Abstract
Background: Sepsis has become one of the main factors inducing the development of acute lung injury (ALI) in clinical practice. Currently, inhibiting the activation of NLRP3 mediated pyroptosis is the target of multiple drugs in the treatment of sepsis induced ALI. This study aimed to explore the effects of METTL14 on the pyroptosis in the sepsis induced ALI progression.
Methods: LPS-stimulated A549 cells and cecal ligation and puncture (CLP)-treated mice were used to establish the ALI model in vitro and in vivo. Then, the cell viability was measured by CCK-8 assay. ELISA kits were used to determine the IL-18 and IL-1β contents. Pyroptosis rate was tested by flow cytometry. M6A dot blot was conducted to analyze the global m6A levels and MeRIP assay was performed to detect the m6A levels of NLRP3. The relationship between METTL14 and NLRP3 was confirmed by RIP and dual-luciferase report assays.
Results: The global m6A levels were significantly increased in the LPS-stimulated A549 cells and CLP-treated mice. METTL14 knockdown decreased the cell viability, IL-18 and IL-1β contents, and pyroptosis rate of the LPS-stimulated A549 cells. Furthermore, the increase of pyroptosis-related proteins in LPS-stimulated A549 cells was significantly decreased after METTL14 knockdown. Additionally, METTL14 knockdown decreased the m6A and mRNA levels of NLRP3, and NLRP3 overexpression reversed the effects of METTL14 knockdown on the pyroptosis in the LPS-stimulated A549 cells. In CLP-treated mice, METTL14 knockdown relieved the injury and decreased the IL-18 and IL-1β contents in the lung tissues, serum and bronchoalveolar lavage fluid.
Conclusion: This study demonstrated that METTL14 knockdown inhibited the pyroptosis in the sepsis-induced ALI progression through decreasing the NLRP3 levels dependent on m6A methylation modification.
Introduction
Sepsis is a life-threatening disease caused by an excessive immune response induced by pathogens, often leading to multiple organ failure.Citation1 Sepsis has become one of the main factors inducing the development of acute lung injury (ALI) in clinical practice.Citation2 Sepsis-induced ALI is a clinical syndrome that includes a variety of acute respiratory failure diseases, characterized by damage to alveolar epithelial and endothelial cells, infiltration of inflammatory cells in the lungs, and the presence of hyperemia and edema.Citation3,Citation4 Currently, the treatment of sepsis induced ALI includes antibiotics, glucocorticoids, and respiratory support.Citation2,Citation5 However, long-term use of antibacterial drugs can produce drug-resistant bacteria, accompanied by a series of adverse reactions, and the treatment effect is not ideal.Citation6,Citation7 These factors lead to the high mortality rate of sepsis-induced ALI patients. In the pathological process of sepsis-induced ALI, the imbalance of inflammatory response-induced cell death leads to the destruction of alveolar epithelial cells and increased epithelial permeability, ultimately promoting the process of lung injury.Citation8,Citation9 Therefore, therapeutic strategies targeting inflammatory response-induced cell death may be an effective method to improve sepsis-induced ALI.
Pyroptosis, also known as inflammatory necrosis of cells, is a natural immune response that plays an important role in the fight against infection.Citation10 The inflammatory complex of NOD-like receptor protein 3 (NLRP3) promotes the release of various inflammatory factors, which was the main inducing factor for the occurrence pyroptosis.Citation11 Activated NLRP3 cleaves and activates caspase-1, which can not only promote the production of multiple inflammatory factors but also cleave the N-terminal domain of Gasdermin D (GSDMD), mediating the occurrence of pyroptosis. Previous studies have shown that inhibiting the NLRP3/caspase-1/GSDMD pathway can prevent the occurrence of pyroptosis.Citation12 Wang et al.Citation13 have shown that NLRP3 inflammasome was activated, and pyroptosis was occurred in the lung tissue of mice with ALI caused by sepsis, which induced the inflammatory infiltration and other injuries. Currently, inhibiting the activation of NLRP3 inflammasome is the target of multiple drugs in the treatment of sepsis-induced ALI.
