Abstract
Background
To investigate the protective effect of p14ARF in a nitric acid (NA) aerosol inhalation-induced bronchiolitis obliterans (BO) mouse model and its potential regulatory mechanism.
Methods
A BO mouse model was established by NA aerosol inhalation. The expressions of p14ARF, phosphatidylinositol-3-kinase (PI3K), and protein kinase B (AKT) were detected by quantitative reverse transcription PCR (qRT-PCR) and western blot (WB). Hematoxylin (HE) staining, Masson staining, and periodic acid-Schiff (PAS) staining observed pulmonary histological changes. TdT-mediated dUTP nick end labeling (TUNEL) staining detected pulmonary cell apoptosis, and enzyme-linked immunosorbent assay (ELISA) measured matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), interleukon-6 (IL-6), and transforminh growth factor-β (TGF-β) levels in lung tissue and bronchoalveolar lavage fluid (BALF).
Results
The expressions of p14ARF, PI3K, and AKT showed a time gradient change, with a decrease trend (*P < 0.05 and **P < 0.01). Severe inflammatory infiltration and tracheal fibrosis were found in lung tissue in the modeling group (BO group) compared with the control group (Con group). The pH, PaO2, and PaO2/FiO2 values significantly reduced, while the PaCO2 value and the number of TUNEL-positive cells increased in BO group (P < 0.05). In addition, MMP-2, MMP-9, IL-6, and TGF-β levels remarkably increased, with an increase in the number of white blood cells, neutrophils, and lymphocytes in BO group (P < 0.05). Furthermore, p14ARF up-regulation reversed the trend of the aforementioned indexes in BO mice.
Conclusions
p14ARF ameliorated the inflammatory response and airway remodeling in a BO mouse model via the PI3K/AKT pathway.
Introduction
Bronchiolitis obliterans (BO) is an airway obstructive disease secondary to severe damage of the lower respiratory tract,Citation1,Citation2 and its pathological characteristics are bronchiolar fibrosis and inflammation infiltration between the tracheal wall and adjacent tissues, resulting in small-airway stenosis or even occlusion.Citation3 Current studies suggest that the incidence of BO is mainly related to transplantation,Citation4 adenovirus infection,Citation5 inhalation factors,Citation6 and connective tissue diseases,Citation7 causing symptoms such as shortness of breath, exercise intolerance, wheezing, and cyanosis, and thus seriously threatening the survival of patients.Citation8 However, the pathogenesis of BO is still unclear and needs to be further elucidated.
As a tumor suppressor gene located at the human 9p21 gene locus,Citation9 p14ARF can not only inhibit cancer cells through the ARF/MDM2/p53 pathwayCitation10 but also promote cell apoptosis and cell cycle arrest through the p53-independent pathway.Citation11,Citation12 Due to promoter hypermethylation and genetic deletion, p14ARF is lowly expressed in non-small cell lung cancer, prostate cancer, and gastric cancer,Citation13 so it is also regarded as a predictor of poor prognosis. Based on the fact that p14ARF has advantages of remarkable negative cell cycle regulation, short cDNA length, and easy cloning, p14ARF expression vector can be transfected into lung adenocarcinoma, hepatoma, and pancreatic cancer cell lines to promote apoptosis.Citation14–15
The PI3K/AKT pathway not only plays an important role in tumor chemoradiotherapy antagonism, but also is the core pathway of tumor cell proliferation.Citation16 The activation of mTORC1 and mTORC2 depends on the PI3K/AKT pathway, increasing collagen deposition and causing mesenchymal fibrosis.Citation17 AKT phosphorylation regulates the stability of ARF, thereby promoting ARF to further inhibit MDM2 expression.Citation18 Furthermore, the up-regulation of PDGFRα promoted the occurrence of Ink4a/ARF-deficient glioblastoma, and this process was related to the activation of the PI3K/AKT/mTOR pathway.Citation19
In present study, we constructed a BO mouse model induced by NA aerosol inhalation and transfected the p14ARF lentivirus vector to up-regulate p14ARF expression. Then, we studied the effects of p14ARF on apoptosis, pathological changes, pulmonary dysfunction, inflammatory infiltration, and fibrosis in BO mice, so as to clarify the protective effect and potential related mechanism of p14ARF, thus providing a new theoretical basis for the clinical treatment of BO.
Materials and methods
Animals
A total of 125 female BALB/c mice (SPF grade, 6–8 weeks old, weight about 20 g) were purchased from Shanghai Animal Research Center and fed adaptively and conventionally 3 days before the experiment. All animal experiments were conducted following the NIH Animal Research Guidelines.Citation20 Our study was approved by the institutional ethics committee.
