Abstract
Aim of the study: Force adaptation is a process whereby the contractile capacity of the airway smooth muscle increases during a sustained contraction (aka tone). Tone also increases the response to a nebulized challenge with methacholine in vivo, presumably through force adaptation. Yet, due to its patchy pattern of deposition, nebulized methacholine often spurs small airway narrowing heterogeneity and closure, two important enhancers of the methacholine response. This raises the possibility that the potentiating effect of tone on the methacholine response is not due to force adaptation but by furthering heterogeneity and closure. Herein, methacholine was delivered homogenously through the intravenous (i.v.) route. Materials and Methods: Female and male BALB/c mice were subjected to one of two i.v. methacholine challenges, each of the same cumulative dose but starting by a 20-min period either with or without tone induced by serial i.v. boluses. Changes in respiratory mechanics were monitored throughout by oscillometry, and the response after the final dose was compared between the two challenges to assess the effect of tone. Results: For the elastance of the respiratory system (Ers), tone potentiated the methacholine response by 64 and 405% in females (37.4 ± 10.7 vs. 61.5 ± 15.1 cmH2O/mL; p = 0.01) and males (33.0 ± 14.3 vs. 166.7 ± 60.6 cmH2O/mL; p = 0.0004), respectively. For the resistance of the respiratory system (Rrs), tone potentiated the methacholine response by 129 and 225% in females (9.7 ± 3.5 vs. 22.2 ± 4.3 cmH2O·s/mL; p = 0.0003) and males (10.7 ± 3.1 vs. 34.7 ± 7.9 cmH2O·s/mL; p < 0.0001), respectively. Conclusions: As previously reported with nebulized challenges, tone increases the response to i.v. methacholine in both sexes; albeit sexual dimorphisms were obvious regarding the relative resistive versus elastic nature of this potentiation. This represents further support that tone increases the lung response to methacholine through force adaptation.
Introduction
The contractile lability of the airway smooth muscle (ASM) is well documentedCitation1,Citation2 and likely contributes to airway hyperresponsiveness in inflammatory lung disorders, such as asthma.Citation2 A sustained contraction itself, hereinafter called tone, was repeatedly shown to increase the contractile capacity of the ASM in vitro.Citation3–9 This phenomenon was coined ‘force adaptation’. There have been fewer reports investigating the impact of force adaptation on the response to methacholine in vivo. Yet, in both miceCitation6,Citation7 and humans,Citation8 a methacholine dose staggered over 20–30 min always leads to a markedly greater response compared to the same dose delivered in one bolus. Startingly though, the potentiating effect of tone is mainly seen when the response is monitored using parameters reflecting changes in the lung periphery (e.g., lung elastance), without much effect on parameters reflecting changes in large airways (e.g., airway resistance to airflow).Citation6–8 This raises concerns about previous interpretations, because parameters reflecting changes in the lung periphery are also very sensitive to other determinants of the methacholine response, such as small airway narrowing heterogeneityCitation10–15 and closure.Citation11,Citation16–18 Together, it implies that the potentiating effect of tone on the methacholine response in vivo may occur through mechanisms other than force adaptation.
Previous studies also opted for the nebulized route of delivery to test the impact of force adaptation on the in vivo response to methacholine.Citation6–8 This amplifies the above concerns for at least two reasons. First, although the deposition pattern of certain nebulized nanoparticles, such as melamine resin, can be impressively widespread and homogeneous throughout the lungCitation19 using specific ventilation profile,Citation19,Citation20 nebulized methacholine is typically thought to accumulate predominantly in large airways in a patchily manner; alike tantalum dust,Citation21 technetium-99m-labeled pentetic acid,Citation22 and iodine.Citation23 Early after the nebulization, this means that some conducting airways are constricting while the surrounding peripheral airways are still compliant, an imbalance furthering airway narrowing heterogeneityCitation24,Citation25 and small airway closure.Citation26,Citation27 Second, the stimulation of ASM by methacholine through the inhaled route and the epithelium for a longer period in the challenge with tone, compared to the challenge without tone, may concurrently further mucus secretion.Citation28–32 The latter promotes heterogeneity and closure, chiefly by increasing the propensity of plugging,Citation33 either by directly drowning small airways or by increasing surface tension and the formation of stretchable menisci across the luminal area that also functionally blocks airflow in subtending airways.