M6A methylation is a common form of epigenetic modification of RNA, which regulates gene expression through RNA translation and degradation, potentially regulating the occurrence and progression of diseases.Citation14 This biological process involves three catalytic enzymes, namely, "write" methyltransferase, "erase" demethylase, and "read" methylated reading protein.Citation15 Among these enzymes, Methyltransferase is a complex composed of METTL3 and METTL14, which forms a heterodimer catalytic core and regulates the Wilms tumor 1 associated protein (WTAP) to jointly promote methylation reactions.Citation16 Previous research on m6A has mainly focused on embryonic abnormalities and tumors.Citation17,Citation18 In recent years, more and more researchers have focused on the role of m6A in lung diseases, including pulmonary fibrosisCitation19 and lung cancer,Citation20 and demonstrated that m6A plays an important role in the occurrence and development of lung diseases.
Therefore, in this study, we established the ALI model in vitro and in vivo to explore the specific mechanisms of METTL14. We hypothesized that METTL14 knockdown inhibited the pyroptosis through down-regulating the m6A levels of NLRP3.
Material and method
Cell culture and treatment
Human lung type II epithelial cell line (A549) were provided by Procell Life Science&Technology Co., Ltd (Wuhan, China). A549 cells were cultured in Dulbecco’s modified eagle medium (DMEM), which was supplemented with 10% fetal bovine serum (Thermo Fisher, USA) and penicillin/streptomycin (Thermo Fisher). After 24 h culture, the cells were treated with LPS (20 μg/mL, Sigma, USA) for 12 h to establish the sepsis-induced ALI model.
Establishment of sepsis-induced ALI mice model
A total of 24 male C57BL/6 mice (30 ± 5) were provided by the Experimental Animal Center of Hebei Medical University. All mice were kept in a clean-grade animal room under alternating light and dark conditions of 12 h/12 h, with a temperature of 18–22 °C and a humidity of 40–60%. Mice were allowed to eat and drink freely. After 1 week of adaptive feeding, mice were used for the experiment.
The mice were divided into four groups: sham group, cecal ligation and puncture (CLP) group, CLP + lentivirus short hairpin RNA NC (lv-shNC) group, CLP + lentivirus short hairpin RNA METTL14 (lv-shMETTL4) group. The CLP assay was used to establish the sepsis-induced ALI model. In brief, mice were fasted for 12 h before surgery and were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg). Mice were fixed in a supine position, and after abdominal disinfection, an incision of about 1 cm was made along the midline of the abdominal wall. Then, the cecum was separated and penetrated using 18-gauge needle for three times. After that, the punctured cecum was returned to the abdominal cavity, and the abdominal incision was sutured layer by layer. The mice in the sham group were subjected to the same procedure without puncture treatment. For METTL14 knockdown globally, lentivirus (lv) containing shMETTL14 and shNC (0.2 ml, 1 × 109 pfu/ml) were injected into the caudal vein 4 days before modeling, respectively. The animal experiment was approved by the Animal Ethical and Welfare Committee (AEWC) (No. MDKN-2022-066).
Sample collection
After modeling and treating, the mice were weighed and sacrificed. The 2 ml abdominal aortic blood were obtained and centrifuged for 10 min (4 °C, 4000 r/min), and the serum was separated and stored at − 80 °C. The left lung was lavaged with sterile PBS for 3 times and bronchoalveolar lavage fluid (BALF) was obtained at a total volume of 1.5 mL. Then the BALF was centrifuged for 5 min (4 °C, 1500 r/min). In addition, the right lung tissue was collected and frozen in liquid nitrogen for hematoxylin–eosin (HE) staining and protein expression determination.
HE staining
The right lung tissues were fixed with 4% paraformaldehyde. Next, the samples were embedded with paraffin. After that, the tissue sections were cut into 5 μm and stained with hematoxylin and eosin for 5 min. Finally, the sections were observed by a light microscope.
Cell transfection
In order to knockout the METTL14 expressions and overexpress the NLRP3 levels, the small interfering RNA METTL14 (si-METTL14), NLRP3 overexpressed vector (oe-NLRP3) and their controls (si-NC and oe-NC) were synthesized by Shanghai GenePharma Company (Shanghai, China). The A549 cells were transfected with siRNAs and vectors using LipofectamineTM 2000 (Hengdai Lao Biotechnology Co., Ltd., China). Subsequently, the transfection efficiency was verified using RT-qPCR assay.