Construction of BO model
A BO mouse model induced by NA atomization was constructed referring to the literature,Citation21 and the lethal ratio of this method was less than 15%. Diluted the analytical pure NA solution (aladdin, Shanghai, China) with distilled water to 5% NA solution. 125 BALB/c mice were randomly divided into the control group (Con group; n = 25) and the modeling group (BO group; n = 100). The mice were placed in a translucent container with holes on the lid and side wall. The ventilation hole was on the top, and the hole on the side wall was connected with an oxygen meter (the oxygen concentration controlled at 21%, MB-20 type). The bottom was connected with an air-compressed atomization pump (capable of transporting 0.5–5 μm aerosol particles, PARI Vios compression pump + PARI LC Plus atomizer). Then, the mice in the BO group were exposed to 5% NA aerosol for 3 h. Meanwhile, the mice in the Con group were atomized and inhaled with distilled water. All mice after aerosol administration were transferred to the cage for routine feeding. 12 mice in the BO group died after 48 h of NA inhalation, while no death occurred in the Con group. The presence of small-airway lumen stenosis and/or occlusion, and inflammation infiltration and fibrosis in the small airway confirmed the successful establishment of our model.
Experimental grouping
Three mice were randomly selected from the BO group and sacrificed after 0, 3, 7, 14, 28, and 56 days. The expressions of p14ARF and PI3K/AKT pathway–related proteins in lung tissues were detected. On the 27th day, Lv-con (Cobioer, Nanjing, China) and Lv-p14ARF were transfected into BO mice by tail vein injection, and LV-con was used as a control. The experiments involved four groups: the Con group, the BO group, the Lv-Con group, and the Lv-p14ARF group. On the 28th day, 20 mice in each group were sacrificed, 10 were prepared for lung tissue sections and homogenization, and the other 10 were used to retain bronchoalveolar lavage fluid (BALF). The remaining surviving mice were used for detecting pulmonary function indexes and for standby.
Preparation of lung tissue homogenate
The left lungs of mice were frozen in liquid nitrogen, rinsed with pre-cooled normal saline, dried, weighed, and cut into pieces. Then, 2 mL/g normal saline was added, and the tissues were ground with TissuePrep rapid tissue and cell disruptor (Bio-xplorer). Centrifuged at 12,000 rpm for 10 min, and collected the supernatant with a pipette and stored in a refrigerator at −70 °C.
Acquisition of BALF
Through tracheal intubation, 10 mice were subjected to pulmonary bronchoalveolar lavage with 1 mL of 1× phosphate-buffered saline (PBS) (Beyotime, Shanghai, China), and the thoracic cavity of mice was slowly massaged for 30 s to make PBS fully contact with the lung tissue. This step was repeated twice. BALF was collected and centrifuged at 1000 rpm for 12 min to obtain the supernatant, which was stored at −70 °C.
RT-qPCR
Total RNA in lung tissue homogenate was extracted with the RNA Extraction Kit (Thermo Fisher, USA) and reverse transcribed into cDNA. The cDNA of each sample was amplified referring to the RT-qPCR Kit (Sangon, Shanghai, China) protocol, and the relative expression levels of p14ARF, PI3K, and AKT mRNA were calculated using the 2-△△CT method. All samples were tested in three replicate wells. The primer sequences are listed as follows:
Western blot
The lung tissue protein was extracted and quantified with the BCA Protein Quantification Kit (Thermo Fisher, USA). After SDS-PAGE, the target proteins (p14ARF, p-PI3K, PI3K, p-AKT, and AKT) were transferred to the PVDF membrane. Incubated the PVDF membrane in 5% skimmed milk powder sealing solution for 2 h, then added the primary antibodies (β-actin diluted at 1:2000, and the other primary antibodies were diluted at 1:1000, Abcam, USA), and incubated overnight at 4 °C. Washed the membrane three times with TBST, added HRP-labeled secondary antibody (1:3000, Abcam, USA), and incubated for 2 h. The membrane was washed again with TBST, developed, and fixed with the ECL kit. Then, the relative expression of each protein was calculated.
HE staining
After disinfection with 75% ethanol (aladdin, Shanghai, China), the thoracic cavities of the mice were opened in an ultra-clean platform, and the intact lung was stripped out. The lungs were washed with pre-cooled normal saline, the left lungs were separated for the histopathological examination, and the lung tissue homogenate was prepared (the left lung was usually more severely damagedCitation22) Then 4% formaldehyde (Solarbio, Beijing, China) was added to fix the left lungs for 24 h, and the lungs were cut into 4-mm-thick lung sections. Dehydrated for 10 h, xylene transparent treatment for 2 h, embed for 1 h, and cut 5 μm paraffin sections. After dewaxing, hematoxylin dye solution and 1% eosin dye solution (Beyotime, Shanghai, China) were added successively for dyeing. The sections were dehydrated, made transparent, and mounted, and the pathological changes in lung tissues were observed under a light microscope.
