Abstract
Backgroud: Aspergillus fumigatus (A. fumigatus) is a clinically important fungal pathogen. Invasive pulmonary aspergillosis (IPA) is the main fungal infection with increased morbidity and mortality in immunocompromised populations, although treatments are available. An innate DNA sensor known as cyclic GMP-AMP Synthase (cGAS) has recently been discovered that senses invading pathogens and has a significant impact on innate immunity. It can activate the cGAS-STING signaling pathway to stimulate downstream signals. But it is still unclear what role it plays in IPA’s pathogenesis.
Methods: An investigation into the infection of A. fumigatus was conducted by inhibiting cGAS activity in vivo and in vitro using siRNA and RU.521(an inhibitor of cGAS).
Results: We discovered that suppressing cGAS increased the host’s susceptibility to A. fumigatus and harmed those with infections by enhancing pulmonary tissue damage and edema, as well as decreasing fungal clearance. Furthermore, our findings show that inhibiting or silencing cGAS can exacerbate the inflammatory response in IPA mouse models and human bronchi epithelial cells (HBECs) treated with A. fumigatus by upregulating the production of inflammatory genes with non-type 1 interferon.
Conclusion: Based on our analysis, we conclude that activating cGAS might increase host resistance to A. fumigatus, protect against pulmonary illnesses brought on by A. fumigatus and that exploring the cGAS-STING signaling pathway is beneficial not only for the immunological investigation of IPA but also may be a potential therapeutic objective.
Introduction
Aspergillus fumigatus is a highly concerning pathogen that can cause severe and potentially fatal illnesses in up to 90% of individuals with immunosuppressed and immunocompromised states, invasive pulmonary aspergillosis is a common type of pulmonary aspergillosis infection and causes severe destruction of lung tissue.Citation1–2 With a high frequency in both hospital and community settings, it is a substantial source of increased mortality in poor clinical outcome.Citation3 Which is related both to diagnostic difficulties and to the deterioration of the disease due to treatment difficulties resulting from increased drug resistance found in clinical aspergillosis strains, further emphasizes the urgent need to develop diagnostic strategy and new therapies.Citation4–5 Therefore, a deeper analysis of the interaction between A. fumigatus and host immunity and fully exploring the mechanisms of infection by pathogenic microorganisms entering the organism are particularly crucial to facilitate the development of potential new therapies and better management techniques to benefit the improvement of disease in clinically high-risk patients and lessen its effects.Citation6–7
Conidia are the main component of Aspergillus fumigates and are ubiquitous in the environment. A daily intake of 100 to 1000 conidia is estimated for every individual. Their tiny size allows them to reach the deepest parts of the lungs, measuring between 2 and 3 μm. When our immune function is impaired or weakened, the fungus that colonizes our respiratory system can exert its disease-causing virulence to harm our physical health.Citation8 When we breathe, microorganisms can enter the airway and interact with epithelial cells for the first time. Apart from serving as physical barriers to remove dangerous substances from the body, they also act as sensors to interact with and respond to microbes, an important key to host immune defense.Citation9 On lung epithelial cells, pattern recognition receptors (PRRs) regulate chemokines and cytokines by identifying pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and serve as a bridge between humoral immunity and cellular immunity to make it more effective at eliminating hazardous chemicals.Citation10–11 Of course, when our cells were stimulated by conidia, receptors on the lung epithelium are triggered to recognize the microorganism, which in turn releases cytokines or chemokines, and recruits immune cells to protect the host. In some cases, however, the body’s excessive inflammatory response can be fatal, leading to serious tissue damage and the persistence of fungi.Citation12 As a result, a moderate response from the immune system and inflammatory conditions is required in patients who have Aspergillus fumigatus infection.
cGAS is an innate DNA sensor that recognizes double-stranded DNA mislocated in the cytoplasm during infection, cGAS and interferon gene stimulating factor (STING) and TANK-binding kinase 1 (TBK1) are activated and phosphorylated by cellular stress and tissue damage to enhance mRNA transcription of type I interferons and other inflammation chemical cytokines.Citation13 The cGAS-STING pathway works differently depending on pathogens and infection patterns and is involved in a variety of pathogen infections.Citation14 It is believed that the suppression of the cGAS-STING pathway results in diminished immune defenses against pathogens. However, overactivation can lead to severe inflammatory storms and tissue damage.Citation15 When infected with cytomegalovirus (CMV) and Pseudomonas aeruginosa (PA), this pathway can promote pathogen clearance; But when infected with dengue virus (DENV) and monocytosis listeria, many pathogens have evolved effective strategies to counter cGAS and STING function to promote themselves persistent in host cells.Citation16–17 Nevertheless, whether cGAS is involved in the pathogenesis and development of IPA, as well as its function and mechanism have not been studied.
Materials and methods
Preparation of Aspergillus fumigatus conidia
Aspergillus fumigatus strains (clinical isolates 20150725185) were incubated from the Department of Laboratory center, the First Affiliated Hospital of Chongqing Medical University on Sabouraud agar plates (1% penicillin, streptomycin double antibody to prevent bacterial contamination) at 37 °C for 7 days. According to the previous method, Rinsing, scraping, filtrating, and adjusting the conidia suspension concentration.
