?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Study Aim: As the geriatric population rapidly expands, there has been a concurrent increase in elderly admissions to intensive care units (ICUs). Acute lung injury (ALI) is a prevalent reason for these admissions and carries poorer survival rates for the aged population compared to younger counterparts. The aging lung is subject to physiological, cellular, and immunological changes. However, our understanding of how aging impacts the clinical progression of ALI is limited. This study explored the effect of aging using a murine model of ALI. Methods: Female C57BL/6J mice, aged 7–8 wk (young) and 18 months (aged), were divided into four groups: young controls, aged controls, young with ALI (YL), and aged with ALI (AL). ALI was induced via intratracheal administration of lipopolysaccharide (LPS, 0.5 mg/kg). The animals were euthanized 72 h after LPS exposure. Results: The AL group exhibited a significantly increased wet/dry ratio compared to the other three groups, including the YL group. The bronchoalveolar lavage (BAL) fluid in the AL group had more cells overall, including more neutrophils, than the other groups. Inflammatory cytokines in BAL fluid showed similar trends. Histological analyses demonstrated more severe lung injury and fibrosis in the AL group than in the other groups. Increased transcription of senescence-associated secretory phenotype markers, including PAI-1 and MUC5B, was more prominent in the AL group than in the other groups. This trend was also observed in BAL samples from humans with pneumonia. Conclusions: Aging may amplify lung damage and inflammatory responses in ALI. This suggests that physicians should exercise increased caution in the clinical management of aged patients with ALI.
Introduction
Population aging is a global concern. Aging is defined as the gradual loss of balanced functions.Citation1 In South Korea, one of the fastest-aging countries globally, 11.7% of the population was over 65-year old in 2015, and it is projected to exceed 40% of the total population by 2050.Citation2 Aging impacts organ systems both physiologically and physically. The aging lung progressively loses its anatomic, physiologic, and immunologic functions.Citation3–5 These changes affect lung function and reduce its ability to repair itself, making the elderly population more susceptible to lung diseases.Citation6–9
As the population ages, the hospitalization rate of elderly patients and their admission to intensive care units (ICUs) are increasing.Citation2,Citation10,Citation11 Acute lung injury (ALI) in elderly patients is associated with higher mortality rates compared to younger patients.Citation12,Citation13 The factors in aged lungs that exacerbate lung damage following infection remain unclear.Citation14 Some researchers have studied ALI in older mice compared to younger mice and have found significant deterioration of lung damage and function in older mice.Citation14–16 Despite these research efforts, there are still many gaps in our understanding of the aging mechanism in lung tissue and cells. Genome telomere shortening, stem cell exhaustion, compromised autophagy, senescence, and altered intercellular communication have been discussed as potential mechanisms, but it remains challenging to discern what truly drives aging and to distinguish cause from effect.Citation17,Citation18 Furthermore, there is a lack of studies on actual elderly patients with acute lung damage.
Considering this background, we explored the impact of and mechanisms related to, aging in acute lung damage using a mouse model. In addition to animal experiments, we analyzed bronchoalveolar lavage (BAL) fluid from elderly human patients with pneumonia.
Materials and methods
Experimental design and animal model
Male C57BL/6J mice were obtained from Deahan Bio-link (Chungcheongbuk-do, Korea), with young mice being 8-wk old and aged mice being 72-wk old. After a week of acclimation, the mice were randomly assigned to four groups: young control (YC), aged control (AC), young with ALI (LPS; YL), and aged with ALI (AL). To induce ALI, we intratracheally injected a dose of 0.5 mg/kg LPS (Escherichia coli 055:B5; Sigma-Aldrich, St. Louis, MO, USA) in 50 µL phosphate-buffered saline (PBS); control mice received an equal volume of PBS alone. Subsequently, the mice were euthanized 72 h post-LPS or saline instillation. The Ethical Committee on Animal Experiments of the Catholic University of Korea approved all experimental animal protocols in this study, in accordance with animal welfare guidelines (EPSMH2019300101FA).
Histopathology
Lung tissues from the left side of each group were fixed in 10% formalin, embedded in paraffin wax, and sliced into 4 m sections using a microtome. After deparaffinization, the tissue sections were subjected to hematoxylin and eosin (H&E) staining for histological analysis and Masson’s trichrome staining for detection and localization of fibrotic responses. Evaluations focused on congestion, focal thickening, intra-alveolar hemorrhage, modifications of alveolar membranes, and intra-alveolar neutrophil infiltration. The Szapiel score was used to assess the extent of parenchymal alveolitis, while the Ashcroft scale was performed to evaluate fibrotic alterations. Five randomly selected fields from each lung section were observed under either x100 or x200 magnification. Two blinded observers independently scored each specimen. The average Szapiel or Ashcroft score for each mouse was subsequently used for statistical analysis.