M6A dot blot assay
Total RNA of the A549 cells or lung tissues were obtained using Trizol (Beyotime, Shanghai, China). The global m6A levels in the RNA were detected using EpiQuik M6A RNA Methylation Quantification Kit (AmyJet Scientific, Wuhan, China). Briefly, 4 µg RNA was purified using the RNeasy Mini Kit (Qiagen, Germany). Then, the RNA was separated on 1.2% formaldehyde-agarose gels and transferred onto Hybond N + membranes. Next, the membranes were incubated with m6A antibody overnight and then treated with horseradish peroxidase conjugate anti-rabbit immunoglobulin G for 2 h. Finally, the blots were washed and developed on PhosphorImager screens and quantited with ImageQuant.
RT-qPCR
The quality of the separated RNA was tested by Nanodrop 2000 spectrophotometer, and the RNA concentration was adjusted to 500 ng/μL. To obtain cDNA, the reverse transcription was carried out using the Reverse transcription Kit (Vazyme, Nanjing, China). Then, the RT-qPCR was performed using the obtained cDNA using HiScript® Q RT SuperMix for qPCR kit (Vazyme). The reaction conditions are set to: 95 °C, 10 min; 94 °C, 15 s, 40 cycles; 55 °C, 30 s; 70 °C, 30 s. The results were analyzed by 2−ΔΔCt method and GAPDH was used as the internal parameter.
For the NLRP3 mRNA stability determination, the A549 cells were treated with 5 μg/ml actinomycin D. After METTL14 knockdown, the mRNA levels of NLRP3 were detected at 2, 4, 8, 12 h by RT-qPCR.
Cell counting kit (CCK)-8 assay
The A549 were plated into 96-well plates, followed by incubating for 48 h. Then, the cells were treated with 10 μL CCK-8 solution (KeyGEN, Jiangsu, China) and cultured for 4 h. Finally, the absorption was determined at 450 nm using a microplate reader (BioTek, USA).
IL-18 and IL-1β content determination
The IL-18 and IL-1β content in the cells, serum, BALF and lung tissues were detected using corresponding kits, which were provided by Nanjing Jiangcheng Bioengineering Institute (Nanjing, China). All operations are strictly in accordance with the instructions of the kits.
Caspase1 FLICA/PI double staining for pyroptosis detection
The pyroptosis of A549 cells was analyzed by flow cytometry analysis with the aid of caspase-1 detection kit (ImmunoChemistry Technologies, USA). Cells in each group were digested with trypsin, washed with PBS twice, and centrifuged at 1000 rpm for 5 min. According to the instructions, the cells were treated with 5 μL caspase1 FLICA and incubated in dark for 1 h. Then, the cells were washed with PBS, centrifuged at 1000 rpm for 5 min, and then treated with 5 μL PI in dark for 15 min. Finally, the cell pyroptosis rate was detected by FACS CaliburTM flow cytometry (BD, USA).
Methylated RNA immunoprecipitation (MeRIP)
The m6A levels of related genes in the A549 cells transfected with si-METTL14 or si-NC were detected using Magna MeRIP™ m6A Kit (Millipore, USA). Briefly, 200 μg RNAs were fragmented into 100 nucleotides. Then, the nucleotides were incubated with m6A antibody (or IgG) which was linked to Magna ChIP Protein A/G Magnetic Beads for immunoprecipitation. Finally, the RNAs were purified and reverse-transcribed into cDNA. After that, the expression of related genes was detected by RT-qPCR.
RNA immunoprecipitation (RIP) assay
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) was utilized to analyzed the interaction between METTL14 and NLRP3. In brief, the A549 cells were lysed using the RIP lysis buffer. After that, the cell extract was incubated with IgG or METTL14 antibody and RIP buffer solution for 1 h. Next, the cells were treated with magnetic beads and digested with proteinase K to obtain the immunoprecipitated RNA. Finally, the RT-qPCR was performed to detect the NLRP3 expressions.
Dual-luciferase reporter assay
3′-UTR of wild or mutant fragments from NLRP3 (M6A binding site 1, site2, site3, site4 mutation, respectively) were cloned into pGL3-control vectors (Promega). Then, the cells were co-transfected with vectors and si-METTL14 using Lipofectamine 3000. After 24 h transfection, the cells were collected, and luciferase activity was measured by using dualluciferase reporter assay system (Promega, Massachusetts, USA).