The mouse model with Aspergillus fumigatus infection
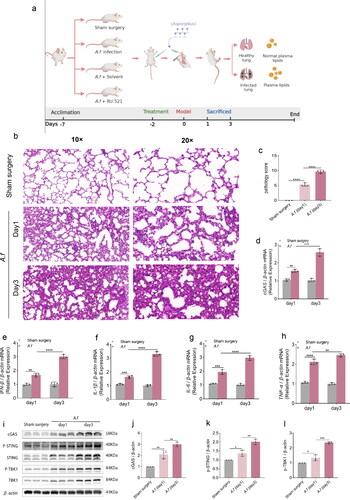
The C57/BL 6 mice used in the experiment (male, 6–8 wk, 22–26 g) were purchased from Chongqing Bernerswell Biotech Co., Ltd. Under specified pathogen-free (SPF) conditions, light/dark cycles are 12h, respectively. and water and food are provided free of charge throughout the experiment. Monitor the weight, survival, and health status of the mice daily. After 1 week of acclimation before the start of the experiment, the mice were randomly grouped into 4 groups (5 mice per group). (1) Sham surgery group: Mice were only intrathecally injected with 50ul sterile PBS. (2) A. fumigatus infection group: Mice were intratracheally injected with A. fumigatus spore suspension. (3) A. fumigatus +Solvent group: A. fumigatus infection group + Solvent for RU.521 (an inhibitor of cGAS, 5 mg/kg, MedChemExpress), (4) A. fumigatus +RU.521 group: A. fumigatus infection group + RU.521. According to the drug instructions, the solvent formulation of RU.521 is 10% DMSO + 40% PEG300 + 5% Tween-80 + 45% saline. Two days before the A. fumigatus infection model was constructed, mice were intraperitoneally injected RU.521 until they were sacrificed. Then, we lightly anesthetized and placed the mice in a supine position, a volume of 50ul of an infection dose of 6 × 107 A. fumigatus spores suspension diluted in sterile PBS were injected into the trachea as a research model for A. fumigatus infection.Citation18 Within 2 h after injection, the mice fully recover. Finally, euthanasia is performed 24 and 72 h after surgery, respectively. All mice were collected retroorbital blood, alveolar lavage fluid, and lung tissue for follow-up studies. The constitution of their group and the course of the experiment shows in .
Figure 1. (a) The constitution of four group and the courses of the experiment. (b–c) H&E staining images (× 10 and × 20) and clinical pathology scores in lung tissues. (d–h) Quantification analysis of mRNA levels of cGAS, IFN-β, IL-1β, IL-6, and TNF-α in lung tissues. (i–l) Protein detected by Western Blot and densitometry analysis of cGAS, p-STING, p-TBK1 in lung tissues. (Data are presented as the mean ± SD, *p < 0.05, **p <0 .01, ***p <0 .001, ****p <0 .0001, n = 3).
Cell culture and infection
From the Cell Bank of the Chinese Academy of Sciences, Human bronchial epithelial cell lines (HBECs) were acquired and cultured in a complete RPMI-1640 medium (Gibco, Grand Island, USA), supplied with 10% fetal bovine serum (Excell Bio, Shanghai, China) in an incubator with the humid environment (37 °C, 5% CO2). To evaluate the effect of A. fumigatus on HBECs, cells were infected with A. fumigatus at a multiplicity of infection (MOI) of 5:1. They were further used for qPCR, Western blot, and cellular immunofluorescence.
Silencing cGAS
siRNA for Human cGAS (si cGAS) was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The sequences of the siRNA designed as follows: si cGAS-1, sense: 5′-CUGCCUUCUUU CACGUAUGUATT-3′ antisense:5′-UACAUACGU GAAAGAAGGCAGTT-3′; si cGAS-2, sense: 5′-CAGCUUCUAAGAUGCUGUCAATT-3′ antiense:5′-UUGACAGCAUCUUAGAAGCUGTT-3′, si cGAS-3, sense:5′-CGUGAAGAUUUCUGCACCUAATT-3′ antisense: 5′-UUAGGUGCAGA AAUCUUCACGTT-3′. Following instructions from the manufacturer, si NC was used as control, Lipofectamine2000 (Invitrogen) was used to transfect cells.
Real-time quantitative polymerase chain reaction (RT-qPCR)
RNAiso Plus (Takara Bio, Inc.) is used to isolate total RNA from lung tissue and cells, RNA continues to be reverse-transcribed into cDNA (Takara Bio, Inc.), qPCR was performed on a CFX96 device (Bio-rad) using Takara’s SYBR Green Premix Ex Taq following the manufacturer’s instructions. Using β-actin as an internal reference, relative quantification (2−ΔΔCt) was used to analyze the expression of target genes. The RT-qPCR primers of cGAS, IFN-β, IL-1β, IL-6, TNF-α, and β-actin are shown in .
Table 1. A sequence of nucleotides used in qPCR amplification for the particular primers.
Enzyme-linked immunosorbent assay (ELISA)
The serums of sham surgery group, A. fumigatus infection group and group with A. fumigatus infection treated with solvent or RU.521 were collected to detect the serum concentrations of IFN-β, IL-1β, IL-6, and TNF-α using the detection kit at day 3. (Quanzhou Jiubang Biotechnology Co., LTD.).