Mean linear intercept and wet/dry weight ratio
To quantify edema in ALI, a lobe from the right lung was excised and immediately weighed on an electronic scale following euthanasia. Then, it was desiccated in an oven at 65 °C for 48 h. The wet/dry weight ratio (W/D) was subsequently calculated for each individual mouse lung. For analysis of the mean linear intercept (Lm), five nonoverlapping fields from lung sections stained with H&E were randomly selected. A grid of vertical and horizontal lines was overlaid on these images. Counting proceeded from left to right, starting from the top left corner of the grid. The Lm was quantified as the average distance between the airspace wall and the walls of pulmonary alveoli, respiratory bronchioles, or blood vessels. The total length of the grid lines was divided by the number of intersections to derive the Lm in micrometers.
BAL fluid in animal model
After anesthetizing the mice, blood was collected sufficiently via the abdominal aorta, followed by the immediate collection of BAL fluid in lung lavage, using 0.8 mL ice-cold PBS. The trachea was cannulated with a 23-gauge catheter for BAL fluid retrieval. A hemocytometer was used to count the total number of cells in the BAL fluid. Subsequently, the BAL fluid was subjected to cytospinning (700 rpm, 5 min) and stained with Diff Quick (Sysmex, Tokyo, Japan) for differential cell counts. Inflammatory cells were identified as macrophages, neutrophils, eosinophils, and lymphocytes based on their typical morphological characteristics. Blood was centrifuged at 3000 rpm for 20 min, and serum was collected for the measurement of substances such as albumin. The supernatant of BAL fluid was stored in a −80 °C freezer for cytokine analysis experiments.
Measurement of hydroxyproline content and TGF-β
The hydroxyproline content in lung tissue was measured using a hydroxyproline assay kit (Biovision Inc., Milpitas, CA, USA). Lung tissue was homogenized, and then, treated with 12 N HCL for 16 h at 110 °C. Then, the mixture was centrifuged at 13,000 rpm for 3 min. Chloramine T solution and DMAB reagent were subsequently added. Then, the mixture was incubated for 90 min at 60 °C. All procedures were carried out in accordance with the manufacturer’s instructions.
The level of acid-activated latent transforming growth factor (TGF)-β in the BAL fluid was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific, Bartlesville, OK, USA), following the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
The concentrations of inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, albumin, and mucin 5B in BAL fluid were measured using ELISA kits from ThermoFisher Scientific and Abcam, and MyBioSource, respectively. Additionally, myeloperoxidase (MPO) levels were quantified using a Biovision ELISA kit, while 8-hydroxy-deoxyguanosine (8-OH-dG) concentrations were measured with an ELISA kit from Cayman Chemical. The ratios of phospho-p38/p38, phospho-ERK/ERK, and phospho-JNK/JNK in lung tissue, along with the expression of transient receptor potential cation channel subfamily V (TRPV4), were also assessed using commercially available ELISA kits. Finally, serum levels of plasminogen activator inhibitor-1 (PAI-1) were quantified using a Thermo Fisher Scientific ELISA kit. All samples and standards were analyzed in duplicate.
Oxidative stress assay
A commercial ELISA kit was utilized to measure the levels of enzyme proteins related to oxidative stress in lung tissue. The lung tissue was homogenized using the lysis buffer provided in the kit, following the manufacturer’s instructions. The ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG), as well as the levels of superoxide dismutase (SOD), lipid peroxidation (MDA), and catalase (CAT) were determined using assays from Bioassay Systems, Hayward, CA, USA. These measurements were taken at the wavelengths of 412, 440, 532, and 412 nm, respectively. All samples and standards were analyzed in duplicate.
Quantitative reverse transcription polymerase chain reaction
Total RNA from lung tissue was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using the QuantiFast SYBR Green PCR kit (Qiagen, Valencia, CA, USA) on the Bio-Rad real-time PCR system. The PCR conditions included initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 10 s, and annealing at 60 °C for 30 s. RT-qPCR was performed to analyze changes in mRNA expression of senescence markers (p53, p21, and p16), toll-like receptor 4 (TLR4), TRPV4, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), PAI1, and Mucin-5b genes. The primer sequences were designed based on the National Center for Biotechnology Information (NCBI) database. Amplification of β-actin was used for normalization of each gene’s cycle threshold values. Relative mRNA expression was calculated using the 2-ΔΔCT method.