Sidestepping the confounding effect of narrowing heterogeneity and closure would thus be important to reinforce previous interpretations suggesting that force adaptation is the underlying mechanism whereby tone increases the methacholine response. The objective of the present study is to test the effect of tone on the in vivo methacholine response using intravenous challenges, instead of the nebulized challenges used in previous studies.Citation6–8 This strategy should minimize the confounding effect of narrowing heterogeneity and closure. First, because it ensures a homogenous distribution of methacholine in peripheral airways and, this, irrespective of the constricted state of the lung prior to the delivery of the final dose.Citation23,Citation34 And second, because it limits mucus secretion, since methacholine is then stimulating ASM without first passing through the epithelium. Findings from several previous studies testify the effectiveness of this strategy, showing that intravenous (i.v.) challenges triggered very little, if not at all, narrowing heterogeneity and closure, especially in healthy animals, such as the ones used in the present study.Citation24,Citation25,Citation35–37
Methods
Mice
Seven-week-old female and male BALB/c mice were purchased from Charles River Laboratory (Bar Harbor, ME). Mice were provided food and water ad libitum and were housed one week before experiments. They were thus tested at 8 wk. All protocols were approved by the Committee of Animal Care of Université Laval in accordance with guidelines of the Canadian Council on Animal Care (2022-977).
Changes in respiratory mechanics during the methacholine challenge
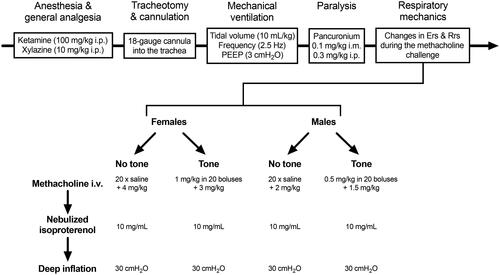
Mice were weighted, anesthetized, tracheotomized, mechanically ventilated and paralyzed as previously described.Citation38 The outline is depicted in . Within each sex, the degree of airway responsiveness was compared between two groups of mice subjected to a methacholine challenge that started with a ∼20-min period either with or without tone. There was no deep inspiration before and throughout the methacholine challenge. The 20-min duration was chosen based on a previous in vitro study with excised mouse tracheas, showing that the gain in ASM’s contractile capacity induced by tone is significant from 20 min post-exposure and beyond.Citation6 The approach was very similar to our previous studies,Citation6,Citation7 except that the methacholine was delivered i.v. instead of nebulized into the trachea. For injecting the i.v. boluses, a 25-gauge cannula was inserted into the right jugular vein. The injected doses were twice higher in females than males owing to their inherently lower degree of responsiveness.Citation7,Citation39 Since the effect of methacholine is waning more rapidly when administered i.v. than by nebulization,Citation40 it was added more frequently during the period of tone. More precisely, a bolus (5 µL) of methacholine was injected every 65 s (). The dose delivered over ∼20 min (in a total volume of 100 µL) was 0.5 and 1 mg/kg for males and females, respectively. The group without tone was receiving an equivalent amount of saline using the same dosing regimen. The degree of responsiveness was then assessed by injecting a high dose of methacholine. This final high dose was adjusted so that each mouse, exposed or not to tone, received the same cumulative dose. In females, the final dose was either 3 or 4 mg/kg in mice exposed or not to tone, respectively, for a total cumulative dose of 4 mg/kg. In males, the final dose was either 1.5 or 2 mg/kg in mice exposed or not to tone, respectively, for a total cumulative dose of 2 mg/kg.
Figure 1. Outline of the experimental design. Abbreviations: Ers, elastance of the respiratory system; i.m., intramuscularly; i.p., intraperitoneally; i.v.; intravenously; PEEP, positive end-expiratory pressure; Rrs, resistance of the respiratory system.
Respiratory mechanics was monitored before and throughout the methacholine challenge with the flexiVent (FX Module 2, SCIREQ, Montreal, Canada) using an oscillometric perturbation called the Snapshot-150. This perturbation was actuated twice at baseline and at 13-s intervals throughout the methacholine challenge. Parameters obtained from the Snapshot-150 include the elastance of the respiratory system (Ers) and the resistance of the respiratory system (Rrs).Citation41 Only changes from baseline (Δ) are shown in figures, representing the methacholine response.
Five minutes after the administration of the final dose of methacholine, isoproterenol (10 mg/mL) was nebulized to pharmacologically relax the ASM. The nebulizer for small particle size (volume median diameter between 2.5 and 4 µm; Aeroneb Lab; Aerogen Inc, Ireland) was operating for a duration of 10 s at a duty cycle of 50% under regular ventilation. One deep inflation (DI) was then imposed 5 min afterwards to mechanically relax the ASM. The DI maneuver consisted of inflating the lung by increasing the pressure at a constant rate until it reaches 30 cmH2O. Respiratory mechanics were still monitored at regular intervals (38 s) after these interventions. The goal was to determine whether any alterations on the methacholine response caused by tone were reversible using interventions known to inhibit ASM contraction.Citation42–49
Statistical analyses
Data shown are means and SD. Unpaired t tests were used to compare weight, as well as baseline Rrs and Ers between sexes. Unpaired t tests were also used to compare ΔRrs and ΔErs between challenges with and without tone at specific time-points (more precisely, at the very end of the period with or without tone, at the peak response after the final dose, 5 min after the final dose of methacholine, 5 min after the administration of isoproterenol, and immediately after the DI). Repeated measures two-way ANOVAs were also used to assess the effect of tone on the methacholine response before and after isoproterenol + the DI, two interventions known to synergistically inhibit ASM contraction.Citation42–49 Values of ΔErs and ΔRrs before these interventions were taken at the peak methacholine response, and values after these interventions were taken immediately after the DI. For each readout (ΔErs and ΔRrs), this repeated measures two-way ANOVA assessed the effect of tone, the combined effect of isoproterenol + the DI, as well as their interaction on the methacholine response. A significant interaction means that the effect of tone on the methacholine response is different before versus after the inhibition of ASM, thereby implying that the potentiating effect of tone on the methacholine response is mediated through a mechanism relying on ASM. All statistical analyses and graphs were performed using Prism 9 (version 9.1.1, GraphPad, San Diego, CA). p < 0.05 was considered significant to reject the null hypothesis.