Western blotting assay
With the help of RIPA lysis buffer (Beyotime, Shanghai, China), protein lysates for tissues and cells were prepared along with 1% (vol/vol) phosphatase inhibitors and phenylmethanesulfonyl fluoride (PMSF). Concentrations were estimated by using the Bicinchoninic Acid (BCA) Protein detection Kit (Beyotime, Shanghai, China). Electrophoresis was performed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a polyvinylidene difluoride (PVDF) membrane. Blocking with 5% (w/v) skims milk for 2h at room temperature (RT), followed by rabbit anti-mouse β-actin (cat. 20536-1-AP,1:1000, Proteintech), cGAS (cat.26416-1-AP,1:1000, Proteintech), p-STING (cat.50907T(Ser366,); cat.62912S1(Ser365,),1:1000, Cell Signaling Technology), STING (cat. 19851-1-AP,1:1000, Proteintech), p-TBK1 (cat. 5483 T,1:1000, Cell Signaling Technology), TBK1 (cat.28397-1-AP 1:1000, Proteintech) overnight at 4 °C, then incubate the secondary antibody (BA1054, 1:5000, Boster) for 1 h at RT. Furthermore, the levels of the internal reference gene grayscale values were used to estimate the target genes’ protein expression levels.
Staining
Infected lungs of C57BL/6 mice were treated at 4% paraformaldehyde for paraffin-embedded segments (n = 3/group/times). Immunohistochemical(IHC) staining was performed using the immunohistochemical test kit (Zsbio, Beijing, China), following the manufacturer’s protocol. For immunofluorescence(IF) staining, lung tissue sections, and cell slides were combined with a primary antibody and incubated with a secondary antibody: Alexa Fluor-488(cat. A-23210, 1:200, Abbkine) and Alexa Flu-594 (Cat. A-23420, 1:200, Abbkine), followed by nuclear staining using DAPI(Sigma). Primary antibodies used in immunostaining: cGAS (cat. 26416-1-AP, 1:50, Proteintech), Uteroglobin-Epithelial cell markers-Epithelial cell marker (cat. ab213203, 1:500, Abcam), For histological analysis, hematoxylin and Eosin(H&E) 、Gomori Methenamine Silver (GMS) stain were used to stain samples of lung tissue. With minor modifications, a previously described scoring system was used to classify lung injury and historical scoring was performed blindly. lung injury was scored according to criteria defined as follows: (1) alveolar hyperemia, (2) hemorrhage, (3) interstitial or aggregation of interstitial or neutrophils, and (4) thickening of the alveolar septum or hyaline membrane formation. Each item was scored on a 4-point scale except (3) as follows: 0 = minimal damage, 1 = mild damage, 2 = moderate damage, 3 = severe damage, and 4= maximal damage. Pneumoniae pulmonary infection scores were approximated through the method by the scoring standard. The scoring standard is based on (1) the infiltration degree of inflammatory cells around the trachea and bronchiole (2) quality of trachea and bronchiole infiltrate (3) infiltration degree of inflammation in trachea and bronchiole cavity (4) infiltration of inflammatory cells around blood vessels, and (5) inflammation of the lung parenchyma. The severity of the inflammation is directly proportional to the magnitude of the score. The lung injury score of neutrophil infiltration was based on the 5-point scale of lung infection. The overall histological score was also determined in ().Citation19 All sections were observed with a Carl Zeiss microscope (Carl Zeiss, Inc., Oberkochen, Germany).
Table 2. Scoring criteria for pathological scores.
Determination of A. fumigatus CFU numbers
Mouse lungs were separated aseptically and tissue was proportionally homogeneously diluted. Three dishes were inoculated with each concentration gradient and cultured at 37 °C for 72 h. The pulmonary fungal load of mice was measured by colony count on the plate.
Wet-to-dry weight ratio
After the operation, the mouse lung tissue was placed in a tube and weighed as wet weight. It was then left at 60 °C for at least 7 days until the tissue was dry and weighed again for dry weight.
Statistical analysis
SPSS 20.0 (IBM, NY, USA) and GraphPad Prism 8.0.2(GraphPad Software, California, USA) were performed to conduct the statistical analysis. The Student’s unpaired two-tailed test was used to compare the two groups while one-way variance analysis (ANOVA) was conducted to evaluate the overall differences between the groups. Every experiment was done independently at least three times. All data are performed as arbitrary deviations. It was determined that the two-tailed p-value was statistically significant at 0.05.