Western blotting
Total protein was extracted from homogenized lung tissue using RIPA lysis buffer (Thermo Scientific). Equal amounts of protein sample were separated on 8–15% SDS-PAGE gels via electrophoresis and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Then, the membranes were blocked with 5% skim milk and incubated with primary antibodies for p53, p21, p16, TLR4, NF-κB, phospho-NF-κb, I-κb, and β-actin at a 1:1000 dilution (Cell Signaling Technology, Inc., Danvers, MA, USA) for 4 h at room temperature. Next, membranes were incubated with a secondary antibody for 2 h at room temperature. Target proteins were detected using the enhanced chemiluminescence Western blotting method (Bio-Rad, Hercules, CA, USA) and images were captured on an ImageQuant LAS 500 Imaging System.
Terminal deoxynucleotidyl transferase dUTP nick end labeled assay
Terminal deoxynucleotidyl transferase dUTP nick end labeled (TUNEL) assay was used to detect apoptotic cells within lung tissues. An in situ apoptosis detection kit (Promega, Madison, WI, USA) was utilized in accordance with the manufacturer’s instructions. Semi-quantitative evaluation of TUNEL-positive cells, exhibiting these distinct dark brown-stained nuclei, was performed through light microscopy in ten randomly selected areas of lung sections observed under a microscope with a x400 magnification field.
BAL fluid from humans
BAL fluid was collected from inpatients with pneumonia by experienced pulmonologists using a flexible bronchoscope, specifically targeting areas of lung infiltration. A minimum of 100 cc normal saline was instilled, and at least a 30% return was required for sampling. Eligible participants were cognitively capable of signing a consent form for sample collection. Patients requiring more oxygen supply than could be provided through nasal prongs were excluded. Additionally, patients were disqualified if a diagnosis other than pneumonia was identified following analysis of bronchoscopy specimens. This process was approved by the Institutional Review Board at the Catholic University of Korea (PC21SISI0193).
Data analyses
All analyses between groups were performed using two-way ANOVA with post hoc test including BAL cell differentiation. The Kruskal–Wallis test was used in clinical sample analysis, followed by Dunn’s multiple comparison test. The GraphPad Prism software (version 7.0; GraphPad Software Inc., San Diego, CA, USA) was utilized for these analyses. The data are presented as mean ± standard deviation (SD). A P-value < .05 was considered significant.
Results
Effects of lipopolysaccharide and aging on lung injury, inflammation, and fibrosis
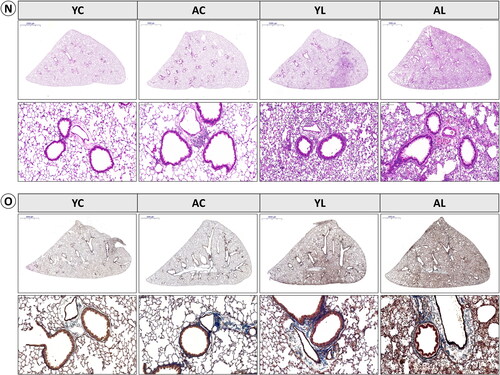
The W/D weight ratio was notably higher in the AL group than in all other groups ((1A)). The mean Lm exhibited a trend similar to that of the W/D ratio ((1B)). In BAL fluid analysis, the AL group had significantly more cells overall, as well as higher neutrophil and eosinophil counts, than the other groups ((1C)). Albumin levels did not significantly vary between control and LPS groups in young or aged mice, and MPO activity remained consistent across all groups ((1D,E)). Levels of 8-hydroxy-2′-deoxyguanosine (8-OH-dG) were significantly higher in LPS groups than in control groups in both young and aged mice ((1 F)). Although the difference between the AL and YL groups was not statistically significant, the value for the former was higher. TNF-α levels were significantly elevated in the AL group compared to the other three groups; IL-1β and IL-6 levels followed a similar trend ((1G–I)). Hydroxyproline levels exhibited no statistically significant differences among the four groups; however, the AL group demonstrated the highest observed value ((1J)). TGF-β levels were notably higher in the AL group compared to the other three groups, being significantly higher than in the YL group ((1K)). Histological analyses indicated more severe lung injury in the AL group, including increased inflammatory cell infiltration and thickened alveolar septa, compared to the other groups ((2N)). Masson’s trichrome staining revealed that the AL group also had more collagen accumulation and interstitial cells than the other groups ((2O)). Consistent with the histological observations, both the Szapiel score and Ashcroft score exhibited markedly elevated values in the AL group when compared to the remaining three groups ((1L,M)).
