?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
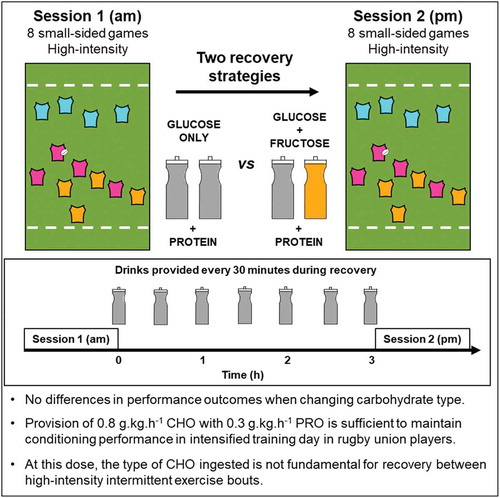
This study assessed the effects of glucose-fructose co-ingestion during recovery from high-intensity rugby training on subsequent performance. Nine professional, senior academy Rugby Union players performed two trials in a double-blind, randomized, crossover design. Identical rugby training sessions were separated by a 3-hour recovery period, during which participants ingested protein (0.3 g×kg BM×h-1) and carbohydrate-containing (0.8 g×kg BM×h-1) recovery drinks, comprised of glucose polymers (GLUCOSE ONLY) or a glucose-fructose mixture (GLUCOSE+FRUCTOSE). Performance outcomes were determined from global positioning systems combined with accelerometry and heart rate monitoring. Mean speed during sessions 1 (am) and 2 (pm) of GLUCOSE ONLY was (mean±SD) 118±6 and 117±4 m×min-1, respectively. During GLUCOSE+FRUCTOSE, mean speed during session 1 and 2 was 117±4 and 116±5 m×min-1, respectively (time x trial interaction, p = 0.61). Blood lactate concentrations were higher throughout recovery in GLUCOSE+FRUCTOSE (mean ±SD: 1-h 3.2 ±2.0 mmol×L-1; 3-h 2.1 ±1.2 mmol×L-1) compared to GLUCOSE ONLY (1-h 2.0 ±1.0 mmol×L-1; 3-h 1.4 ±1.0 mmol×L-1; trial effect p = 0.05). Gastrointestinal discomfort low in both conditions. These data suggest glucose-fructose mixtures consumed as protein-carbohydrate recovery drinks following rugby training do not enhance subsequent performance compared to glucose-based recovery drinks.
ABSTRACT
Introduction
Endogenous carbohydrate availability is an important determinant of performance during and in recovery from moderate-to-high-intensity exercise (Gonzalez et al., Citation2016). Therefore, when optimal exercise performance is required on multiple occasions within a 24-h period (such as during intensified training and multi-stage sporting events), the restoration of endogenous glycogen stores is a major factor determining recovery (Gonzalez et al., Citation2017). To maximise recovery of endogenous glycogen stores it is recommended that carbohydrates are consumed at a rate of at 1–1.2 g ⸱ kg BM⋅h−1 for the first 4 h of recovery (Thomas et al., Citation2016). Alternatively, with lower rates of carbohydrate intake (~0.8 g ⸱ kg BM⋅h−1), protein co-ingestion (~0.3 g ⸱ kg BM ⸱ h−1) can accelerate muscle glycogen storage, thereby restoring muscle glycogen resynthesis rates to those seen at higher carbohydrate ingestion rates (Betts & Williams, Citation2010).