Results
A. fumigatus infection causes lung damage
We established lung infection with A. fumigatus in mice. As shown in , Compared to the sham surgery group, we observed the enhanced quantities of inflammatory cell infiltration in the bronchioles, perivascular, and vascular lumen of the A.fumigatus infected lungs, and the increased lung pathology scores were shown in the A. fumigatus infection group on day 1 and day 3 (p < 0.0001 and p < 0.0001, ). Meanwhile, Infection like this kind of fungal dramatically has increased many cytokine mRNA production, including IFN-β (p < 0. 01 and p < 0.0001, ), IL-1β (p < 0.001 and p < 0.0001, ), IL-6 (p < 0.0001 and p < 0.01, ), TNF-α (p < 0.0001 and p < 0. 01, ) in the lung tissue at day 1 and day 3.
cGAS expression in mouse lung tissue
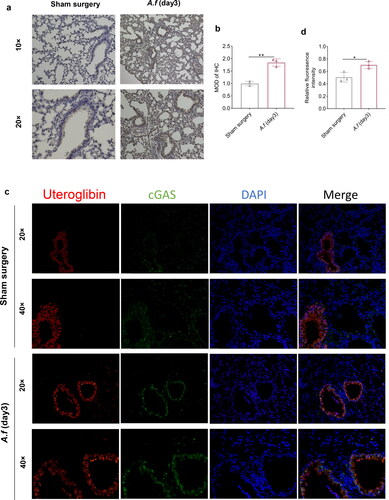
To investigate whether the lungs infected with conidia produced an immune response by activating cGAS and downstream molecules, the differences between pre-and post-infection were detected at the mRNA and protein expression by qPCR, Western blot, immunostaining, etc. our study showed that the mRNA expression of cGAS after A. fumigatus infection was elevated (p < 0.01 and p < 0. 0001, ), and the corresponding pathway downstream protein expression was up-regulated with increasing infection time (), including cGAS (p < 0.01 and p < 0.01, ), p-STING (p < 0.05 and p < 0. 01, ) and p-TBK1 (p < 0.05 and p < 0. 001), ). These results illustrate downstream pathway of cGAS was activated in the lungs of fungi. Data from the immunohistochemistry (IHC) revealed that cGAS levels in infected lungs were significantly higher than those in uninfected ones on day 3. (p < 0.01, ). Meanwhile, tissue immunofluorescence (IF) data also showed that A. fumigatus infection group has higher cGAS levels than the sham surgery group (p < 0.05, ), which was consistent with previous Western blot data. These results demonstrated that cGAS was activated in A. fumigatus-infected mouse lung tissue together.
Figure 2. (a) IHC staining images in lung tissues (× 10 and × 20). (b) Quantitative analysis MOD of cGAS. (c) IF co-staining images: cGAS (green), Uteroglobin-Epithelial cell markers (red), and DAPI (blue) (× 20 and × 40). (d) Quantitative analysis of relative fluorescence intensity of cGAS. (Data are presented as the mean ± SD, *p < 0.05, **p <0 .01, ***p <0 .001, ****p <0 .0001, n = 3).
Inhibiting cGAS accelerated the disease process
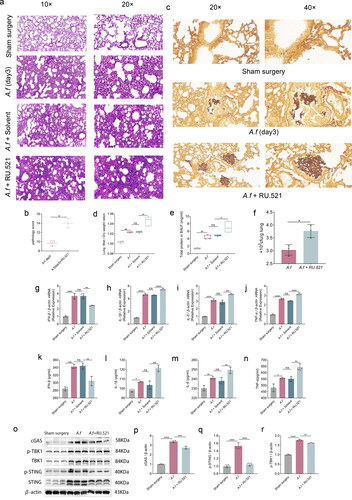
To explore the function of cGAS, RU.521 was used to inhibit the cGAS in C57BL/6 mice. The RU.521-treated infected group showed more severe alveolar hemorrhage and severe lung inflammation on day 3 than in the infected group (). On day 3 postinfection, cGAS inhibition increased the disease severity score, as evidenced by the mean clinical score. (p < 0.01, ), and when comparing the infected group with RU.521 to the infected group on day 3, the clearance of fungi decreased was showed by GMS staining (), the lung wet weight/dry weight ratio, a marker of pulmonary edema, was higher (p < 0.05, ). Moreover, the respiratory tract of mice experienced an increase in capillary leakage by inhibiting cGAS, leading to a significant increase in albumin levels in bronchoalveolar lavage fluid (BALF) in the lungs of these mice (p < 0.05, ). The lungs of mice who received RU.521 treatment had a higher fungi load after A. fumigatus infection compared to the infection group (p < 0.05, ). Through RT-qPCR and ELISA, they determined how RU.521 impacted those inflammatory cytokines in lungs. Inhibition of cGAS, according to mRNA data, increased the transcription of IL-1β (p < 0.0001, ), IL-6 (p < 0.001, ), and TNF-α (p < 0.0001, ). Moreover, ELISA’s data revealed that the increased translation of IL-1β (p < 0.001, ), IL-6 (p < 0.01, ), and TNF-α (p < 0.01, ). In addition, the inhibitor also suppressed the downstream pathway protein expression by reducing the expressions of cGAS (p < 0.001, ) p-STING (p < 0.0001, ), p-TBK1 (p < 0.01, ) which was showed in western blot () and the level of IFN-β mRNA and IFN-β protein (p < 0.01 and p < 0.01, , respectively) in infected lung tissues. Incorporating these findings suggests that the fungus’s pulmonary clearance could be reduced when cGAS is suppressed, which would result in tissue damage and host death.