Masson staining
The slides containing 5-μm paraffin-embedded sections were dried in the oven at 60 °C for 1 h and then dewaxed. The sections were stained with hematoxylin for 5 min and Masson Ponceau acid compound solution (Beyotime, Shanghai, China) for 8 min. The slides were differentiated in 1% phosphomolybdic acid solution for 3 min, and then stained with Bright Green for 5 min. At last, the slides were dehydrated, and the lung tissue structure was observed under a light microscope.
PAS staining
The sections were stained with periodic acid-Schiff (PAS) referring to the instructions of the PAS kit (Solarbio, Beijing, China). The 5-μm paraffin-embedded sections were routinely dewaxed, immersed in periodic acid oxidation solution for 16 min, and washed twice with distilled water. Added Schiff solution for staining for 25 min, rinsed with running water for 5 min, and added hematoxylin nuclear staining for 3 min. Subsequently, the sections were differentiated in hydrochloric acid and ethanol and washed with distilled water until the nuclei turned blue. They were dehydrated, made transparent, and sealed. The mouse airway mucosa goblet cells and mucus secretion were observed under a microscope.
Detection of pulmonary dysfunction indexes
On the 28th day of NA inhalation, three mice in each group were connected to the HX-300S small animal ventilator (Techman, Shanghai, China), and the parameters were adjusted as follows: the respiratory ratio, 1:1; FiO2, 1.0; tidal volume, 8 mL/kg. When the ventilation was stable for 1 h, 0.5 mL of carotid blood was collected, and the pH, PaCO2, and PaO2 were measured with an iSTAT rapid blood-gas analyzer (Abbott, Shanghai, China) and the PaO2/FiO2 value was calculated.
ELISA
The concentrations of MMP-2, MMP-9, TIMP-1, IL-6 and TGF-β in the lung tissue supernatant and BALF were measured with enzyme-linked immunosorbent assay (ELISA) kits (Beyotime, Shanghai, China). Added 40 μL sample and 10 μL biotin-labeled antibody working solution into the plate, then added 50 μL streptavidin-HRP. Sealed the plate, gently shaken to mix, and incubated for 1 h. The sealing film was removed, washed, and shaded, and color was developed for 10 min. Furthermore, 50 μL of termination solution was added to stop the reaction, and the OD450 value was determined.
Tunel staining
After routine dewaxing, the paraffin-embedded sections were immediately immersed in 1% Triton X-100 solution, and the infiltration was promoted at room temperature for 5 min. The sections were washed three times with PBS and blocked. Subsequently, 100 μL of Proteinase K working solution (Solarbio) was added to permeate for 0.5 h, and 100 μL of DNase I was incubated for 20 min. The sections were rinsed again with PBS, the sample was dried, and 50 μL of TdT enzyme reaction solution for 1 h in the dark, then added 50 μL Streptavidin-HRP for 0.5 h in the dark. Afterward, 50 μL of DAB working buffer was added to develop color for 3 min, and the sections were stained with hematoxylin for 3 min. The sections were dehydrated and dried. The slides were sealed and observed under an optical microscope.
Inflammatory cell count
The cell sediment of BALF was collected, mixed with 50 μL of ACK lysis buffer (Solarbio), and lysed on ice for 1 min. Then, 1 mL of 1× PBS was added, centrifuged at 1000 rpm for 10 min, and aspirated, and the supernatant was discarded. Next, 50 μL of 1× PBS was added to make a cell suspension. Further, 10 μL of 0.1% trypan blue (Solarbio) was added to 10 μL of cell suspension for staining and then the suspension was placed on a cell counting plate for observation and counting. The round white blood cells were observed under a low-power microscope. The cytoplasm was transparent, but the nucleus was purple-black in color. The number of white blood cells = (total number of cells in 4 large squares/4) × 104 cells/mL. Took another 20 μL cell suspension, dropped it on the glass slide, and dragged the suspension into a monolayer with a tip. Dried and fixed with 95% ethanol for 25 min, and dried again. The cell smear was stained with Wright-Giemsa (Wright’s) for 5 min, washed with distilled water, dried, and inspected by microscopy. A uniformly distributed area was selected for cell classification and count. That is, 200 cells were continuously counted, the percentages of lymphocytes and neutrophils were determined according to the cell morphology, and then the absolute value was calculated.