Results
As expected, males were heavier than females (23.3 ± 1.4 vs. 18.0 ± 1.0 g, respectively; p < 0.0001). However, there were no differences between males and females for baseline respiratory system resistance (1.27 ± 0.33 vs. 1.33 ± 0.23 cmH2O·s/mL, respectively; p = 0.65) and respiratory system elastance (60.9 ± 13.8 vs. 61.2 ± 12.8 cmH2O/mL, respectively; p = 0.96).
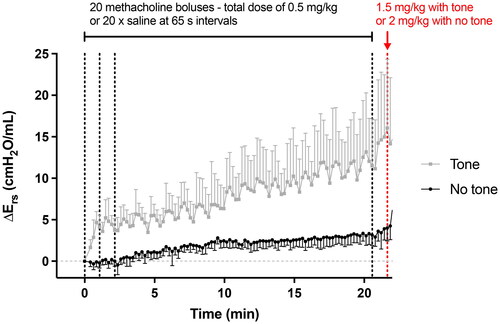
The new dosing regimen through the i.v. route was successful to maintain tone. The mean traces of ΔErs in males during the first ∼20 min for both methacholine challenges are depicted in . Compared to mice receiving saline (i.e., not exposed to tone), the ΔErs in mice exposed to tone was obvious after the first dose of methacholine. The effect of each dose was transient, as can be seen from the 20 clearly distinguished peaks in ΔErs. Yet, the consistent rate of increase over a longer time-scale () indicated that the next dose was usually delivered before the effect of the previous dose had completely vanished. Ers was also creeping up in the challenge without tone, presumably due to a progressive stiffening of the lung tissue and/or accretion of small airway closure. However, the total rise after ∼20 min without tone was nowhere near the one caused by tone (4.0 ± 1.6 vs. 16.1 ± 8.3 cmH2O/mL, respectively; p = 0.01).
Figure 2. Tone (i.e., a sustained activation of the airway smooth muscle) induced by methacholine. Changes in respiratory system elastance relative to baseline (ΔErs) caused by 20 serial i.v. doses of saline (black) or methacholine (gray). Vertical black dotted lines, 65 s apart, indicate when doses were delivered. Only the first 3 doses and the 20th one prior to the final higher dose are indicated for clarity. The red vertical dotted line indicates when the higher final i.v. dose was delivered. These results are also shown in (first ∼20 min of the upper left graph), but on a different scale. Data are means + or - SD for a n = 6. The dosing regimen was identical in females, except that all doses were twice lower.
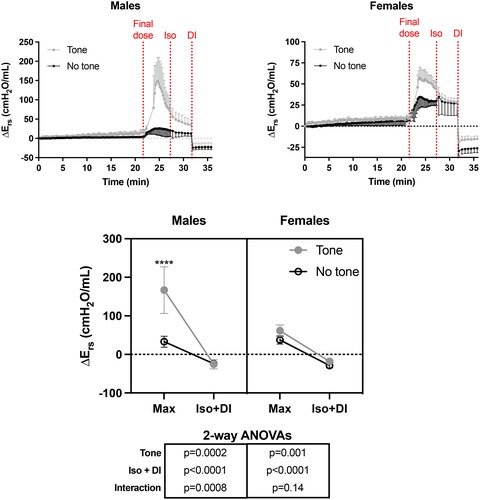
ΔErs during the whole protocol is depicted in , showing the responses to the i.v. methacholine challenge, the nebulized isoproterenol, and the DI. Tone potentiated the response to methacholine in both sexes. The potentiating effect was markedly greater in males than females (note the different scales on the y-axis). In fact, tone increased the methacholine response (i.e., the peak response) by a mean of 405% in males (t test, p = 0.0004) and 64% in females (t test, p = 0.01). As observed for each dose for inducing tone, the response to the final (and higher) dose was transient, chiefly reflecting the quick clearance of methacholine. Yet, ΔErs was still higher in mice exposed to tone than in mice not exposed to tone 5 min after the final dose (p = 0.008 and 0.003 in females and males, respectively). Isoproterenol then slightly accelerated the rate of recovery. Yet, even after 5 min of isoproterenol exposure, ΔErs was still clearly visible (i.e., Ers was still above baseline in both sexes). Notably, the difference between mice with and without tone was mostly preserved after isoproterenol in males (p = 0.004), while it was gone in females (p = 0.87). The DI then completely abolished the methacholine-induced ΔErs in both sexes. In fact, ΔErs became negative post-DI, presumably reflecting recruitment and/or a transient increase in lung compliance post-DI. Interestingly, the difference between mice with and without tone was completely abrogated by DI in males (t test, p = 0.80), while it was back observed in females (t test, p = 0.004). Note also the significant interaction in the repeated two-way ANOVA (, underneath the bottom graph) between the effect of tone and the combined effect of the two interventions inhibiting ASM (i.e., isoproterenol + the DI) in males, but not in females.