The type of carbohydrate ingested can have a profound influence on tissue-specific storage of glycogen, when ingested alone, glucose is preferentially stored as muscle glycogen, whereas fructose is preferentially stored as liver glycogen (Fuchs et al., Citation2019). The co-ingestion of glucose and fructose therefore seems to provide a more balanced rate of total (both liver and muscle) glycogen synthesis post-exercise (Gonzalez et al., Citation2017). Glucose-fructose co-ingestion accelerates liver glycogen recovery post-exercise ~2-fold, compared to isocaloric glucose polymer (maltodextrin and dextrose) ingestion alone, without compromising muscle glycogen repletion rates (Fuchs et al., Citation2016). Recent evidence has shown that this co-ingestion of fructose with glucose during recovery can also enhance subsequent endurance running (Maunder et al., Citation2018) and cycling capacity (Gray et al., Citation2019). Therefore, the co-ingestion of fructose with glucose-based carbohydrates may be useful for maintaining high exercise intensities when multiple bouts of exercise are performed within 24 h. However, to date, this has only been demonstrated during continuous, endurance-type exercise.
Rugby Union is a team sport characterised by intermittent running interspersed with high-intensity bouts of sprinting, isometric muscle actions and physical contact, combined with the execution of motor skills (Austin et al., Citation2011; Read et al., Citation2018), the unique physical demands of rugby and unique physical characteristics of rugby players limit the ability to directly extrapolate evidence from continuous, endurance-type exercise to rugby nutrition practices. Whilst it is acknowledged that carbohydrates provide an important fuel source during rugby (league) match-play (Bradley et al., Citation2016) and simulations (Bradley et al., Citation2017), little is known about the role of carbohydrates in the immediate recovery from rugby. Neither is it known whether glucose-fructose co-ingestion in recovery from intermittent team-sport training provides any benefit for subsequent performance. Accordingly, this study aimed to establish whether glucose-fructose co-ingestion during recovery from an intensified rugby training session could enhance performance during subsequent training sessions later the same day. It was hypothesised that glucose-fructose co-ingestion would improve recovery of rugby performance when compared to isocaloric ingestion of glucose-based carbohydrates.
Methods
Participants and sample size
Using data from Maunder et al. (Maunder et al., Citation2018), glucose-fructose co-ingestion during recovery from exhaustive exercise increased subsequent exercise performance compared to glucose ingestion alone with a large effect size of d = 1.84. In a within-subject design, six participants would provide >90% power to detect such an effect with an alpha level of 0.05 using a 2-tailed t-test. Maunder et al. (Maunder et al., Citation2018) used exercise to exhaustion with a 4-h recovery period. As the present study employed a less intense training protocol and 3-h recovery period, we anticipated that smaller effects may still be worthwhile; therefore, a conservative estimate of an effect size of d = 1.00 would require 12 participants to provide 88% power with an alpha level 0.05 in this design. We, therefore, recruited 12 male Rugby Union players from Bath Rugby Union Football Club. However, two dropped out of the second training session of one trial due to injury and one dropped out due to circumstances unrelated to the study procedures, resulting in a final sample size of n = 9 for primary outcome measures. For blood lactate concentrations and gut discomfort, we obtained full datasets for the recovery period for n = 11.
Study design
The study was a triple-blind (researchers, participants, and analysts blinded to treatments), randomised crossover design, with experimental conditions separated by 72 h. The protocol was approved by the Leeds Beckett University Research Ethics Committee, was conducted in accordance with the latest version of the Declaration of Helsinki, and was carried out in line with the PRESENT 2020 checklist for sport nutrition studies (Betts et al., Citation2020). All participants provided informed, written consent prior to participation. Four days prior to the first main trial, a familiarisation session was performed which was identical to the main trials, including the provision of a randomly allocated test drink.
Main trials
On each trial, participants arrived at the training session at 09:00. Habitual food and fluid intake were permitted on the morning of the trial prior to the session. Participants were asked to consume their habitual breakfast choice as prepared by club chefs and to record this on the first trial for replication on subsequent trials. This was confirmed verbally with players and checked visually by one of the coaches on site.