Statistical analysis
The obtained data were analyzed with the SPSS 20.0 software. The results are expressed as mean ± standard deviation. The t-test was used to compare the two groups, and the difference was statistically significant with P < 0.05.
Results
Expressions of the p14ARF and PI3K/AKT pathway
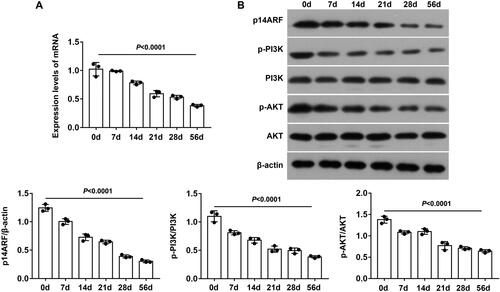
We detected the expression levels of p14ARF and PI3K/AKT pathway–related proteins in lung tissue on days 0, 3, 7, 14, 28, and 56 of NA aerosol inhalation. The results showed that the expression levels of p14ARF, p-PI3K and p-AKT mRNA and protein presented a time-dependent decrease (*P < 0.05 and **P < 0.01), but the decreasing trend slowed down after the 28th day (). This demonstrated that p14ARF was involved in the occurrence and development of BO, and it was related to the PI3K/AKT pathway. Due to the damage of NA atomization inhalation to lung tissue, we uniformly sacrificed mice of each group on the 28th day, and obtained lung tissue (section or homogenate) and BALF for subsequent experiments.
P14arf ameliorated histology
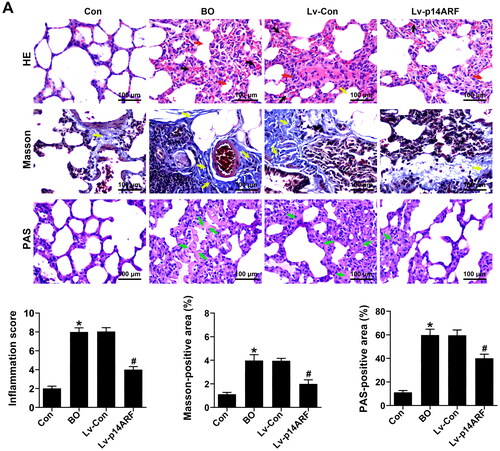
To clarify the role of p14ARF in BO, p14ARF lentiviral vector and empty lentivirus were transfected into BO mice on the 27th day of NA aerosol inhalation, and p14ARF, p-PI3K, and p-AKT expressions in the lung tissue homogenate of each group was detected 24 h later. As shown in , the p14ARF, p-PI3K and p-AKT levels were significantly reduced in the BO group (P < 0.05), which were reversed by p14ARF overexpression in the Lv-p14ARF group (P < 0.05). As shown in , the lung tissue of the Con group was normal in shape, that is, the structure of the bronchiolar lumen was normal, without inflammatory cell infiltration, fibrosis, and increased mucus secretion. However, typical BO pathological features were observed in the BO group and the Lv-Con group. HE staining indicated that disorderly shedding of epithelial cells, and accompanied by a large number of inflammatory cell infiltration of bronchiole and alveolar were observed in the BO group and the Lv-Con group. Moreover, the results from Masson staining showed the peribronchial fibrosis and a large amount of collagen deposition around the bronchioles in the modeling group. PAS staining displayed the increased mucus secretion in the BO group and the Lv-Con group. After transfection with p14ARF, although there was still collagen deposition around the bronchioles, the inflammation infiltration and fibrosis were greatly ameliorated ().
Figure 2. p14ARF regulated the PI3K/AKT pathway in BO mice. WB detected the expression of p14ARF and PI3K/AKT pathway–related proteins (n = 3).
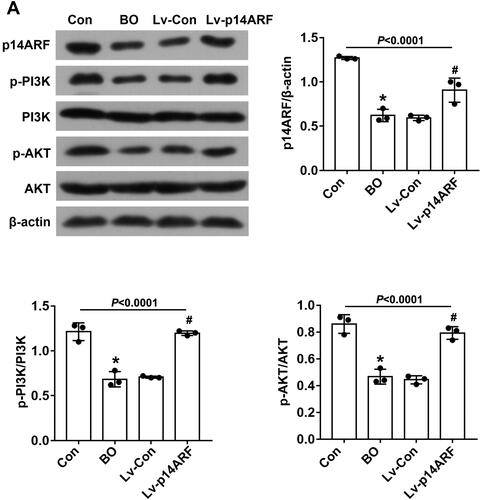
Figure 3. p14ARF ameliorated the pathological morphology of lung tissue in BO mice. On the 28th day, 10 mice in each group (Con group, BO group, Lv-Con group, and Lv-p14ARF group) were sacrificed, and HE staining (Black arrows indicated inflammatory cell infiltration, and red arrows indicated alveolar atrophy and dissolution), Masson staining (Yellow arrows indicated collagen deposition), and PAS staining (Green arrows indicated the glycogen deposition) were performed.