Western blot
Primary anti-NLRP3 (1:800), anti-ASC (1:1200), anti-Pro-caspase-1 (1:1500), anti-Cleaved-caspase-1 (1:1000), anti-GSDMD-N (1:1000), and anti-GAPDH (1:3000) were purchased from Abcam (USA). The protein of the cells or lung tissues was extracted by using RIPA reagents (Beyotime). Then, the protein concentration was determined with a BCA Kit (Beyotime). After that, the proteins were separated by 10% SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes. Next, the membranes were blocked with 5% skim milk for 1 h and incubated with primary antibodies at 4 °C overnight. Next day, the membranes were incubated with secondary antibodies for 1.5 h. Finally, the protein expression was determined by an ECL kit using Scion Image v. 4.0.2 software (Scion Corporation).
Statistical analysis
SPSS 25.0 was used to carried out the statistical analysis. The results are shown as the mean ± SD. The difference analysis was conducted using Student’s t test, and analysis of variance (ANOVA). p < 0.05 was regarded to be statistically significant.
Results
LPS stimulation increased the m6A levels in the A549 cells
After 20 μg/mL LPS stimulation, the global m6A levels in the A549 cells were significantly increased (). Then, we analyzed the mRNA expressions of m6A modification-related genes (ALKBH5, FTO, RBM15, WTAP, METTL14, METTL3) in the LPS-stimulated A549 cells, which was shown as hot map. Among them, METTL14 showed the most significant difference (). Furthermore, western blot assay showed that the protein levels of METTL14 were significantly increased in the LPS-stimulated A549 cells ().
Figure 1. LPS stimulation increased the m6A levels in the A549 cells.
The A549 cells were stimulated with 20 μg/mL LPS. (A) The m6A levels were tested by m6A dot blot assay. (B) The mRNA expressions of m6A modification-related genes were detected by RT-qPCR and expressed as hot map. (C) The protein levels of METTL14 were measured by western blot.
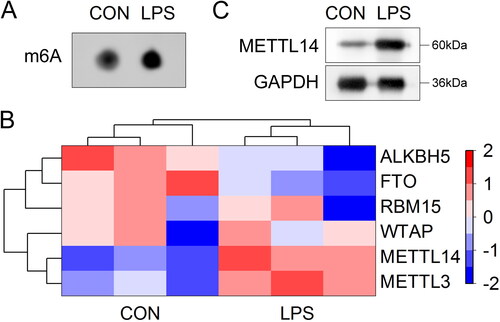
METTL4 knockdown inhibited the pyroptosis in the LPS-stimulated A549 cells
After si-METTL14 transfection, the METTL14 levels were significantly decreased and si-METTL14 #1 was selected for the next experiment (). Then, we found that LPS stimulation significantly decreased the cell viability of the A549 cells, while METTL14 knockdown significantly increased it (). Additionally, after LPS stimulation, the IL-18 () and IL-1β () content, and pyroptosis rate () were significantly increased. METTL14 knockdown significantly decreased the IL-18 () and IL-1β () content, and pyroptosis rate () in the LPS stimulated A549 cells. Furthermore, the protein levels of NLRP3, ASC, Cleaved-caspase-1 and GSDMD-N were significantly up-regulated in the LPS-stimulated A549 cells, while METTL14 knockdown significantly down-regulated them ().
Figure 2. METTL4 knockdown inhibited the pyroptosis in the LPS-stimulated A549 cells.
(A) The verification of transfection efficiency of si-METTL14 #1 and #2. The A549 cells were stimulated with LPS and treated with si-METTL14. After that, (B) the cell viability was detected by CCK-8 assay. The IL-18 (C) and IL-1β (D) contents were detected by ELISA kits. (E, F) The pyroptosis rate was measured by flow cytometry. (G) The protein levels of NLRP3, ASC, Cleaved-caspase-1 and GSDMD were detected by western blot. **P < 0.01 VS control group. ##P < 0.01 VS LPS + si-NC group.