Figure 3. (a–b) H&E staining images and histopathology scores in lung tissues (× 10 and × 20). (c) GMS stain images in lung tissues (× 20 and × 40). (d) Lung wet-to-dry weight ratios. (e) Albumin concentrations in BALF. (f) Colony-forming units (CFU) in the lungs of A. fumigatus + RU.521 and A. fumigatus infection at days 3. (g–j) Quantification analysis of mRNA levels of cGAS, IFN-β, IL-1β, IL-6, and TNF-α in lung tissues. (k–n) Quantification analysis of protein levels of IFN-β, IL-1β, IL-6, and TNF-α detected by Elisa. (o–r) Protein detected by Western Blot and densitometry analysis of cGAS, p-STING, p-TBK1 in lung tissues. (Data are presented as the mean ± SD, *p < 0.05, **p <0 .01, ***p <0 .001, ****p <0 .0001, n = 3).
cGAS expression in HBECs
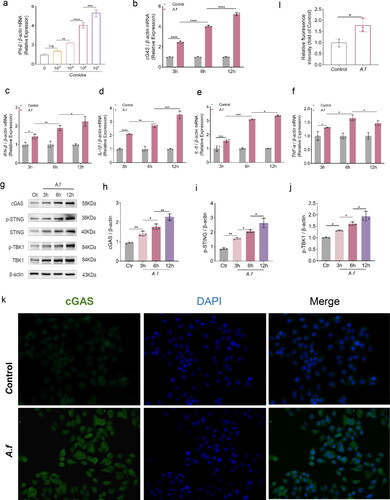
To understand the activation of cGAS in vitro, Western blot, qPCR and immunostaining were carried out to determine the concentration of cGAS in HBECs before and after A. fumigatus infection. First, the IFN-β mRNA expression was upregulated in a manner that significantly depends on the dose when the fungi stimulated HBECs (). Then, we focused on the immune response of HBECs. The mRNA level of many cytokine was increased with time at 3h, 6h, and 12h after A. fumigatus stimulation by qPCR: IFN-β (p < 0.05, p < 0.01, p < 0.05, , respectively), IL-1β (p < 0.0001, p < 0.01, p < 0.001, , respectively), IL-6 (p < 0.001, p < 0.001, p < 0.05, , respectively), and TNF-α (p < 0.05, p < 0.05, p < 0.05, , respectively). Furtherly, we investigated whether the downstream pathway of cGAS was activated after infection. According to the mRNA expression, cGAS mRNA was significantly elevated when A. fumigatus infection occurred ((p < 0.0001, p < 0.0001, p < 0.0001, , respectively)). cGAS protein relative fluoresence in A. fumigatus infections lung were higher than usual, according to IF data (p < 0.05, ). Last but not least, our data in western blot revealed that the protein levels of this pathway also increased post-infection (): cGAS (p < 0.01, p < 0.05, p < 0.01 , respectively), p-STING (p < 0.01, p < 0.05, p < 0.05 , respectively), and p-TBK1 (p < 0.05, p < 0.05, p < 0.05 , respectively). In light of our findings, it appears that cGAS and its downstream pathway were activated in HBECs fungi-infection and may be crucial.
Figure 4. (a) Quantification of relative mRNA expression of IFN-β in HBECs inoculated with the indicated concentrations of A. fumigatus conidia for 12 h. (b–f) Quantification analysis of mRNA expression of cGAS, IFN-β, IL-1β, IL-6, and TNF-α in HBECs infected with A. fumigatus for 3, 6 and 12h. (g) Protein of cGAS, STING, p-STING, TBK1, and p-TBK1 were detected by Western Blot. (h–j) Quantification of relative protein levels of of cGAS, p-STING, p-TBK1 in HBECs infected with A. fumigatus for 3, 6, and 12h. (k–l) IF co-staining images and relative fluorescence intensity analysis: cGAS (green) and DAPI (blue) (× 40). (Data are presented as the mean ± SEM, *p < 0.05, **p <0 .01, ***p < 0.001, ****p <0 .0001, n = 3).
cGAS suppressed the inflammatory cytokines in vitro
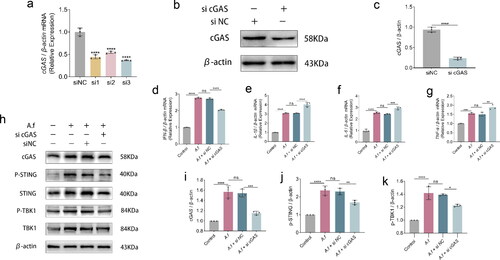
When cGAS was suppressed in HBECs in vitro to investigate its function in the regulation of inflammation, inflammatory cytokines mRNA levels were detected after cGAS was silenced in HBECs in vitro. Compared with siNC group, the interference efficiency of the three sequences of si cGAS is shown in (p < 0.0001, p < 0.0001, p < 0.0001, respectively). The interference capability of si cGAS-3 is optimal. And the effect was validated at the protein level again (p < 0.0001, ). By combining the data of qPCR and Western blot, si cGAS performance was verified. Western blot analysis revealed a simultaneous decrease in the pathway downstream molecules following HBECs transfected with si cGAS (), including cGAS protein (p < 0.0001 and p < 0.001, , p-STING protein (p < 0.001 and p < 0.01, ), p-TBK1 protein (p < 0.0001 and p < 0.05, ), The cGAS-knocked down HBECs were infected with conidia for 6 h, and the mRNA expression were observed by qPCR. Results from our study showed that lowering cGAS raised the levels of inflammatory cytokines mRNA in HBECs following infection, including IL-1β (p < 0.0001, ), IL-6 (p < 0.001, ), TNF-α (p < 0.01, ), while the level of IFN-β mRNA was decreased (p < 0.0001, ). Ultimately, suppressing cGAS may negatively influence the regulation of the immune response to fungi-infection in vitro.