Figure 3. The effect of tone on the methacholine-induced changes in respiratory system elastance relative to baseline (ΔErs). The upper left and right graphs demonstrate the actual changes in Ers throughout the whole protocol in both males and females, respectively. Note that the scale on the y-axis is smaller in females than males. The three vertical red dotted lines from left to right indicate when: 1- the final i.v. dose of methacholine was administered; 2- the nebulized dose of isoproterenol (Iso) was administered; and 3- the deep inspiration (DI) was imposed. In the bottom graph, the max Ers after the final dose (i.e., peak response), as well as the minimal Ers after Iso and the DI, are compared between challenges with and without tone in each sex. Results of two-way ANOVAs are shown below the graph, which were used to evaluate the effect of tone, the combined effect of Iso and the DI, and their interaction within each sex. Asterisks are from post hoc analyses, showing significant differences between challenges with and without tone for the max response (**** is p < 0.0001). Data are means + or - SD for a n = 6.
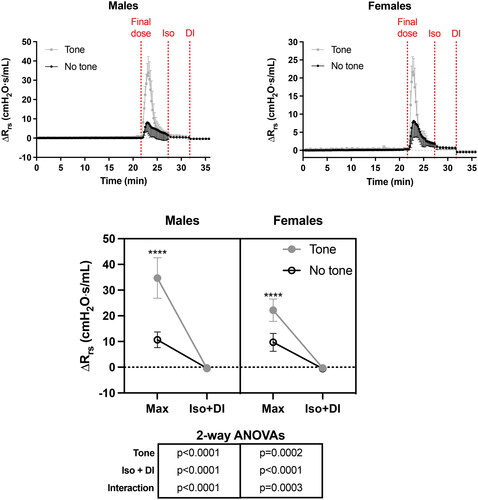
ΔRrs during the whole protocol is depicted in . As per Ers, tone potentiated the response of Rrs to methacholine in both sexes. The potentiating effect was again greater in males than females (note the different scales on the y-axis). In fact, tone increased the methacholine response (i.e., the peak response) by a mean of 225% in males (t test, p < 0.0001) and 129% in females (t test, p = 0.0003). The effect of methacholine on ΔRrs was peaking earlier (between 65 and 91s) than for ΔErs (between 130 and 195 s). It was also receding more quickly, reaching nearly baseline values 5 min after the administration of the final dose. Isoproterenol still slightly hastened the recovery and the DI completely abolished the small remaining effect of methacholine on ΔRrs. In contrast to ΔErs, the kinetics of changes for ΔRrs was qualitatively similar between sexes and, most relevant to the present study, the difference between mice with and without tone was completely abrogated before the administration of isoproterenol (p = 0.33 and 0.83 in females and males, respectively).
Figure 4. The effect of tone on the methacholine-induced changes in respiratory system resistance relative to baseline (ΔRrs). The upper left and right graphs demonstrate the actual changes in Rrs throughout the whole protocol in both males and females, respectively. Note that the scale on the y-axis is smaller in females than males. The three vertical red dotted lines from left to right indicate when: 1- the last i.v. dose of methacholine was administered; 2- the nebulized dose of isoproterenol (Iso) was administered; and 3- the deep inspiration (DI) was imposed. In the bottom graph, the max Rrs after the final dose (i.e., peak response), as well as the minimal Rrs after Iso and the DI, are compared between challenges with and without tone in each sex. Results of two-way ANOVAs are shown below the graph, which were used to evaluate the effect of tone, the combined effect of Iso and the DI, and their interaction within each sex. Asterisks are from post hoc analyses, showing significant differences between challenges with and without tone for the max response (**** is p < 0.0001). Data are means + or - SD for a n = 6.
Discussion
This study used the intravenous route to deliver methacholine in the lung and onto the ASM in order to limit small airway narrowing heterogeneity and closure.Citation24,Citation25,Citation35–37 As previously reported with nebulized challenges,Citation6–8 tone still markedly potentiated the response to methacholine. The potentiating effect of tone on the methacholine response was observed by monitoring changes in both elastance and resistance of the respiratory system. It was also observed in both sexes, although the magnitude of the potentiated response was greater in males. The nature of the response also differed between sexes, being predominantly elastic in males, but rather resistive in females. The potentiating effect was also quickly reversed, at least to a great extent, by interventions inhibiting the ASM. Together, the results lend further support to the notion that tone potentiates the methacholine response in vivo by an ASM-mediated mechanism, likely force adaptation, and not by furthering small airway narrowing heterogeneity and closure.