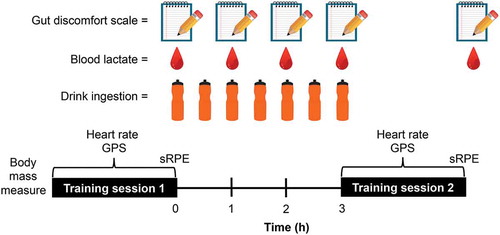
Participants then performed two training sessions separated by a 3-h recovery period. The training sessions comprised small-sided games and were developed by coaching staff and standardised for pitch area per player, rules and objectives, game length, and between-game rest. Each training session consisted of eight standardised small-sided games. During training, only ad libitum water intake was permitted. During recovery, participants ingested drinks containing 0.8 g ⸱ kg BM ⸱ h−1, plus 0.3 g ⸱ kg BM ⸱ h−1 of whey protein (chocolate flavour whey protein isolates with stevia sweetener; MyProtein, Cheshire, UK). Nutritional content per 100 g of protein powder was 392 kcal, 7.6 g fat, 5.0 g saturated fat, 6.3 g carbohydrate, 4.6 g sugars, 3.2 g fibre, 73 g protein, 0.25 g salt. Mean ± SD, body mass of participants was 106 ± 11 kg. Drinks were provided at 30 min intervals by the research team. Carbohydrate and protein were made to volume with water to produce a 20% solution, with the addition of 20 mmol ⸱ L−1 sodium chloride to assist with the maintenance of serum sodium concentrations. On the GLUCOSE ONLY trial, the carbohydrate source was maltodextrin (glucose polymer; MyProtein, Cheshire, UK) plus dextrose (MyProtein, Cheshire, UK) in a 1.5:1 ratio. On the GLUCOSE+FRUCTOSE trial, the carbohydrate source was maltodextrin plus fructose (Bulk Powders, Essex, UK) in a 1.5:1 ratio (). During the recovery phase, participants were only permitted to consume the drinks provided and no other supplements.
Performance measures
The primary objective of the training sessions was to improve player physical conditioning and the goal was to maintain high-intensity performance during sessions. Performance outcomes, therefore, included mean speed, mean heart rate, and the number of accelerations per minute. Locomotive characteristics were measured using GPS sampling at 10 Hz while external forces were assessed using tri-axial accelerometers, both housed within the micro-technology devices (Optimeye S5, Catapult Innovations, Melbourne, Australia). Locomotive characteristics for each player were made relative to their most-recently measured maximum velocity (Vmax) and 30–15 Intermittent Fitness Test score assessed by the strength and conditioning staff at the club. During all training sessions, players wore a specialised vest to house and position the micro-technological unit in the upper-back region between the shoulder blades, and a heart rate belt around the chest (Polar T31; Polar Warwick England, UK). The units were turned on 10 min prior to the beginning of the activity being quantified to allow for satellite connection. Accelerometer and GPS data were downloaded to a laptop and analysed using Catapult Openfield software (Catapult Innovations, Melbourne, Australia).
Subjective ratings
Subjective internal load was measured using the session rating of perceived exertion method (sRPE) (Foster et al., Citation2001) and differential RPE method (McLaren et al., Citation2016) within 15–30 min of each session on a modified Borg scale. These values are multiplied by the duration of the session by the club to calculate an arbitrary training load value in arbitrary units (AU). Ratings of gastrointestinal symptoms were assessed immediately following the first training session, every hour during recovery and immediately following the second training session by employing a 15-item, 7-point scale adapted from Revicki et al. (Revicki et al., Research Citation1997).
Blood lactate concentrations
Blood lactate concentrations were determined immediately following the first training session, every hour during recovery and immediately following the second training session by finger-prick samples using a portable lactate analyser (Lactate Plus, Nova Biomedical, US), which is reliable and valid at a range of concentrations (Hart et al., Citation2013).