Figure 5. (a–c) Quantification analysis of mRNA(a), protein(c) expression and western blot images after cGAS interference, IFN-β, IL-1β, IL-6, and TNF-α in HBECs infected with A. fumigatus for 3, 6 and 12h. (d–g) Quantification analysis of mRNA expression of IFN-β, IL-1β, IL-6, and TNF-αin cells transfected si cGAS. (h) Protein of cGAS, STING, p-STING, TBK1, and p-TBK1 were detected by Western Blot. (i–k) Quantification of relative protein levels of cGAS, p-STING, p-TBK1. (Data are presented as the mean ± SEM, *p < 0.05, **p <0 .01, ***p <0 .001, ****p <0 .0001, n = 3).
Discussion
There is growing evidence that the epidemiology of invasive aspergillosis is changing and that the general population is at increased risk of developing more aggressive infections.Citation20 To better understand the interaction between A. fumigatus and the host, we established non-immunocompromised mice model of pulmonary infection with A. fumigatus to avoid any experimental immunosuppression that might affect the host response to infection and reduce the complexity of model understanding.Citation21 In our study, we have demonstrated that cGAS acts as a protective factor in the response of host immune against A. fumigatus. We found that cGAS were activated and up-regulated the expression of type I interferon IFN-β during Aspergillus fumigatus infection. Compared with the sham surgery group, the infected mice treated with RU.521 were more susceptible to A. fumigatus, with reduced fungal clearance, more severe tissue edema and protein leakage in BALF. Thus, cGAS is involved in regulating the disease development of fungal infections. In vitro experiments, cGAS can also be found in many cells, including macrophages and epithelial cells. Additionally, epithelial cells have been shown to be crucial to how the host immune system responds to fungal infections. Therefore, using HBECs as the research model, we found that cGAS and its downstream pathways also showed a corresponding increase trend in epithelial cell infection, and cGAS knockdown could increase the level of pro-inflammatory factor of non-type I interferon (IL-1β、IL-6 and TNF-α), which was consistent with the vitro results. Therefore, all the results suggest that inhibition of cGAS activity has an adverse effect. In conclusion, appropriate activation of the cGAS may contribute to the treatment of patients with invasive pulmonary aspergillus.
A limited number of antifungal drugs and the increasing resistance of clinically isolated strains drive the need to develop alternative and effective strategies to combat fungal infections.Citation22 Adjust the host immune against invasive fungal infection is a very promising antifungal treatment strategies. Cytokines are essential for the activation of host immunity against multiple classes of pathogens including fungi. In addition to its important role in host defense against viruses, type I interferons (IFNα/IFNβ) and type III interferons (IFN-λs) have diverse effects on innate and adaptive immune cells during infection with fungi, directly and/or through the induction of other mediators.Citation15–23–Citation26 Several findings suggest a host protective contribution of IFNα/β to immunity to Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans. A study found that Aspergillus fumigatus conidia induces IFNβ signaling in respiratory epithelial cells, which is consistent with our study.Citation27 Additionally, IFNβ can modulate dendritic cells to enhance T-helper cell type 1’s anti-aspergillus response.Citation28 Moreover, exogenous stimulation of type I interferon protects mice with chronic granulomatous disease (CGD) from aspergillus by early recruitment of host protective neutrophils into the lungs, which partially supports the role of cGAS receptors in IPA in our study.Citation29 IFNα/β signaling also was found to be required variously for induction of reactive oxygen intermediates that are necessary for killing of C. albicans by phagocytic cells.Citation30–31 Interestingly, IFNα/β signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis.Citation32 And conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages.Citation33 It is well known that neutrophils are essential for defense against infection by extracellular pathogens, especially fungi. And type III IFNs are crucial regulators of innate antifungal immunity via direct effects on neutrophil function.Citation25 Upon infection with Aspergillus fumigatus, early production of IFN-α/β promotes optimal induction of the type III IFNs which act as critical activators of ROS generation of pulmonary neutrophils, an essential effector killing mechanism in control of fungal infection, to direct elimination of Aspergillus spores together. Importantly, their study demonstrated that proper antifungal defense could be restored by exogenous administration of these cytokines.Citation25 Host immunity may be regulated by IFNs in a variety of methods, and various elements of this regulation are accomplished through selective activation receptor.