P14arf improved pulmonary dysfunction
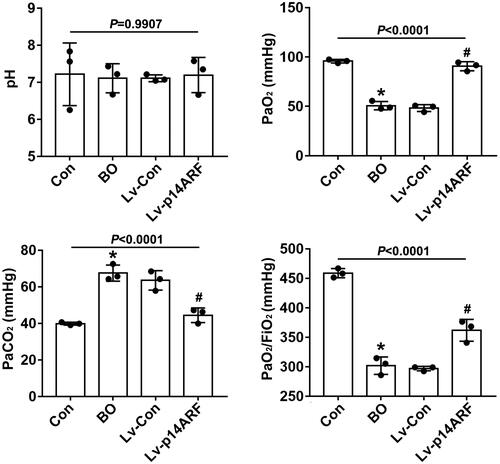
The values of pH, PaO2, and PaO2/FiO2 were decreased while that of PaCO2 was increased in the BO group compared with the Con group (P < 0.05), indicating that the mice had a tendency to be hypoxic, the lung oxygen exchange efficiency decreased, and the lung oxygen diffusion disorder appeared.Citation23 However, few differences in indicators were noted between the BO and Lv-Con groups (P > 0.05). In addition, the values of pH, PaO2, and PaO2/FiO2 were increased but that of PaCO2 was decreased in the Lv-p14ARF group compared with the BO group (P < 0.05), that is, the lung oxygenation function and the lung diffusion function of the mice both improved ().
Levels of MMP-2, MMP-9, and TIMP-1 in BALF and lung tissue
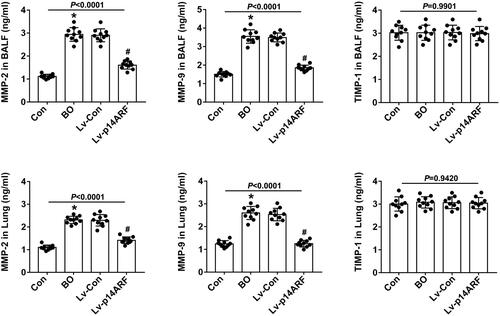
MMP-2, MMP-9, and TIMP-1 can regulate the remodeling and degradation of extracellular matrix,Citation24,Citation25 while the imbalance of MMP/TIMP leads to airway fibrosis and remodeling. As shown in , whether in BALF or lung tissue, the levels of MMP-2 and MMP-9 were significantly higher in the BO group than that in the Con group (P < 0.05), while TIMP-1 levels kept unchanged (P > 0.05). After further transfection of Lv-p14ARF, the levels of MMP-2 and MMP-9 were significantly down-regulated (P < 0.05).
Figure 5. ELISA detected the levels of MMP-2, MMP-9, and TIMP-1 in BALF and lung tissue. On the 28th day, 20 mice in each group were sacrificed, and 10 mice were prepared for lung tissue homogenization and the other 10 mice were used to collect BALF. The levels of MMP-2, MMP-9, and TMP-1 in BALF and lung tissues were detected using ELISA (n = 10).
P14arf inhibited pulmonary cell apoptosis
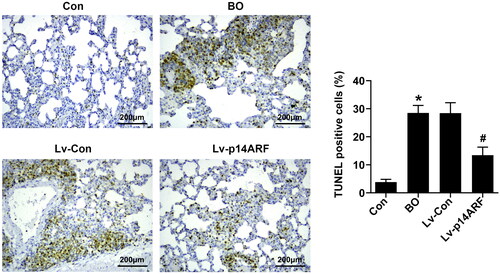
As shown in , the number of TUNEL-positive cells was higher in the BO group than in the Con group (P < 0.05). The number of TUNEL-positive cells significantly reduced in the Lv-p14ARF group compared with the BO group (P < 0.05), indicating that up-regulation of p14ARF inhibited lung cell apoptosis.