On isolated ASM preparations in vitro, the gain in ASM’s contractile capacity induced by force adaptation is quickly reversible by abating tone through spasmogen removal.Citation6,Citation50 Controlling tone in vivo is more difficult because of the inevitable clearance of methacholine, due to the washing effect of circulating blood and/or methacholine degradation. Yet, owing to the reversible nature of force adaptation,Citation6,Citation50 the maintenance of tone was absolutely required in order to assess its impact on respiratory mechanics. One way to keep the level of ASM activation above baseline is to deliver serial doses of methacholine. During a nebulized challenge, a dose every 5 min was shown sufficient to keep oscillometric readouts (e.g., Ers & Rrs) continuously above baseline.Citation6–8 Preliminary experiments (not shown) clearly established that this was not the case for an i.v. challenge. In fact, the kinetics of changes in oscillometric readouts following methacholine delivery is strikingly different between nebulized and i.v. challenges.Citation40 During a nebulized challenge, the effects of methacholine are manifested slower, and also last longer. Based on a computational model, this was predicted to be entirely due to the epithelium, which the inhaled methacholine has to traverse before reaching the ASM40. The effect of i.v. methacholine is more transient, acting more rapidly and also vanishing more quickly. For this reason, the dosing regimen during the period of tone was changed. Instead of delivering methacholine every 5 min, it was delivered at 65-s intervals. showed that tone was maintained throughout the period of tone. Elastance was actually slightly rising over time. This was also reassuring because the dwindling effect of methacholine on respiratory mechanics outlasts, sometimes by a substantial margin, the vanishing rate of methacholine concentration acting on the ASM.Citation51 The slow progressive rise () thus suggests that the dosing regimen was successful to maintain a degree of ASM activation above baseline throughout the period of tone, rendering the protocol conducive to force adaptation.
Irrespective of whether the response was measured by monitoring the changes in elastance or resistance, tone significantly potentiated the response to i.v. methacholine in both sexes. Therefore, an i.v. challenge – a strategy curbing small airway heterogeneity and closure by delivering methacholine homogenously throughout the lung, as well as by limiting mucus secretion by acting first on the ASM before reaching the epithelium – was just as effective as a nebulized challenge to demonstrate the potentiating effect of tone on the methacholine response. This represents further evidence that the underlying mechanism whereby tone potentiates the methacholine response is force adaptation.
The potentiating effect of tone on the methacholine response was short-lived. This is mainly due to the quick clearance of methacholine, and the resulting transient nature of its effect. Yet, the proposal is that when tone is mediated by the production and release of endogenous spasmogens, such histamine and leukotrienes, which are typically overexpressed in asthma,Citation52 the phenomenon may contribute to airway hyperresponsiveness. In other words, the fleeting effect of tone on the methacholine response observed in the present study may persist in diseased lungs as long as those inflammation-derived spasmogens are overexpressed.
Differences between sexes were observed though. The potentiating effect of tone on the methacholine response was greater in magnitude for males than females. This happened despite lower methacholine doses in the former. The nature of the response also varied markedly, being predominantly elastic in males but rather resistive in females. The reason for these sexual dimorphisms is uncertain. We have recently demonstrated in this mouse strain (BALB/c) that males have more ASM than females.Citation53 We have also recently confirmed findings of others,Citation46,Citation54–57 showing that the ASM undergoes an elastic transition during a sustained contraction.Citation43 We previously argued that ‘force adaptation’ and this ‘elastic transition’ are two monikers used to describe two phenomena in different experimental contexts but that probably rely on the same underlying mechanism.Citation7,Citation43 We therefore speculate that this elastic transition impacts lung mechanics to a greater extent in males merely because their airways are more muscular than females.
The responses to a bronchodilator drug and a DI were also different between sexes, at least for the changes in elastance. While elastance in mice exposed to tone was back to the level seen in mice not exposed to tone 5 min after the delivery of isoproterenol in females, it was still elevated by tone at that time-point in males. This can be due to the fact that the potentiating effect of tone was greater in males than females, implying a longer time to subside after relaxing the ASM with isoproterenol. The greater level of constriction in males may have also decreased the amount of nebulized isoproterenol entering the lungCitation23 and/or foster a more proximal deposition pattern,Citation34 which may have then attenuated its effect in males compared to females. Contrastingly, after the DI, elastance in males exposed to tone settled to the level seen in males not exposed to tone, while it was then elevated anew by tone in females. The complete disappearance of the effect of tone post-DI in males reinforced the proposal that the potentiating effect of tone on the methacholine response occurs through an ASM-mediated mechanism. The remaining effect of tone after the DI in females is more perplexing. It can be due to several things. Perhaps two DIs instead of one would have been required to completely eliminate the mechanical effect of tone on the ASM in females. The different methacholine doses between sexes may also have played a role. Since the doses were twice higher in females, the effect of methacholine may have lingered or may not have been completely inhibited by the used dose of isoproterenol. It is also possible that this higher dose may have triggered mucus secretion. Indeed, the i.v. route may limit mucus secretion by acting first on the ASM, but it may still hit the epithelium after traversing the ASM and the lamina propria if delivered at sufficiently high concentrations. Obviously, the enhancing effect of an excess of mucus on lung elastance should not disappear after a DI, perhaps explaining why elastance was again higher in female mice exposed to tone post-DI.