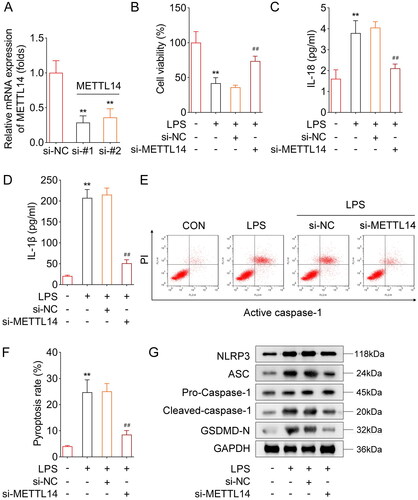
METTL14 regulated the m6A levels of NLRP3
Subsequently, we aimed to explore the specific mechanism of METTL14 regulating pyroptosis. After METTL14 knockdown, the m6A levels of NLRP3 were significantly decreased, while the m6A levels of ASC, caspase-1 and GSDMD showed no difference (). Then, the RIP assay showed that METTL14 can bind to NLRP3 (). In addition, the PCR assay showed that METTL14 knockdown significantly decreased the mRNA levels of NLRP3. Furthermore, through the SRAMP online database, we found that NLRP3 could undergo m6A methylation at multiple sites (), and the six m6A methylation site are shown in . Among them four m6A methylation site (very high confidence) were selected to perform the dual-luciferase report assay. The dual-luciferase reporter assay showed that METTL14 knockdown significantly decreased the luciferase activities of site 2 (), site 3 () and site 4 () of WT-NLRP3, and site 1 () of WT-NLRP3 showed no difference. After site 2, site 3, and site 4 mutation, the significant changes in fluorescence activity were reversed. Besides, Actinomycin D treatment was used to analyze the mRNA stability of NLRP3, we found that METTL14 knockdown dramatically decreased the mRNA stability of NLRP3 ().
Figure 3. METTL14 regulated the m6A levels of NLRP3.
(A) After METTL14 knockdown, the m6A levels of NLRP3, ASC, caspase-1 and GSDMD were detected by Me-RIP assay. (B) The interaction between METTL14 and NLRP3 was confirmed by RIP assay. (C) After METTL14 knockdown, the mRNA levels of NLRP3 were detected by RT-qPCR assay. (D-E) The m6A methylation sites of NLRP3 were obtained from SRAMP online database. The dual-luciferase reporter assay was performed to analyzed the relationship between METTL14 and NLRP3 after site 1 (F), site 2 (G), site 3 (H), and site 4 (I) mutations. (I) The mRNA stability of NLRP3 was determined by RT-qPCR after Actinomycin D treatment. **P < 0.01.
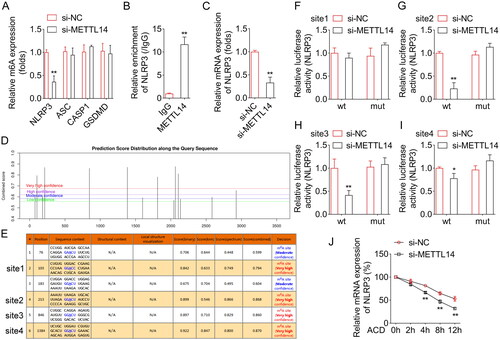
NLRP3 overexpression reversed the role of METTL14 knockdown in the LPS stimulated A549 cells
After NLRP3 overexpressed vector transfection, the NLRP3 levels were significantly increased (). Then we found that NLRP3 overexpression significantly decreased the cell viability (), and increased the IL-18 () and IL-1β content (), and pyroptosis rate ().
Figure 4. NLRP3 overexpression reversed the role of METTL14 knockdown in the LPS-stimulated A549 cells.
(A) The verification of transfection efficiency of oe-NLRP3. The LPS-stimulated A549 cells were transfected with si-METTL14 and oe-NLRP3. After that, (B) the cell viability was detected by CCK-8 assay. The IL-18 (C) and IL-1β (D) contents were detected by ELISA kits. (E) The pyroptosis rate was measured by flow cytometry. **P < 0.01 VS LPS + si-NC + oe-NC group. ##P < 0.01 VS LPS + si-METTL14 + oe-NC group.