Effects of p14ARF on inflammation
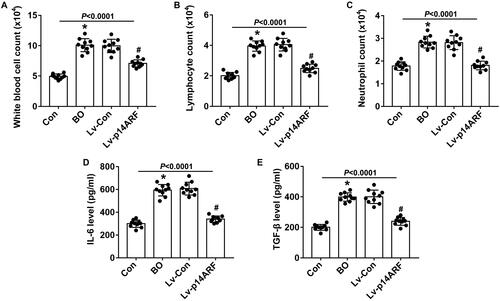
To clarify the effect of p14ARF on the inflammatory infiltration of BO mice, we counted the inflammatory cells in BALF. As shown in , the number of white blood cells, lymphocytes, and neutrophils in the BALF was significantly increased in the BO group (P < 0.05). The up-regulation of p14ARF remarkably reduced the numbers of white blood cells, lymphocytes, and neutrophils in BALF (P < 0.05). As shown in , the levels of IL-6 and TGF-β were higher in the BO group than in the Con group (P < 0.05), which were reversed by up-regulation of p14ARF (P < 0.05), demonstrating that the inflammatory response was alleviated by p14ARF overexpression.
Discussion
BO is an irreversible chronic obstructive pulmonary disease of small airways. Its clinical symptoms are usually serious and even endanger the patient’s life.Citation26 At present, the treatment of BO is mostly based on macrolide antibiotics,Citation27,Citation28 glucocorticoids,Citation29,Citation30 and immunosuppressive agents,Citation30 but the above treatments can only delay the deterioration of lung function in some patients. Therefore, this study intended to explore the specific pathogenesis of BO and provide a theoretical basis for the clinical development of targeted drugs.
P14ARF is a variable–reading frame gene of CDKN2A, which can combine with MDM2 and p53 to form a complex, cause cell cycle arrest in G1/G2 phase,Citation31,Citation32 and promote cancer cell apoptosis. Based on p14ARF's significant cell cycle regulation and cell protection, we investigated whether it could improve BO symptoms. First, we detected the expression changes of p14ARF and PI3K/AKT pathway in lung tissue homogenates of BO mice in 0, 3, 7, 14, 28, and 56 days. And it turned out that p14ARF, p-PI3K, and p-AKT mRNA levels and protein expressions were decreased in a time gradient. NA aerosol treatment induced the thickening of the bronchial wall and basal layer, a large number of inflammatory cell infiltration, the peribronchial fibrosis, narrowing and deforming of the lumen, and increase of mucus secretion in the BO mice, while p14ARF overexpression could alleviate these damage in lung tissues. We increased the level of p14ARF, and as a result, the expressions of p-PI3K and p-AKT protein were remarkably upregulated, and the inflammation infiltration and tracheal fibrosis of lung tissue, lung oxygenation function, and diffusion function were improved, accompanied by a decrease in the apoptosis ratio. In other words, p14ARF improved lung tissue morphology and lung dysfunction in BO mice and inhibited lung cell apoptosis. The protective effect was related to the PI3K/AKT pathway.
The pathogenesis of BO involves airway inflammation,Citation33 fibroblast proliferation,Citation34 epithelial–mesenchymal transition,Citation35 vascular remodeling,Citation36 and airway fibrosis. As one of the pathological features of BO, inflammatory infiltration includes inflammatory cell activation and epithelial cell damage.Citation33 We observed inflammation infiltration, epithelial cell abscission, and tracheal stenosis in the pathological sections of lung tissues in the BO and Lv-Con groups. In addition, the levels of inflammatory cytokines (IL-6 and TGF-β) and the number of inflammatory cells (white blood cells, neutrophils, and lymphocytes) in BALF were increased, indicating that the inflammation persisted. However, the aforementioned inflammation indicators were significantly down-regulated after p14ARF transfection.
With the aggravation of epithelial cell injury, the primordial airway cells are gradually depleted, resulting in the impairment of epithelial regeneration and repair function.Citation37 At this time, tissue fibrous repair is activated to promote the proliferation of fibroblasts. Meanwhile, TGF-β secreted by inflammatory cells (leukocytes and neutrophils) can also promote the growth of fibroblasts, which in turn causes fibrosis of surrounding tissues.Citation38–40 The continuous progression of fibrosis around the bronchioles causes centripetal stenosis to irreversible complete blockage. The imbalance of the MMP/TIMP ratio affects tracheal remodeling by regulating the deposition and degradation of the extracellular matrix.Citation41 MMPs secreted by neutrophils can degrade the extracellular matrix and promote the infiltration of inflammatory cells and fibroblasts into bronchioles.Citation42 However, TIMP can antagonize MMPs. The results of our study also confirmed that the imbalance of MMP/TIMP promoted BO tracheal remodeling and fibrosis. TIMP-1 levels remained unchanged. The levels of MMP-2 and MMP-9 in the lung tissue and BALF were significantly increased in the BO group compared with the Con group. Also, the levels of MMP-2 and MMP-9 reduced in the Lv-p14ARF group, which further revealed that p14ARF improved tracheal remodeling in BO mice.