It is also understood that the effect of tone on the methacholine response may be very different in a context where inflammation is prominent. In asthma and experimental asthma, small airway narrowing heterogeneity and closure upon methacholine challenge are always in the offing.Citation10–18 Whether tone modifies asthmatic hyperresponsiveness to methacholine, and whether it occurs through a gain in ASM’s contractile capacity caused by force adaptation or by altering other determinants of the methacholine response, such as heterogeneity and closure, will require further studies.
Conclusion
The present study dug deeper into the underlying mechanisms whereby tone potentiates the methacholine response in vivo. More precisely, it evaluated whether the potentiating effect of tone on the methacholine response occurs in an experimental setting where small airway narrowing heterogeneity and closure are limited by using an i.v. challenge instead of a nebulized challenge. As previously reported with nebulized challenges,Citation6–8 tone markedly increases the methacholine response to an i.v. challenge in both sexes. The mechanism whereby tone potentiates the methacholine response is thus unlikely due to small airway narrowing heterogeneity and closure. The results rather support the proposal that, at least in healthy mice, tone increases the lung response to methacholine mainly through force adaptation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gazzola M, Flamand N, Bossé Y. [Extracellular molecules controlling the contraction of airway smooth muscle and their potential contribution to bronchial hyperresponsiveness]. Rev Mal Respir. 2020;37(6):462–473. doi:10.1016/j.rmr.2020.03.009.
- Auger L, Mailhot-Larouche S, Tremblay F, Poirier M, Farah C, Bossé Y. The contractile lability of smooth muscle in asthmatic airway hyperresponsiveness. Expert Rev Respir Med. 2016;10(1):19–27. doi:10.1586/17476348.2016.1111764.
- Bossé Y, Chin LY, Pare PD, Seow CY. Adaptation of airway smooth muscle to basal tone: relevance to airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2009;40(1):13–18. doi:10.1165/rcmb.2008-0150OC.
- Bossé Y, Chin LY, Pare PD, Seow CY. Chronic activation in shortened airway smooth muscle: a synergistic combination underlying airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2010;42(3):341–348. doi:10.1165/rcmb.2008-0448OC.
- Pascoe C, Jiao Y, Seow CY, Paré PD, Bossé Y. Force oscillations simulating breathing maneuvers do not prevent force adaptation. Am J Respir Cell Mol Biol. 2012;47(1):44–49. doi:10.1165/rcmb.2011-0429OC.
- Lee-Gosselin A, Gendron D, Blanchet MR, Marsolais D, Bossé Y. The gain of smooth muscle’s contractile capacity induced by tone on in vivo airway responsiveness in mice. J Appl Physiol (1985). 2015;118(6):692–698. doi:10.1152/japplphysiol.00645.2014.
- Boucher M, Dufour-Mailhot A, Tremblay-Pitre S, et al. In mice of both sexes, repeated contractions of smooth muscle in vivo greatly enhance the response of peripheral airways to methacholine. Respir Physiol Neurobiol. 2022;304:103938. doi:10.1016/j.resp.2022.103938.
- Gazzola M, Lortie K, Henry C, et al. Airway smooth muscle tone increases airway responsiveness in healthy young adults. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L348–L357. doi:10.1152/ajplung.00400.2016.
- Gazzola M, Henry C, Lortie K, et al. Airway smooth muscle tone increases actin filamentogenesis and contractile capacity. Am J Physiol Lung Cell Mol Physiol. 2020;318(2):L442–L451. doi:10.1152/ajplung.00205.2019.
- Bates JH. Systems physiology of the airways in health and obstructive pulmonary disease. Wiley Interdiscip Rev Syst Biol Med. 2016;8(5):423–437. doi:10.1002/wsbm.1347.
- Dame Carroll JR, Magnussen JS, Berend N, Salome CM, King GG. Greater parallel heterogeneity of airway narrowing and airway closure in asthma measured by high-resolution CT. Thorax. 2015;70(12):1163–1170. doi:10.1136/thoraxjnl-2014-206387.
- Farrow CE, Salome CM, Harris BE, Bailey DL, Berend N, King GG. Peripheral ventilation heterogeneity determines the extent of bronchoconstriction in asthma. J Appl Physiol (1985). 2017;123(5):1188–1194. doi:10.1152/japplphysiol.00640.2016.