METTL14 knockdown alleviated the sepsis-induced ALI in vivo
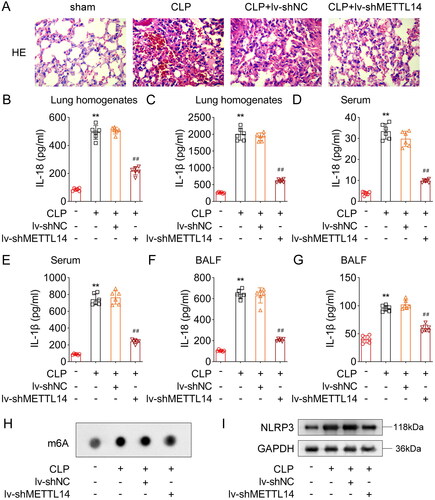
Finally, we established the sepsis-induced ALI model mice to explore the role of METTL14 in vivo. HE staining showed that in the sham group, the alveolar walls of the mice were smooth, the alveolar cavities were free of exudation, and the structure was clear and complete. In the CLP group, a large number of red blood cells were observed in the alveolar cavities, and inflammatory cells were exuded from the alveolar cavity. The alveolar septa and walls were significantly thickened, and partial alveolar cavity collapsed. After METTL14 knockdown, these pathological symptoms were effectively improved (). Then, we found that the IL-18 and IL-1β content were significantly increased in the lung homogenates (), serum () and BALF () of the CLP-treated mice, while METTL14 knockdown significantly decreased the IL-18 and IL-1β content in the lung homogenates (), serum () and BALF () of the CLP treated mice. Furthermore, the m6A dot blot assay showed that global m6A levels () and NLRP3 protein levels (Figure 51) were significantly increased in the lung tissues of the CLP-treated mice, while METTL14 knockdown significantly decreased them.
Figure 5. METTL14 knockdown alleviated the sepsis-induced ALI in vivo.
The CLP treated mice were used to establish the sepsis-induced ALI model. After METTL14 knockdown, (A) the HE staining was performed to observe the lung tissue structure. The IL-18 and IL-1β content the lung homogenates (B, C), serum (D, E) and BALF (F, G) were measured using ELISA kits. (H) The m6A levels were tested by m6A dot blot assay. (I) The protein levels of NLRP3 were detected by western blot assay. **P < 0.01 VS Sham group. ##P < 0.01 VS CLP + lv-shNC group.
Discussion
In the current study, we found that METTL14-mediated m6A modification levels were up-regulated in the LPS-stimulated A549 cells. Knockdown of METTL14 inhibited the pyroptosis in the LPS-stimulated A549 cells through down-regulating the m6A levels of NLRP3, which further promoted the mRNA degradation of NLRP3.
Pyroptosis is a type of programmed cell death caused by the release of inflammatory mediators mediated by caspase-1, which plays an important role in infection, inflammation, and immune diseases.Citation21,Citation22 Previous study found that in a sepsis mouse model, neutrophil extracellular trap networks cause lysosomal breakdown, inducing the formation of NLRP3 inflammatory bodies, and the activation of caspase-1.Citation23 Activated caspase-1 not only stimulates the transformation of IL-18 and IL-1β into mature inflammatory factors, but also cuts GSDMD to form GSDMD-N fragments, inducing cell perforation and rupture, releasing a large number of inflammatory mediators.Citation24 Caspase-1 and GSDMD, as important mediators of cell death pathway, are important indicators for evaluating pyroptosis. Here, we found that in the LPS-stimulated A549 cells, the IL-18 and IL-1β contents, and the protein levels of NLRP3, ASC, Cleaved-caspase-1 and GSDMD-N were significantly increased. Combining with the results of flow cytometry, all results showed that cell pyroptosis was occurred in the LPS-stimulated A549 cells. Similarly, Liu et al.Citation25 and Cao et al.Citation26 also demonstrated that targeting NLRP3-mediated pyroptosis was a new strategy for clinical mitigation of sepsis-induced ALI.