In conclusion, we confirmed that p14ARF ameliorated the inflammatory infiltration, tracheal fibrosis, airway remodeling, and pulmonary dysfunction of lung tissue, but inhibited pulmonary cell apoptosis in a BO model induced by 5% NA aerosol inhalation, thus providing an experimental basis for the development of new BO predictors.
Author contributions
(I) Conception and design: Ting Yang
(II) Administrative support: Chang Xu
(III) Provision of study materials or patients: Niu Ding, Shujuan Luo
(IV) Collection and assembly of data: Bichen Wu, Shijie Jin
(V) Data analysis and interpretation: Yanping Chen
(VI) Manuscript writing: All authors
(VII) Final approval of manuscript: All authors
Competing interests
The authors declare no conflicts of interest.
Acknowledgments
Not applicable.
Availability of data and material
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Moonnumakal SP, Fan LL. Bronchiolitis obliterans in children. Curr Opin Pediatr. 2008;20(3):272–278. doi:10.1097/MOP.0b013e3282ff62e9.
- Colom AJ, Teper AM, Vollmer WM, Diette GB. Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis. Thorax. 2006;61(6):503–506. doi:10.1136/thx.2005.044909.
- Cullinan P, Bush A. Growing old(er) with postinfectious bronchiolitis obliterans. Thorax. 2015;70(2):103–104. doi:10.1136/thoraxjnl-2014-206168.
- Hertz MI, Taylor DO, Trulock EP, et al. The registry of the international society for heart and lung transplantation: nineteenth official report-2002. J Heart Lung Transplant. 2002;21(9):950–970. doi:10.1016/S1053-2498(02)00498-9.
- Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168(11):1277–1292. doi:10.1164/rccm.200301-053SO.
- King TE.Jr. Miscellaneous causes of bronchiolitis: inhalational, infectious, drug-induced, and idiopathic. Semin Respir Crit Care Med. 2003;24(5):567–576. doi:10.1055/s-2004-815604.
- Yousem SA. The pulmonary pathologic manifestations of the CREST syndrome. Hum Pathol. 1990;21(5):467–474. doi:10.1016/0046-8177(90)90002-m.
- Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report–2007. J Heart Lung Transplant. 2007;26(8):769–781. doi:10.1016/j.healun.2007.06.004.
- Ito T, Nishida N, Fukuda Y, Nishimura T, Komeda T, Nakao K. Alteration of the p14(ARF) gene and p53 status in human hepatocellular carcinomas. J Gastroenterol. 2004;39(4):355–361. doi:10.1007/s00535-003-1302-9.
- Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat Res. 2005;576(1–2):22–38. doi:10.1016/j.mrfmmm.2004.08.021.
- Muer A, Overkamp T, Gillissen B, et al. p14(ARF)-induced apoptosis in p53 protein-deficient cells is mediated by BH3-only protein-independent derepression of Bak protein through down-regulation of Mcl-1 and Bcl-xL proteins. J Biol Chem. 2012;287(21):17343–17352. doi:10.1074/jbc.M111.314898.
- Weber JD, Jeffers JR, Rehg JE, et al. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14(18):2358–2365. 2 doi:10.1101/gad.827300.
- Ozenne P, Eymin B, Brambilla E, Gazzeri S. The ARF tumor suppressor: structure, functions and status in cancer. Int J Cancer. 2010;127(10):2239–2247. doi:10.1002/ijc.25511.
- Saito K, Takigawa N, Ohtani N, et al. Antitumor impact of p14ARF on gefitinib-resistant non-small cell lung cancers. Mol Cancer Ther. 2013;12(8):1616–1628. doi:10.1158/1535-7163.MCT-12-1239.
- Zhang H, Nie W, Huang F. The correlation relationship between P14ARF Gene DNA methylation and primary liver cancer. Med Sci Monit. 2015;21:3077–3082. doi:10.12659/MSM.894395.
- Huang SJ, Li ZL, Ma XL, Du B, Wang JL. TREML4: a potential target for immunotherapy of sepsis. Discov Med. 2021;32(166):87–92.
- Walker NM, Belloli EA, Stuckey L, et al. Mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2 as key signaling intermediates in mesenchymal cell activation. J Biol Chem. 2016;291(12):6262–6271. doi:10.1074/jbc.M115.672170.
- Hamilton G, Abraham AG, Morton J, et al. AKT regulates NPM dependent ARF localization and p53mut stability in tumors. Oncotarget. 2014;5(15):6142–6167. doi:10.18632/oncotarget.2178.
- Liu KW, Feng H, Bachoo R, et al. SHP-2/PTPN11 mediates gliomagenesis driven by PDGFRA and INK4A/ARF aberrations in mice and humans. J Clin Invest. 2011;121(3):905–917. doi:10.1172/JCI43690.