Figure 1. (1) Effect of lipopolysaccharide (LPS) and aging on lung injury, inflammation, and fibrosis. (A) Lung wet/dry (W/D) ratio and (B) mean linear intercept (Lm) were analyzed as markers of acute lung injury. (C) Bronchoalveolar lavage (BAL) cells were isolated, and total cells and specific cell types were counted to analyze the number of inflammatory cells. (D) Albumin and (E) myeloperoxidase (MPO) as the inflammatory enzyme, and (F) 8-hydroxy-2’-deoxyguanosine (8-OH-dG) as the cell damage marker were measured in serum or lung homogenates. (G) Tumor necrosis factor (TNF)-α, (H) interleukin (IL)-1β, and (I) IL-6 were measured as proinflammatory cytokines in BAL fluid. (J) Hydroxyproline content in lung tissue and (K) transforming growth factor (TGF)-β in BAL fluid were measured in lung tissue. (L) Szapiel’s score was used to evaluate alveolar inflammation and lung injury. (M) Ashcroft score, and collagen deposition were analyzed. Data are mean ± SD. (*P < .05, **P < .01, ***P < .001 vs. YC; #P < .05, ##P < .01, ###P < .001 vs. AC; $P < .05, $$P < .01, $$$P < .001 vs. YL). (2) Effect of lipopolysaccharide (LPS) and aging on lung injury, inflammation, and fibrosis. Representative photomicrographs of lung tissues were stained with (N) hematoxylin and eosin (H&E). The upper panel shows an image of a whole lung section (Scale bars: 2000 μm), and the images are enlarged in the lower panels (magnification, ×200). (O) Representative Masson’s trichrome staining of lung sections shows collagen accumulation status (upper panels: whole lung section, lower panels: magnification, ×200).
Effects of lipopolysaccharide on oxidative stress, and changes in oxidative stress with aging
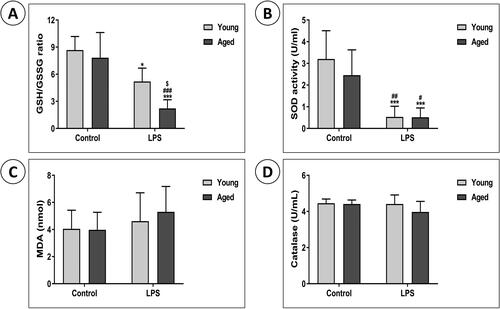
We investigated whether LPS exposure influenced the activity of enzymes typically involved in antioxidant defense in the lungs. The GSH to GSSG ratio, indicating GSH consumption due to reactive oxygen species (ROS) accumulation, was significantly lower in the AL group than in the other three groups, including the YL group (). SOD activity in lung homogenates was significantly lower in the LPS groups than in controls (). However, there were no significant differences in MDA levels or catalase activity between the groups ()).
Figure 2. Effects of LPS on oxidative stress, and changes in oxidative stress with aging. The levels of (A) the reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio, (B) superoxide dismutase (SOD), (C) malondialdehyde (MDA), and (D) catalase (CAT) activity were measured as indicators of oxidative stress in the lung homogenates. Data are mean ± SD. (*P < .05, **P < .01, ***P < .001 vs. YC; #P < .05, ##P < .01, ###P < .001 vs. AC; $P < .05, $$P < .01, $$$P < .001 vs. YL).
Effects of lipopolysaccharide on apoptosis, mitogen-activated protein kinase
Signaling pathways, and the senescence-associated secretory phenotype, and the changes in these with aging
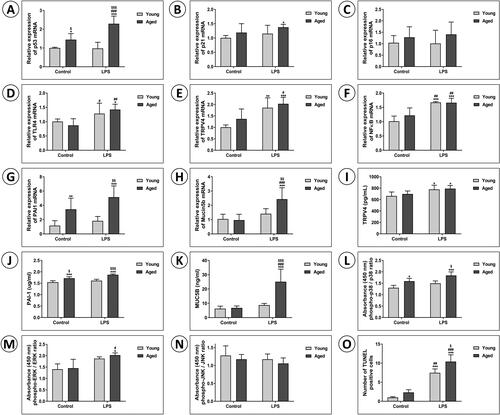
Next, the expression of genes related to apoptosis and cell survival, including p53, p21, and p16, was analyzed. The mRNA expressions of p53, p21, and p16 were consistently higher in aged mice ((1A–C)). Notably, that of p53 was significantly higher in the AL group than in the other three groups, including the YL group (P < .001). The protein levels showed the same trends ((2P)).