Subsequently, we found that METTL14 knockdown effectively inhibited the pyroptosis in the LPS-stimulated A549 cells. METTL14, one of the main members of the methylation complex, has been demonstrated to participate in the cell growth and apoptosis in lung cancers through regulating the m6A levels of target genes.Citation16,Citation27 For instance, Gong et al.Citation28 found that METTL14 knockdown decreased the IC50 non-small cell lung cancer (NSCLC) cells to cisplatin, inhibited NSCLC cell proliferation, and induced the cell apoptosis through inhibiting the nature of pri-miR-19a via down-regulating the m6A level. Additionally, Yang et al.Citation29 suggested that METTL14 silencing prevented cell growth and metastasis of lung cancer cell lines through decreasing the levels of the transcription factor Twist, indicating that METTL14 may be a potential therapeutic target for NSCLC. On the other hand, METTL14-mediated m6A methylation has been demonstrated to regulate the in pyroptosis in diabetic cardiomyopathy progression. Yuan et al.Citation30 found that in the intervertebral disk degeneration progression, METTL14 knockdown decreased the m6A levels ofNLRP3 and promoted the mRNA degradation of NLRP3, which further inhibited the pyroptosis development. Similarly, in this study, we found that METTL14 knockdown decreased the m6A levels of NLRP3, which further promoted the mRNA degradation of NLRP3. In addition, the relationship between METTL14 and NLRP3 was also confirmed by RIP and dual-luciferase report assays. The rescue experiments showed that NLRP3 overexpression reversed the effects of METTL14 knockdown on the pyroptosis of the LPS-stimulated A549 cells.
In conclusion, this study demonstrated that METTL14 mediated m6A modification was the key factor in the sepsis-induced ALI. METTL14 knockdown inhibited the pryoptosis of the alveolar epithelial cells through decreasing the m6A levels and promoting the mRNA degradation of NLRP3. This study provided a solid foundation for accurate targeted treatment of sepsis-induced ALI in the future. However, there were still some limitations in this study, we used tail vein injection to knock out METTL14 in mice. Specific knockout was not performed through intratracheal treatment. In our future research, we will conduct intratracheal shMETTL14 treatment to comprehensively explore the specific mechanism of METTL14 in ALI.
Competing interests
The authors confirm that no conflicts of interest exist in this work.
Ethics approval
The study was approved by Affidavit of the Approval of Animal Ethical and Welfare Committee (AEWC) (No. MDKN-2022-066). All experiments were performed in accordance with relevant guidelines and regulations.
Acknowledgements
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of interest
No potential conflict of interest was reported by the author(s)
Additional information
Funding
References
- Font MD, Thyagarajan B, Khanna AK. Sepsis and septic shock – basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am. 2020;104(4):573–585. doi:10.1016/j.mcna.2020.02.011.
- Park I, Kim M, Choe K, et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur Respir J. 2019;53(3):1800786. doi:10.1183/13993003.00786-2018.
- Wang S, Sun Y, Bai Y, et al. Contribution of connexin hemichannels to the pathogenesis of acute lung injury. Mediat Inflamm. 2020;2020:1–10. doi:10.1155/2020/8094347.
- Qian Y, Wang Z, Lin H, et al. Trim47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via k63-linked ubiquitination of traf2. Signal Transduct Target Ther. 2022;7:148.
- Wepler M, Preuss JM, Merz T, et al. Impaired glucocorticoid receptor dimerization aggravates lps-induced circulatory and pulmonary dysfunction. Front Immunol. 2019;10:3152. doi:10.3389/fimmu.2019.03152.
- Yildiz IE, Topcu A, Bahceci I, et al. The protective role of fosfomycin in lung injury due to oxidative stress and inflammation caused by sepsis. Life Sci. 2021;279:119662. doi:10.1016/j.lfs.2021.119662.
- Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of mrsa bacteremia across patient populations-a review of recent developments in mrsa management and treatment. Crit Care. 2017;21(1):211. doi:10.1186/s13054-017-1801-3.
- Zhao H, Chen H, Xiaoyin M, et al. Autophagy activation improves lung injury and inflammation in sepsis. Inflammation. 2019;42(2):426–439. doi:10.1007/s10753-018-00952-5.
- Liu F, Peng W, Chen J, et al. Exosomes derived from alveolar epithelial cells promote alveolar macrophage activation mediated by mir-92a-3p in sepsis-induced acute lung injury. Front Cell Infect Microbiol. 2021;11:646546. doi:10.3389/fcimb.2021.646546.
- Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004.
- Coll RC, Schroder K, Pelegrín P. Nlrp3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653–668. doi:10.1016/j.tips.2022.04.003.