- Downie SR, Salome CM, Verbanck S, Thompson B, Berend N, King GG. Ventilation heterogeneity is a major determinant of airway hyperresponsiveness in asthma, independent of airway inflammation. Thorax. 2007;62(8):684–689. doi:10.1136/thx.2006.069682.
- King GG, Carroll JD, Muller NL, et al. Heterogeneity of narrowing in normal and asthmatic airways measured by HRCT. Eur Respir J. 2004;24(2):211–218. doi:10.1183/09031936.04.00047503.
- Venegas JG, Winkler T, Musch G, et al. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434(7034):777–782. doi:10.1038/nature03490.
- Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32(6):1563–1569. doi:10.1183/09031936.00114007.
- Farrow CE, Salome CM, Harris BE, et al. Airway closure on imaging relates to airway hyperresponsiveness and peripheral airway disease in asthma. J Appl Physiol (1985)Gov’t. J Appl Physiol. 2012;113(6):958–966. doi:10.1152/japplphysiol.01618.2011.
- Lundblad LK, Thompson-Figueroa J, Allen GB, et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175(8):768–774. doi:10.1164/rccm.200610-1410OC.
- Yang L, Feuchtinger A, Moller W, et al. Three-Dimensional Quantitative Co-Mapping of Pulmonary Morphology and Nanoparticle Distribution with Cellular Resolution in Nondissected Murine Lungs. ACS Nano. 2019;13(2):1029–1041. doi:10.1021/acsnano.8b07524.
- Robichaud A, Fereydoonzad L, Schuessler TF. Delivered dose estimate to standardize airway hyperresponsiveness assessment in mice. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L837–46. doi:10.1152/ajplung.00343.2014.
- Phung TN, Sinclair SE, Makena P, Molthen RC, Waters CM. Dynamic airway constriction in rats: heterogeneity and response to deep inspiration. Am J Physiol Lung Cell Mol Physiol. 2019;317(1):L39–L48. doi:10.1152/ajplung.00050.2019.
- Dugernier J, Reychler G, Wittebole X, et al. Aerosol delivery with two ventilation modes during mechanical ventilation: a randomized study. Ann Intensive Care. 2016;6(1):73. doi:10.1186/s13613-016-0169-x.
- Porra L, Degrugilliers L, Broche L, et al. Quantitative Imaging of Regional Aerosol Deposition, Lung Ventilation and Morphology by Synchrotron Radiation CT. Sci Rep. 2018;8(1):3519. doi:10.1038/s41598-018-20986-x.
- Petak F, Hantos Z, Adamicza A, Asztalos T, Sly PD. Methacholine-induced bronchoconstriction in rats: effects of intravenous vs. aerosol delivery. J Appl Physiol (1985). 1997;82(5):1479–1487. doi:10.1152/jappl.1997.82.5.1479.
- Wagers SS, Haverkamp HC, Bates JH, et al. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol (1985). 2007;102(1):221–230. doi:10.1152/japplphysiol.01385.2005.
- Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol (1985). 1996;80(5):1841–1849. doi:10.1152/jappl.1996.80.5.1841.
- Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol (1985). 1997;83(4):1192–1201. doi:10.1152/jappl.1997.83.4.1192.
- Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi:10.1038/ncomms7281.
- Agrawal A, Rengarajan S, Adler KB, et al. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol (1985). 2007;102(1):399–405. doi:10.1152/japplphysiol.00630.2006.
- Singer M, Martin LD, Vargaftig BB, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10(2):193–196. doi:10.1038/nm983.
- Ishihara H, Shimura S, Satoh M, et al. Muscarinic receptor subtypes in feline tracheal submucosal gland secretion. Am J Physiol. 1992;262(2 Pt 1):L223–8. doi:10.1152/ajplung.1992.262.2.L223.
- Gallagher JT, Kent PW, Passatore M, Phipps RJ, Richardson PS. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975;192(1106):49–76. doi:10.1098/rspb.1975.0151.
- Kuyper LM, Pare PD, Hogg JC, et al. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115(1):6–11. doi:10.1016/s0002-9343(03)00241-9.
- O’Riordan TG, Walser L, Smaldone GC. Changing patterns of aerosol deposition during methacholine bronchoprovocation. Chest. 1993;103(5):1385–1389.
- Nagase T, Moretto A, Ludwig MS. Airway and tissue behavior during induced constriction in rats: intravenous vs. aerosol administration. J Appl Physiol (1985). 1994;76(2):830–838. doi:10.1152/jappl.1994.76.2.830.
- Salerno FG, Moretto A, Dallaire M, Ludwig MS. How mode of stimulus affects the relative contribution of elastance and hysteresivity to changes in lung tissue resistance. J Appl Physiol (1985). 1995;78(1):282–287. doi:10.1152/jappl.1995.78.1.282.