Figure 3. (1) Effects of LPS on apoptosis, mitogen-activated protein kinase signaling pathways, and the senescence-associated secretory phenotype, and the changes in these with aging. The mRNA expressions of (A) p53, (B) p21, and (C) p16 as the senescence markers, and those of (D) TLR4, (E) TRPV4, (F) NF-kB, (G) PAI-1, and (H) mucin 5b were examined by qRT-PCR. The protein levels of (I) TRPV4, (J) PAI-1, and (K) MUC5B, and (L) phospho-p38/p38 ratio, (M) phospho-ERK/ERK ratio, and (N) phospho-JNK/JNK ratio were assessed by ELISA. (O) The TUNEL-positive cells were semi-quantitatively assessed. Data are mean ± SD. (*P < .05, **P < .01, ***P < .001 vs. YC; #P < .05, ##P < .01, ###P < .001 vs. AC; $P < .05, $$P < .01, $$$P < .001 vs. YL). (2) Effects of LPS on apoptosis, mitogen-activated protein kinase signaling pathways, and the senescence-associated secretory phenotype, and the changes in these with aging. (P) The protein levels of p53, p21, and p16 as the senescence markers, and those of (Q) TLR4, NF-kB, p-NF-kB, I-kB, and β-actin were examined by Western blotting. (R) Representative photomicrographs of lung tissues were stained with TUNEL assay kit to determine apoptotic cells (Magnification, x200). The images are enlarged in the lower panels (Magnification, x400).
We also examined the mRNA expression of TLR4, TRPV4, NF-kB, PAI-1, and Muc5b to identify the pathway associated with lung damage due to LPS exposure and aging ((1D–H)). Upon LPS exposure, the mRNA expression of TRPV4 and NF-kB significantly increased in both young and aged mice. That of TLR4 was the same except no significant difference was observed between the AL and YL groups. The AL group had the highest expression of PAI-1 and Muc5b. Relative to the YL group, the AL group had significantly higher expression of PAI-1 and Muc5b. The protein levels of TPRV4, PAI-1, MUC5B, TLR4, and NF-kB paralleled the trends of mRNA expression ((1I–K, 2Q)). The protein level of MUC5B was significantly higher in the AL group than the other groups.
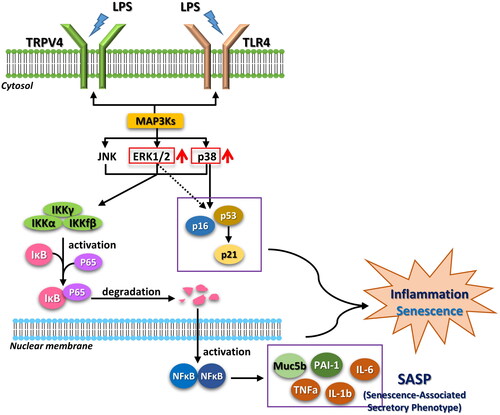
The phosphorylation of p38, ERK, and JNK was assessed via ELISA ((1L–N)). The phospho-p38/p38 ratio was significantly higher in the AL group compared to the AC and YC groups; it was also higher, but not significantly so, than that of the YL group. Moreover, the AL group had the highest phospho-ERK value and lowest value phospho-JNK value. The AL group exhibited significantly more TUNEL-positive cells compared to the other three groups ((1O)). (2R) shows enlarged images after TUNEL staining. The proposed signaling pathways of LPS-induced ALI and senescence are summarized in .
Figure 4. Proposed signaling pathways of LPS-induced ALI and senescence. Stimulation by LPS results in activation of MAP3Ks. MAPKs including ERK1/2 and p38 are activated and induce inflammation and senescence through p16 and p53. Increased transcription of the senescence-associated secretory phenotype (SASP) and inflammation genes are regulated by the IKK/NF-κB pathway.
Analysis of human BAL fluid
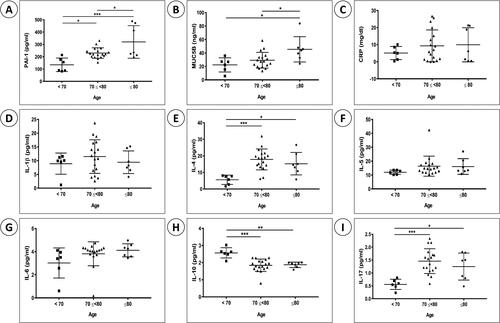
The BAL fluid from 31 inpatients with pneumonia was analyzed (). The levels of PAI-1 and MUC5B were significantly higher in the group aged 80 years or older (elderly group) than in the other age groups (younger groups). Although IL-4 and IL-17 levels did not significantly differ between the elderly and younger groups the 80+ and 70–79 age groups demonstrated significant differences versus the under 70 age group. IL-10 levels decreased with age. There were no significant differences in IL-1β, IL-5, or IL-6 levels, or the C-reactive protein level (a commonly used inflammatory marker), among the groups.