As an important molecule, cGAS has been extensively studied for many years, especially for innate immunity to various pathogen infections.Citation34 Inhibition of the cGAS is often considered to lead to serious defense defects.Citation35 In addition to cGAS, numerous other receptors are also responsible for the type I interferon antifungal response of invasive Aspergillus.Citation36 including Toll-like receptors (TLRs), C-type lectin-like receptors (CLRs) Dectin-1 and RIG-I like receptors (RLRs) MDA5.Citation11–37 It has been shown that TLR3-deficient,Citation38 TLR6-deficien,Citation39 and TLR9-deficient miceCitation40 had a higher risk of invasive pulmonary aspergillosis than WT mice, Moreover, compared with WT mice, a higher fungal durability percentage was observed in mice that lacked TLR and exacerbated tissue damage during invasive lung infection. In the airways challenged by fungi, neutrophil antifungal effector functions are built up and activated, the host’s ability to fight infection with A. fumigatus depends on communication through MDA5/MAVS, and an interesting study shows that MDA5/MAVs-dependent interferon response is essential to induce optimal antifungal neutrophilic killing of Aspergillus fumigatus spores. The dsRNA of Aspergillus fumigatus drives the activation of MDA5/MAVS signaling. Interestingly, the induction of type I interferon after Aspergillus fumigatus excitation is only partially dependent on MDA5/MAVS signaling, while the expression of type III interferon is entirely dependent on MDA5/MAVS signaling.Citation37–41 In addition, dectin-1-mediated recognition of β-glucan on the cell wall of the clinically relevant fungal pathogen Aspergillus fumigatus promotes the activation of a protective cascade of type I and III interferon expression.Citation42 Therefore, all these results demonstrate the partial role cGAS plays in in the progression of A. fumigatus infection from the perspective of our research. In conclusion, our study broadens cGAS’ ability to regulate antifungal immunity.
Despite these limitations, studying is still a little challenging. Firstly, we are unsure of the applicability of the effects observed in mice and cell models to clinical diseases. A second point to note is that the human body is a complex system. During the development and occurrence of each disease, there will be a variety of pattern recognition receptors involved, and we don’t rule out that additional receptors play a role in cGAS’ immunomodulatory function throughout the IPA process. Finally, we did not use knockout mice and conduct rescue experiments in our study.
We conclude that the cGAS-STING pathway, in addition to broadening the previously unexplored role of cGAS in fungal infections, is an attractive target for future treatments for aggressive pulmonary aspergillosis.
Author contributions
MP and LP wrote the manuscript. LP, XL, and MP designed experiments. MP and XZ performed experiments and analyzed data. MP did the statistical analyses. LP supervised the work and reviewed the manuscript.
Ethics statement
Following the recommendations of the Guidelines for the Protection and Use of Laboratory Animals and in accordance with the Animal Protection Law of China and relevant regulations as well as approval from the Animal Protection and Use Committee of Chongqing Medical University in all animal experiments, the number is Lot 2022-K381.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Reasonable requests can be made for access to the study’s data from the corresponding authors.
Additional information
Funding
References
- Margalit A, Kavanagh K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev. 2015;39(5):670–687. doi:10.1093/femsre/fuv018.
- Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2019;200(5):535–550. doi:10.1164/rccm.201906-1185ST.PMC6727169.
- Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–e162. doi:10.1016/S1473-3099(20)30847-1.PMC7833078.
- Verweij PE, Zhang J, Debets AJM, et al. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect Dis. 2016;16(11):e251–e260. doi:10.1016/S1473-3099(16)30138-4.
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24 Suppl 1(Suppl 1):e1–e38. doi:10.1016/j.cmi.2018.01.002.
- van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latge JP. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15(11):661–674. doi:10.1038/nrmicro.2017.90.
- Lehrnbecher T, Kalkum M, Champer J, Tramsen L, Schmidt S, Klingebiel T. Immunotherapy in invasive fungal infection–focus on invasive aspergillosis. Curr Pharm Des. 2013;19(20):3689–3712. doi:10.2174/1381612811319200010.
- Latge JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1):e00140-18. doi:10.1128/CMR.00140-18.PMC6860006.
- Barros B, Almeida BR, Barros DTL, Toledo MS, Suzuki E. Respiratory epithelial cells: more than just a physical barrier to fungal infections. JoF. 2022;8(6):548. doi:10.3390/jof8060548.PMC9225092.
- Leiva-Juarez MM, Kolls JK, Evans SE. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018;11(1):21–34. doi:10.1038/mi.2017.71.PMC5738267.
- Dambuza IM, Levitz SM, Netea MG, Brown GD. Fungal recognition and Microbiol Spectr. 2017;5(4). doi:10.1128/microbiolspec.FUNK-0050-2016. PMID: 28752813
- Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus Conidia with airway epithelial cells: a critical review. Front Microbiol. 2016;7:472. doi:10.3389/fmicb.2016.00472.PMC4823921.
- Ablasser A, Chen ZJ. cGAS in action: expanding roles in immunity and inflammation. Science. 2019;363(6431):eaat8657. doi:10.1126/science.aat8657.
- Cheng Z, Dai T, He X, et al. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Target Ther. 2020;5(1):91. doi:10.1038/s41392-020-0198-7.PMC7293265.
- McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi:10.1038/nri3787.PMC7162685.
- Liu N, Pang X, Zhang H, Ji P. The cGAS-STING pathway in bacterial infection and bacterial immunity. Front Immunol. 2021;12:814709. doi:10.3389/fimmu.2021.814709.PMC8793285.
- Ni G, Ma Z, Damania B. cGAS and STING: at the intersection of DNA and RNA virus-sensing networks. PLoS Pathog. 2018;14(8):e1007148. doi:10.1371/journal.ppat.1007148.PMC6095619.
- Rivera A, Hohl TM, Collins N, et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. 2011;208(2):369–381. doi:10.1084/jem.20100906.PMC3039849.