- Jonasson S, Hedenstierna G, Hedenstrom H, Hjoberg J. Comparisons of effects of intravenous and inhaled methacholine on airway physiology in a murine asthma model. Respir Physiol Neurobiol. 2009;165(2-3):229–236. doi:10.1016/j.resp.2008.12.005.
- Boucher M, Henry C, Khadangi F, et al. Effects of airway smooth muscle contraction and inflammation on lung tissue compliance. Am J Physiol Lung Cell Mol Physiol. 2022;322(2):L294–L304. doi:10.1152/ajplung.00384.2021.
- Card JW, Carey MA, Bradbury JA, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–630. doi:10.4049/jimmunol.177.1.621.
- Lauzon AM, Bates JH. Kinetics of respiratory system elastance after airway challenge in dogs. J Appl Physiol (1985). 2000;89(5):2023–2029. doi:10.1152/jappl.2000.89.5.2023.
- Bates JH. Chap 3: The linear single-compartement model. In Lung Mechanics: An Inverse Modeling Approach. New York: Cambridge University Press; 2009. p. 37–61.
- Rosner SR, Pascoe CD, Blankman E, et al. The actin regulator zyxin reinforces airway smooth muscle and accumulates in airways of fatal asthmatics. PLoS One. 2017;12(3):e0171728. doi:10.1371/journal.pone.0171728.
- Dufour-Mailhot A, Boucher M, Henry C, et al. Flexibility of microstructural adaptations in airway smooth muscle. J Appl Physiol (1985). 2021;130(5):1555–1561. doi:10.1152/japplphysiol.00894.2020.
- Mailhot-Larouche S, Lortie K, Marsolais D, Flamand N, Bossé Y. An in vitro study examining the duration between deep inspirations on the rate of renarrowing. Respir Physiol Neurobiol. 2017;243:13–19. doi:10.1016/j.resp.2017.04.013.
- Zhang W, Gunst SJ. Molecular mechanisms for the mechanical modulation of airway responsiveness. J Eng Sci Med Diagn Ther. 2019;2(010805):1–8.
- Fredberg JJ, Inouye D, Miller B, et al. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156(6):1752–1759. doi:10.1164/ajrccm.156.6.9611016.
- Wang L, Chitano P, Seow CY. Filament evanescence of myosin II and smooth muscle function. J Gen Physiol. 2021;153(3).e202012781. doi:10.1085/jgp.202012781.
- Noble PB, McFawn PK, Mitchell HW. Responsiveness of the isolated airway during simulated deep inspirations: effect of airway smooth muscle stiffness and strain. J Appl Physiol (1985). 2007;103(3):787–795. doi:10.1152/japplphysiol.00314.2007.
- Mitchell RW, Dowell ML, Solway J, Lakser OJ. Force Fluctuation induced Relengthening of Acetylcholine-contracted Airway Smooth Muscle. Proc Am Thorac Soc. 2008;5(1):68–72. doi:10.1513/pats.200705-058VS.
- Bossé Y, Chapman DG, Pare PD, King GG, Salome CM. A ‘Good’ muscle in a ‘Bad’ environment: The importance of airway smooth muscle force adaptation to airway hyperresponsiveness. Respir Physiol Neurobiol. Dec 15 2011;179(2-3):269–275. doi:10.1016/j.resp.2011.09.003.
- Chapman DG, Pascoe CD, Lee-Gosselin A, et al. Smooth muscle in the maintenance of increased airway resistance elicited by methacholine in humans. Am J Respir Crit Care Med. 2014;190(8):879–885. doi:10.1164/rccm.201403-0502OC.
- Bossé Y. Asthmatic airway hyperresponsiveness: the ants in the tree. Trends Mol Med. 2012;18(11):627–633. doi:10.1016/j.molmed.2012.09.002.
- Gill R, Rojas-Ruiz A, Boucher M, Henry C, Bossé Y. More airway smooth muscle in males versus females in a mouse model of asthma: A blessing in disguise?. Exp Physiol. 2023.
- Gump A, Haughney L, Fredberg J. Relaxation of activated airway smooth muscle: relative potency of isoproterenol vs. tidal stretch. J Appl Physiol (1985). 2001;90(6):2306–2310. doi:10.1152/jappl.2001.90.6.2306.
- Fredberg JJ, Jones KA, Nathan M, et al. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol (1985). 1996;81(6):2703–2712. doi:10.1152/jappl.1996.81.6.2703.
- Oliver M, Kovats T, Mijailovich SM, Butler JP, Fredberg JJ, Lenormand G. Remodeling of integrated contractile tissues and its dependence on strain-rate amplitude. Phys Rev Lett. 2010;105(15):158102. doi:10.1103/PhysRevLett.105.158102.
- Gazzola M, Khadangi F, Clisson M, Beaudoin J, Clavel MA, Bossé Y. Airway smooth muscle adapting in dynamic conditions is refractory to the bronchodilator effect of a deep inspiration. Am J Physiol Lung Cell Mol Physiol. 2020;318(2):L452–L458. doi:10.1152/ajplung.00270.2019.