Figure 5. Analysis of BAL fluid from human samples. Protein levels of (A) PAI-1, (B) MUC5b, (C) C-reactive protein (CRP), (D) IL-1β, (E) IL-4, (F) IL-5, (G) IL-6, (H) IL-10, and (I) IL-17 were measured in BAL fluid from 31 in patients with pneumonia. The patients were grouped by age, as follows: <70-year old, 70 ≤ 80-year old, and ≥80-year old. Data are mean ± SD (*P < .05, **P < .01, ***P < .001).
Discussion
We assessed the effects of aging on ALI triggered by LPS. Our results suggest that aging amplifies the severity of lung injury in the ALI mouse model. Notably, several aspects, including inflammation, fibrosis, and oxidative stress, were intensified by aging. The transcription of SASP was particularly prominent in aged mice with lung injury. This trend was also observed in BAL samples from humans with pneumonia.
LPS-induced lung injury in mice typically peaks within 24–36 h, followed by a recovery phase over the next 2–3 days.Citation19 In this study, we report the findings from mice euthanized 72 h after LPS exposure. Although we do not present the data, we also euthanized mice 24 h post-LPS challenge. The results from each time point had similar trends, with the AL group consistently exhibiting the most severe lung injury but the influence of aging was more discernible when mice were euthanized 72 h post-LPS challenge.
Navarro et al.Citation20 noted that the lungs undergo age-related changes, resulting in a decrease in the strength of respiratory muscles, an increase in proinflammatory markers, and a diminished capacity for immune responses. Numerous studies have reported a strong correlation between aging and chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF).Citation20–22 COPD, characterized by emphysema and chronic bronchitis, is associated with an increased prevalence and higher mortality rate with advancing age.Citation23–25 Similarly, IPF, which is associated with aging,Citation25 involves various genes implicated in pathogenesis, including those linked to aging-related telomere length shortening.Citation26 Other potential mechanisms for IPF include oxidative stress, altered proteostasis, and mitochondrial abnormalities.Citation7
Our results align with those of Kling et al.,Citation14 who demonstrated more severe ALI with advanced age in mice, characterized by a more severe inflammatory response, decreased lung function, and heightened blood–air barrier damage. Brandenberger et al.Citation15 proposed that a decrease in stimulators of adaptive immunity with advancing age might be associated with worse ALI prognosis in the elderly. All of these studies, including ours, affirm that lung injury is more severe in aged mice compared to younger mice. This reinforces the notion that acute inflammatory responses are more pronounced in elderly subjects than in their younger counterparts.
ROS, as byproducts of acute pulmonary inflammation and cellular apoptosis, tend to increase in ALI.Citation20 Palumbo et al.Citation16 reported upregulation of the ROS production enzyme Nox4, which led to increased ROS levels in elderly mice with ALI. They found that pharmacological inhibition of Nox4 can reduce endothelial cell damage in the lungs of aged ALI mice. Another indicator of oxidative stress is the GSH/GSSG ratio, with an increase in GSSG levels potentially signifying the severity of intracellular oxidative stress. In our study, we observed a significant decrease in the GSH/GSSG ratio in the AL group compared to the other groups. This indicates higher intracellular oxidative stress in aged mice.
LPS may stimulate TRPV4 or TLR4 to activate MAP3Ks, leading to ALI and senescence.Citation27 The MAP3Ks upregulate ERK1/2 and p38, which in turn induce inflammation and senescence by activating p16 and p53.Citation28 Additionally, ERK1/2 and p38 activate the IKK/NF-κB pathway, leading to upregulation of SASP components such as MUC5B, PAI-1, TNF-α, and interleukins. We found significantly higher mRNA expressions of p53, Muc5b, and PAI-1 in the AL mouse group.
p53 is a tumor suppressor and is notably involved in regulating cellular senescence.Citation29 Impaired p53 expression can lead to the development of cancer, while the expression of full-length p53 is associated with premature aging.Citation30 In our study, we noted higher expression of p53 in the aged mouse groups compared to the young mouse groups.