- Institute of Laboratory Animal Resources (US). Committee on Care, & Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 2011.
- Yin J, Ma X, Huang F, Ma Y, Li Y. Bronchiolitis obliterans murine model induced by nitric acid aerosol inhalation: an economical and reproducible model. Exp Lung Res. 2018;44(3):143–152. doi:10.1080/01902148.2018.1455926.
- Costa CL, Spilborghs GM, Martins MA, Saldiva PH, Mauad T. Nitric acid-induced bronchiolitis in rats mimics childhood bronchiolitis obliterans. Respiration. 2005;72(6):642–649. doi:10.1159/000087363.
- Ege T, Huseyin G, Yalcin O, Us MH, Arar C, Duran E. Importance of pulmonary artery perfusion in cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(2):166–174. doi:10.1053/j.jvca.2004.01.022.
- Mattos W, Lim S, Russell R, Jatakanon A, Chung KF, Barnes PJ. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest. 2002;122(5):1543–1552. doi:10.1378/chest.122.5.1543.
- Hoshino M, Takahashi M, Takai Y, Sim J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J Allergy Clin Immunol. 1999;104(2):356–363. doi:10.1016/S0091-6749(99)70379-9.
- Gozal D. Post-infectious bronchiolitis obliterans in children: is general quality of life the right measure? J Pediatr (Rio J). 2018;94(4):340–341. doi:10.1016/j.jped.2018.01.001.
- Gan CT, Ward C, Meachery G, Lordan JL, Fisher AJ, Corris PA. Long-term effect of azithromycin in bronchiolitis obliterans syndrome. BMJ Open Respir Res. 2019;6(1):e000465. doi:10.1136/bmjresp-2019-000465.
- Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax. 2015;70(5):442–450. doi:10.1136/thoraxjnl-2014-205998.
- Bergeron A, Chevret S, Chagnon K, et al. Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191(11):1242–1249. doi:10.1164/rccm.201410-1818OC.
- Kim SW, Rhee CK, Kim YJ, Lee S, Kim HJ, Lee JW. Therapeutic effect of budesonide/formoterol, montelukast and N-acetylcysteine for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Respir Res. 2016;17(1):63. doi:10.1186/s12931-016-0380-1.
- Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–5774. doi:10.1200/JCO.2008.17.7279.
- Sharma G, Mirza S, Prasad CP, Srivastava A, Gupta SD, Ralhan R. Promoter hypermethylation of p16INK4A, p14ARF, CyclinD2 and Slit2 in serum and tumor DNA from breast cancer patients. Life Sci. 2007;80(20):1873–1881. doi:10.1016/j.lfs.2007.02.026.
- Lu N, Yang Y, Liu H, et al. Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine. 3 Biotech. 2019;9(11):392. doi:10.1007/s13205-019-1917-z.
- Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-beta-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017;18(10):2157. doi:10.3390/ijms18102157.
- Jolly MK, Ward C, Eapen MS, et al. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev Dyn. 2018;247(3):346–358. doi:10.1002/dvdy.24541.
- Semenza GL. Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization. J Investig Med. 2016;64(2):361–363. doi:10.1097/JIM.0000000000000206.
- Swatek AM, Lynch TJ, Crooke AK, et al. Depletion of airway submucosal glands and TP63(+)KRT5(+) basal cells in obliterative bronchiolitis. Am J Respir Crit Care Med. 2018;197(8):1045–1057. doi:10.1164/rccm.201707-1368OC.
- Wang XX, Jiang LL. Silencing PDCD10 protects the uterus against ischemia/reperfusion injury by inhibiting fibrosis, inflammation and apoptosis in rats. J Biol Regul Homeost Agents. 2022;36(3):711–720.
- Jin H, Cai W, Yu D, Fan J, Liu Q, Yu J. Development of proliferative vitreoretinopathy is attenuated by chicken ovalbumin upstream promoter transcriptional factor 1 via inhibiting epithelial-mesenchymal transition. Discov Med. 2022;34(172):103–113.
- Wu Y, Deng H, Sun J, Xu Y. Isoquercitrin alleviates inflammation in diabetic nephropathy by inhibiting TLR4/NF-κB signaling pathway. J Biol Regul Homeost Agents. 2022;36(2):339–351.
- Kennedy VE, Todd JL, Palmer SM. Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am J Transplant. 2013;13(3):552–561. doi:10.1111/ajt.12091.
- Liu X, Yue Z, Yu J, et al. Proteomic characterization reveals that MMP-3 correlates with bronchiolitis obliterans syndrome following allogeneic hematopoietic cell and lung transplantation. Am J Transplant. 2016;16(8):2342–2351. doi:10.1111/ajt.13750.