. GATA6 regulates differentiation of distal lung epithelium. Development 2002; 129: 2233–2246
- Morrisey E E, Ip H S, Lu M M, et al. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol 1996; 177: 309–322
- Fischer A, Klattig J, Kneitz B, et al. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol Cell Biol 2005; 25: 8960–8970
- Zhao R, Watt A J, Li J, et al. GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Mol Cell Biol 2005; 25: 2622–2631
- Suzuki E, Evans T, Lowry J, et al. The human GATA-6 gene: structure, chromosomal location, and regulation of expression by tissue-specific and mitogen-responsive signals. Genomics 1996; 38: 283–290
- Perlman H, Suzuki E, Simonson M, et al. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J Biol Chem 1998; 273: 13713–13718
- Mano T, Luo Z, Malendowicz S L, et al. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ Res 1999; 84: 647–654
- Morrisey E E. GATA-6: the proliferation stops here: cell proliferation in glomerular mesangial and vascular smooth muscle cells. Circ Res. 2000; 87: 638–640
- Sokabe T, Yamamoto K, Ohura N, et al. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2004; 287: H2027–H2034
- Lepore J J, Cappola T P, Mericko P A, et al. GATA-6 regulates genes promoting synthetic functions in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2005; 25: 309–314
- Mishra T K, Routray S. Current perspectives on statins. J Indian Med Assoc 2003; 101: 381–383
- Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000; 6: 1399–1402
- Bustos C, Hernandez-Presa M A, Ortego M, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol 1998; 32: 2057–2064
- Indolfi C, Cioppa A, Stabile E, et al. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol 2000; 35: 214–221
- Laufs U, Marra D, Node K, et al. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J Biol Chem 1999; 274: 21926–21931
- Laufs U, Fata V L, Liao J K. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem 1997; 272: 31725–31729
- Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 2000; 6: 1004–1010
- Nishimura T, Vaszar L T, Faul J L, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation JT – Circulation 2003; 108: 1640–1645
- Nishimura T, Faul J L, Berry G J, et al. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med 2002; 166: 1403–1408
- Girgis R E, Li D, Zhan X, et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003; 285: H938–H945
- Wada H, Abe M, Ono K, et al. Statins activate GATA-6 and induce differentiated vascular smooth muscle cells. Biochem Biophys Res Commun 2008; 374: 731–736
- Okada K, Tanaka Y, Bernstein M, et al. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 1997; 151: 1019–1025
- Faul J L, Nishimura T, Berry G J, et al. Triptolide attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2000; 162: 2252–2258
- Nishimura T, Faul J L, Berry G J, et al. 40-O-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2001; 163: 498–502
- Hoshikawa Y, Ono S, Suzuki S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol 2001; 90: 1299–1306
- Fishman A P, Fishman M C, Freeman B A, et al. Mechanisms of proliferative and obliterative vascular diseases. Insights from the pulmonary and systemic circulations. NHLBI Workshop summary. Am J Respir Crit Care Med 1998; 158: 670–674
- Newman J H, Lane K B. Hypertensive pulmonary vascular disease: dawn of the age of prevention?. Am J Respir Crit Care Med 2000; 162: 2020–2021
- Kao P N, Faul J L. Emerging therapies for pulmonary hypertension: striving for efficacy and safety. J Am Coll Cardiol 2003; 41: 2126–2129
- Rubin L J. Therapy of pulmonary hypertension: the evolution from vasodilators to antiproliferative agents. Am J Respir Crit Care Med. 2002; 166: 1308–1309
- Newman J H, Fanburg B L, Archer S L, et al. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation 2004; 109: 2947–2952
- Chan A W, Bhatt D L, Chew D P, et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation 2002; 105: 691–696
- Heeschen C, Hamm C W, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105: 1446–1452
- Epstein M, Campese V M. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis 2005; 45: 2–14
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 2001; 81: 153–208
- Rikitake Y, Liao J K. Rho GTPases, statins, and nitric oxide. Circ Res 2005; 97: 1232–1235
- Stark W W, Jr, Blaskovich M A, Johnson B A, et al. Inhibiting geranylgeranylation blocks growth and promotes apoptosis in pulmonary vascular smooth muscle cells. Am J Physiol. 1998; 275(1)L55–L63, Pt 1
- Hernandez-Perera O, Perez-Sala D, Soria E, et al. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res 2000; 87: 616–622
- Xu C B, Stenman E, Edvinsson L. Reduction of bFGF-induced smooth muscle cell proliferation and endothelin receptor mRNA expression by mevastatin and atorvastatin. Biochem Pharmacol 2002; 64: 497–505
- Kalinowski L, Dobrucki L W, Brovkovych V, et al. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation 2002; 105: 933–938
- Weissmann N, Winterhalder S, Nollen M, et al. NO and reactive oxygen species are involved in biphasic hypoxic vasoconstriction of isolated rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2001; 280: L638–L645
- Sun-Wada G H, Kamei Y, Wada Y, et al. Regulatory elements directing gut expression of the GATA6 gene during mouse early development. J Biochem (Tokyo) 2004; 135: 165–169
- Narita N, Heikinheimo M, Bielinska M, et al. The gene for transcription factor GATA-6 resides on mouse chromosome 18 and is expressed in myocardium and vascular smooth muscle. Genomics 1998; 36: 345–348
- Arora M, Chen L, Paglia M, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci U S A 2006; 103: 7777–7782