MUC5B, a major gel-forming mucin in the lungs, plays a crucial role in mucosal clearance and host defense.Citation31 Its overproduction is associated with the development of fibrosis.Citation32 Because the AL mouse group exhibited fibrotic changes in the lungs, our results suggest overproduction of MUC5B in this group.
Another key component of the SASP is PAI-1, which is considered both a marker and a significant mediator of cellular aging.Citation33 Studies on subjects spanning from murine models to humans have demonstrated correlations between PAI-1 and aging.Citation33 In our study, PAI-1 was significantly elevated in both aged mouse groups, corroborating the results of previous studies.Citation34
PAI-1 and interleukin levels are associated with ALI and acute respiratory distress syndrome.Citation35 Given the scarcity of research on human samples, we conducted a study with a small human cohort to ascertain if humans would demonstrate the same trends observed in mice. Despite the limitations of a small sample size and an imprecise assessment of pneumonia severity, our data indicated a correlation between aging and levels of PAI-1, MUC5B, IL-4, IL-17, and IL-10. However, CRP, a traditional inflammatory marker, did not show any difference related to aging. Further human studies are required to identify suitable biomarkers that can accurately reflect the impact of aging.
Our study had several limitations. First, aging is a complex biological process influenced by a multitude of factors including molecular and cellular changes.Citation6 Therefore, the variables, including aging, that can affect the inflammatory pathway are not yet fully understood. Second, it is challenging to directly apply our results to clinical practice because factors damaging lung tissue in humans are more complex than those in mice. Unlike laboratory mice, humans may have comorbidities such as diabetes, hypertension, and obesity. Third, the analysis of our human data was based on a small sample size. While our study does demonstrate a correlation between some inflammatory factors and aging, it does not definitively establish that all aging patients with lung damage would exhibit higher inflammatory factors.
We found that aging exacerbates ALI by increasing inflammation, fibrosis, and oxidative stress, suggesting that aging could be a risk factor for lung injury. Aging influences apoptosis and MAPK signaling pathways, thereby making our bodies more susceptible to damage. This implies that ALI in aged patients should be treated with increased seriousness. Understanding the factors that affect inflammatory signaling pathways is crucial for the treatment of aged patients who develop acute respiratory distress syndrome. Therefore, further studies are needed to comprehend aging and related factors.
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/WHUibt.
Declaration of interest
All authors declare that they have no conflict of interest.
Additional information
Funding
References
- Brandsma C-A, de Vries M, Costa R, Woldhuis RR, Königshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26(146):170073. doi:10.1183/16000617.0073-2017.
- Lim JU, Lee J, Ha JH, Kang HH, Lee SH, Moon HS. Demographic changes in intensive care units in Korea over the last decade and outcomes of elderly patients: a single-center retrospective study. Korean J Crit Care Med. 2017;32(2):164–173. doi:10.4266/kjccm.2016.00668.
- Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–260. doi:10.2147/ciia.2006.1.3.253.
- Freitas FS, Ibiapina CC, Alvim CG, Britto RR, Parreira VF. Relationship between cough strength and functional level in elderly. Rev Bras Fisioter. 2010;14(6):470–476. doi:10.1590/S1413-35552010000600004.
- Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013;8:1489–1496. doi:10.2147/cia.S51152.
- Brandenberger C, Muhlfeld C. Mechanisms of lung aging. Cell Tissue Res. 2017;367(3):469–480. doi:10.1007/s00441-016-2511-x.
- Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am J Physiol Lung Cell Mol Physiol. 2018;314(4):L642–Ll653. doi:10.1152/ajplung.00275.2017.
- Lee JY, Yoo CG, Kim HJ, Jung KS, Yoo KH. Disease burden of pneumonia in Korean adults aged over 50 years stratified by age and underlying diseases. Korean J Intern Med. 2014;29(6):764–773. doi:10.3904/kjim.2014.29.6.764.
- Meyer KC. Aging. Proc Am Thorac Soc. 2005;2(5):433–439. doi:10.1513/pats.200508-081JS.
- Brunker LB, Boncyk CS, Rengel KF, Hughes CG. Elderly patients and management in intensive care units (ICU): Clinical challenges. Clin Interv Aging. 2023;18:93–112. doi:10.2147/cia.S365968.
- Laporte L, Hermetet C, Jouan Y, et al. Ten-year trends in intensive care admissions for respiratory infections in the elderly. Ann Intensive Care. 2018;8(1):84. doi:10.1186/s13613-018-0430-6.
- Guillon A, Hermetet C, Barker KA, et al. Long-term survival of elderly patients after intensive care unit admission for acute respiratory infection: a population-based, propensity score-matched cohort study. Crit Care. 2020;24(1):384. doi:10.1186/s13054-020-03100-4.
- Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136(1):25–36. doi:10.7326/0003-4819-136-1-200201010-00007.
- Kling KM, Lopez-Rodriguez E, Pfarrer C, Muhlfeld C, Brandenberger C. Aging exacerbates acute lung injury-induced changes of the air-blood barrier, lung function, and inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L1–Ll12. doi:10.1152/ajplung.00347.2016.
- Brandenberger C, Kling KM, Vital M, Christian M. The role of pulmonary and systemic immunosenescence in acute lung injury. Aging Dis. 2018;9(4):553–565. doi:10.14336/ad.2017.0902.
- Palumbo S, Shin YJ, Ahmad K, et al. Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L297–Ll308. doi:10.1152/ajplung.00305.2016.
- Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019;177:37–45. doi:10.1016/j.mad.2018.03.013.
- Guo J, Huang X, Dou L, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391. doi:10.1038/s41392-022-01251-0.
- Wu C, Evans CE, Dai Z, et al. Lipopolysaccharide-induced endotoxemia in corn oil-preloaded mice causes an extended course of lung injury and repair and pulmonary fibrosis: A translational mouse model of acute respiratory distress syndrome. PLoS One. 2017;12(3):e0174327. doi:10.1371/journal.pone.0174327.
- Navarro S, Driscoll B. Regeneration of the aging lung: a mini-review. Gerontology. 2017;63(3):270–280. doi:10.1159/000451081.
- Cho WK, Lee CG, Kim LK. COPD as a Disease of Immunosenescence. Yonsei Med J. 2019;60(5):407–413. doi:10.3349/ymj.2019.60.5.407.
- Breen M, Nwanaji-Enwerem JC, Karrasch S, et al. Accelerated epigenetic aging as a risk factor for chronic obstructive pulmonary disease and decreased lung function in two prospective cohort studies. Aging (Albany NY). 2020;12(16):16539–16554. doi:10.18632/aging.103784.
- Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance–United States, 1999-2011. Chest. 2013;144(1):284–305. doi:10.1378/chest.13-0809.
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi:10.1016/s0140-6736(07)61377-4.
- Thannickal VJ, Murthy M, Balch WE, et al. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med. 2015;191(3):261–269. doi:10.1164/rccm.201410-1876PP.
- Glass DS, Grossfeld D, Renna HA, et al. Idiopathic pulmonary fibrosis: Molecular mechanisms and potential treatment approaches. Respir Investig. 2020;58(5):320–335. doi:10.1016/j.resinv.2020.04.002.
- Gao H, Xiao D, Gao L, Li X. MicroRNA‑93 contributes to the suppression of lung inflammatory responses in LPS‑induced acute lung injury in mice via the TLR4/MyD88/NF‑kappaB signaling pathway. Int J Mol Med. 2020;46(2):561–570. doi:10.3892/ijmm.2020.4610.
- Mao B, Guo W, Tang X, et al. Inosine pretreatment attenuates LPS-induced lung injury through regulating the TLR4/MyD88/NF-kappaB signaling pathway in vivo. Nutrients. 2022;14(14):2830. doi:10.3390/nu14142830.
- Hernández Borrero LJ, El-Deiry WS. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188556. doi:10.1016/j.bbcan.2021.188556.
- Tabibzadeh S. Signaling pathways and effectors of aging. Front Biosci (Landmark Ed). 2021;26(1):50–96. doi:10.2741/4889.
- Ostedgaard LS, Moninger TO, McMenimen JD, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci USA. 2017;114(26):6842–6847. doi:10.1073/pnas.1703228114.
- Hancock LA, Hennessy CE, Solomon GM, et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun. 2018;9(1):5363. doi:10.1038/s41467-018-07768-9.
- Vaughan DE, Rai R, Khan SS, Eren M, Ghosh AK. Plasminogen activator inhibitor-1 is a marker and a mediator of senescence. Arterioscler Thromb Vasc Biol. 2017;37(8):1446–1452. doi:10.1161/atvbaha.117.309451.
- Eren M, Boe AE, Klyachko EA, Vaughan DE. Role of plasminogen activator inhibitor-1 in senescence and aging. Semin Thromb Hemost. 2014;40(6):645–651. doi:10.1055/s-0034-1387883.
- Tsangaris I, Tsantes A, Bonovas S, et al. The impact of the PAI-1 4G/5G polymorphism on the outcome of patients with ALI/ARDS. Thromb Res. 2009;123(6):832–836. doi:10.1016/j.thromres.2008.07.018.