- Teng JF, Mei QB, Zhou XG, et al. Polyphyllin vi induces caspase-1-mediated pyroptosis via the induction of ros/nf-kappab/nlrp3/gsdmd signal axis in non-small cell lung cancer. Cancers (Basel). 2020;12(1):193. doi:10.3390/cancers12010193.
- Wang YC, Liu QX, Zheng Q, et al. Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting nlrp3 inflammasome-dependent pyroptosis in mice model. Inflammation. 2019;42(4):1301–1310. doi:10.1007/s10753-019-00990-7.
- Zhang H, Shi X, Huang T, et al. Dynamic landscape and evolution of m6a methylation in human. Nucleic Acids Res. 2020;48(11):6251–6264. doi:10.1093/nar/gkaa347.
- Sendinc E, Shi Y. Rna m6a methylation across the transcriptome. Mol Cell. 2023;83(3):428–441. doi:10.1016/j.molcel.2023.01.006.
- Li Q, Li X, Tang H, et al. Nsun2-mediated m5c methylation and mettl3/mettl14-mediated m6a methylation cooperatively enhance p21 translation. J Cell Biochem. 2017;118(9):2587–2598. doi:10.1002/jcb.25957.
- Li C, Jiang Z, Hao J, et al. Role of n6-methyl-adenosine modification in mammalian embryonic development. Genet Mol Biol. 2021;44(2):e20200253. doi:10.1590/1678-4685-GMB-2020-0253.
- Sun T, Wu R, Ming L. The role of m6a rna methylation in cancer. Biomed Pharmacother. 2019;112:108613. doi:10.1016/j.biopha.2019.108613.
- Deng MS, Chen KJ, Zhang DD, Li GH, Weng CM, Wang JM. M6a rna methylation regulators contribute to predict and as a therapy target of pulmonary fibrosis. Evid Based Complement Alternat Med. 2022;2022:2425065–2425067. doi:10.1155/2022/2425065.
- Li H, Zhang Y, Guo Y, et al. Alkbh1 promotes lung cancer by regulating m6a rna demethylation. Biochem Pharmacol. 2021;189:114284. doi:10.1016/j.bcp.2020.114284.
- Wen S, Deng F, Li L, Xu L, Li X, Fan Q. Vx‐765 ameliorates renal injury and fibrosis in diabetes by regulating caspase‐1‐mediated pyroptosis and inflammation. J Diabetes Investig. 2022;13(1):22–33. doi:10.1111/jdi.13660.
- Li S, Sun Y, Song M, et al. Nlrp3/caspase-1/gsdmd-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021;6(23):e146852. doi:10.1172/jci.insight.146852.
- Chen L, Zhao Y, Lai D, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9(6):597. doi:10.1038/s41419-018-0538-5.
- Jiang S, Zhang H, Li X, et al. Vitamin d/vdr attenuate cisplatin-induced aki by down-regulating nlrp3/caspase-1/gsdmd pyroptosis pathway. J Steroid Biochem Mol Biol. 2021;206:105789. doi:10.1016/j.jsbmb.2020.105789.
- Liu B, Wang Z, He R, et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting nlrp3-mediated pyroptosis through an ampk-dependent pathway. Clin Sci (Lond). 2022;136(4):273–289. doi:10.1042/CS20211156.
- Cao Z, Qin H, Huang Y, et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered. 2022;13(3):4810–4820. doi:10.1080/21655979.2022.2033381.
- He M, Lei H, He X, et al. Mettl14 regulates osteogenesis of bone marrow mesenchymal stem cells via inducing autophagy through m6a/igf2bps/beclin-1 signal axis. Stem Cells Transl Med. 2022;11(9):987–1001. doi:10.1093/stcltm/szac049.
- Gong S, Wang S, Shao M. Mechanism of mettl14-mediated m6a modification in non-small cell lung cancer cell resistance to cisplatin. J Mol Med (Berl). 2022;100(12):1771–1785. doi:10.1007/s00109-022-02268-2.
- Yang F, Yuan WQ, Li J, Luo YQ. Knockdown of mettl14 suppresses the malignant progression of non-small cell lung cancer by reducing twist expression. Oncol Lett. 2021;22(6):847. doi:10.3892/ol.2021.13108.
- Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous mir-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through mettl14/nlrp3. Mol Med. 2021;27(1):91. doi:10.1186/s10020-021-00355-7.