- Mikawa K, Nishina K, Takao Y, Obara H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg. 2003;97(6):1751–1755. doi:10.1213/01.ANE.0000086896.90343.13.
- Denning DW, Chakrabarti A. Pulmonary and sinus fungal diseases in non-immunocompromised patients. Lancet Infect Dis. 2017;17(11):e357–e366. doi:10.1016/s1473-3099(17)30309-2.
- Desoubeaux G, Cray C. Rodent models of invasive aspergillosis due to Aspergillus fumigatus: still a long path toward standardization. Front Microbiol. 2017;8:841. doi:10.3389/fmicb.2017.00841.PMC5432554.
- Salazar F, Brown GD. Antifungal innate immunity: a perspective from the last 10 years. J Innate Immun. 2018;10(5–6):373–397. doi:10.1159/000488539.PMC6784043.
- Pekmezovic M, Dietschmann A, Gresnigt MS. Type I interferons during host-fungus interactions: is antifungal immunity going viral? PLoS Pathog. 2022;18(8):e1010740. doi:10.1371/journal.ppat.1010740.PMC9409562.
- Kotenko SV, Rivera A, Parker D, Durbin JE. Type III IFNs: beyond antiviral protection. Semin Immunol. 2019;43:101303. doi:10.1016/j.smim.2019.101303.PMC7141597.
- Espinosa V, Dutta O, McElrath C, et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol. 2017;2(16):eaan5357. doi:10.1126/sciimmunol.aan5357.PMC5880030.
- Broggi A, Granucci F, Zanoni I. Type III interferons: balancing tissue tolerance and resistance to pathogen invasion. J Exp Med. 2020;217(1):e20190295. doi:10.1084/jem.20190295.PMC7037241.
- Beisswenger C, Hess C, Bals R. Aspergillus fumigatus conidia induce interferon-beta signalling in respiratory epithelial cells. Eur Respir J. 2012;39(2):411–418. doi:10.1183/09031936.00096110.
- Homma T, Kato A, Bhushan B, et al. Role of Aspergillus fumigatus in triggering protease-activated receptor-2 in airway epithelial cells and skewing the cells toward a T-helper 2 bias. Am J Respir Cell Mol Biol. 2016;54(1):60–70. doi:10.1165/rcmb.2015-0062OC.PMC4742929.
- Seyedmousavi S, Davis MJ, Sugui JA, et al. Exogenous stimulation of type I interferon protects mice with chronic granulomatous disease from Aspergillosis through early recruitment of host-protective neutrophils into the lung. mBio. 2018;9(2):9. doi:10.1128/mBio.00422-18.PMC5874922.
- Biondo C, Signorino G, Costa A, et al. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol. 2011;41(7):1969–1979. doi:10.1002/eji.201141490.
- Majer O, Bourgeois C, Zwolanek F, et al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8(7):e1002811. doi:10.1371/journal.ppat.1002811.PMC3406095.
- Biondo C, Midiri A, Gambuzza M, et al. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J Immunol. 2008;181(1):566–573. doi:10.4049/jimmunol.181.1.566.
- Inglis DO, Berkes CA, Hocking Murray DR, Sil A. Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages. Infect Immun. 2010;78(9):3871–3882. doi:10.1128/IAI.00204-10.PMC2937464.
- Tan X, Sun L, Chen J, Chen ZJ. Detection of microbial infections through innate immune sensing of nucleic acids. Annu Rev Microbiol. 2018;72:447–478. doi:10.1146/annurev-micro-102215-095605.
- Schoggins JW, MacDuff DA, Imanaka N, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–695. doi:10.1038/nature12862.PMC4077721.
- Gow NA, Netea MG. Medical mycology and fungal immunology: new research perspectives addressing a major world health challenge. Phil Trans R Soc B. 2016;371(1709):20150462. doi:10.1098/rstb.2015.0462.PMC5095541.
- Wang X, Caffrey-Carr AK, Liu KW, et al. MDA5 is an essential sensor of a pathogen-associated molecular pattern associated with vitality that is necessary for host resistance against Aspergillus fumigatus. J Immunol. 2020;205(11):3058–3070. doi:10.4049/jimmunol.2000802.PMC7785165.
- Carvalho A, De Luca A, Bozza S, et al. TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood. 2012;119(4):967–977. doi:10.1182/blood-2011-06-362582.
- Rubino I, Coste A, Le Roy D, et al. Species-specific recognition of Aspergillus fumigatus by toll-like receptor 1 and toll-like receptor 6. J Infect Dis. 2012;205(6):944–954. doi:10.1093/infdis/jir882.
- Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe. 2011;9(5):415–424. doi:10.1016/j.chom.2011.04.007.PMC3100664.
- Wang X, Cunha C, Grau MS, et al. MAVS expression in alveolar macrophages is essential for host resistance against Aspergillus fumigatus. J Immunol. 2022;209(2):346–353. doi:10.4049/jimmunol.2100759.PMC9307106.
- Dutta O, Espinosa V, Wang K, Avina S, Rivera A. Dectin-1 promotes type I and III interferon expression to support optimal antifungal immunity in the lung. Front Cell Infect Microbiol. 2020;10:321. doi:10.3389/fcimb.2020.00321.PMC7360811.