Statistical analysis
Data were analysed using GraphPad Prism v8 (GraphPad Software Inc, San Diego, CA). Data were checked for normality using the Shapiro–Wilk test combined with visual analysis of residuals. Subsequently, data were checked for order effects and an order effect between trials was detected for distance covered (order effect: p = 0.012), due to small differences in session duration (maximum 39 seconds of work different across sessions [mm:ss 24:39 to 25:18], order effect: p < 0.01). Therefore, hereafter, statistics reported are normalised to the duration of the sessions (e.g., mean speed, mean heart rate, and accelerations per minute). No order effects were detected for mean speed (order effect: p = 0.21), mean heart rate (order effect: p = 0.45) or accelerations per minute (order effect: p = 0.91). Time-series data were analysed using two-way repeated measures ANOVA. Where interaction effects were observed, post hoc comparisons were adjusted for multiple comparisons using the Bonferonni correction. Data are means ± 95% confidence intervals (CI) unless otherwise stated. Statistical significance was accepted if p 0.05.
Results
Performance
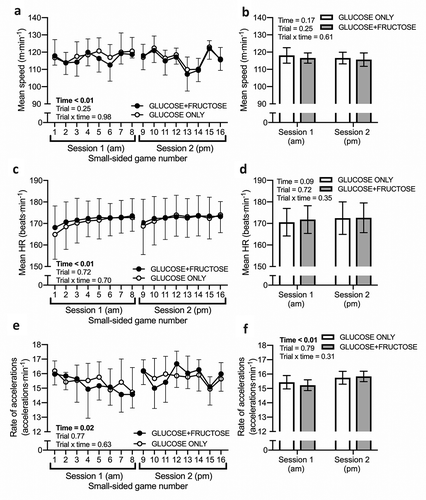
When assessed at the level of each small-sided game, mean speed ()), mean heart rate ()), and the rate of accelerations ()) all displayed main effects of time (all p < 0.05, p-values displayed in ), but no main effects of trial or trial x time interaction effects were observed (all p > 0.05, p-values displayed in ). When assessed at the whole session level (small-sided games pooled into one session), the main effect of time was no longer detected for mean speed (); p = 0.17) or mean heart rate (); p = 0.05), but was preserved for the rate of accelerations (); p < 0.01), whereby the rate of accelerations was marginally higher, by less than 1 acceleration per minute, in session 2 (pm) compared to session 1 (am).
Figure 2. Mean speed (a, b), mean heart rate (HR) (c, d) and rate of accelerations (e, f) during two sessions of small-sided games separated by a 3-h recovery period, during which carbohydrate-protein drinks containing either glucose-based carbohydrates (GLUCOSE ONLY) or glucose-fructose mixtures (GLUCOSE+FRUCTOSE) were ingested. HRmax, maximum heart rate. Data are means ± 95%CI. n = 9 rugby players
Subjective ratings of exertion following training sessions
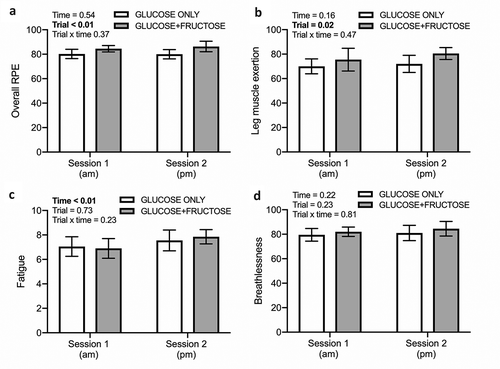
Overall RPE ()) and leg muscle exertion ()) displayed a main effect of the trial (both p < 0.05), whereby overall RPE and leg muscle exertion were both higher during the GLUCOSE+FRUCTOSE trial compared to the GLUCOSE ONLY trial. Ratings of fatigue displayed a main effect of time (); p < 0.01). No time × trial interaction effects were observed for any of the above ratings, nor for breathlessness (; all p > 0.05).
Figure 3. Overall RPE (a), leg muscle exertion (b), fatigue (c) and breathlessness (d) ratings after two sessions of small-sided games separated by a 3-h recovery period, during which carbohydrate-protein drinks containing either glucose-based carbohydrates (GLUCOSE ONLY) or glucose-fructose mixtures (GLUCOSE+FRUCTOSE) were ingested. Data are means ± 95%CI. n = 9 rugby
Blood lactate concentrations and gut discomfort during recovery
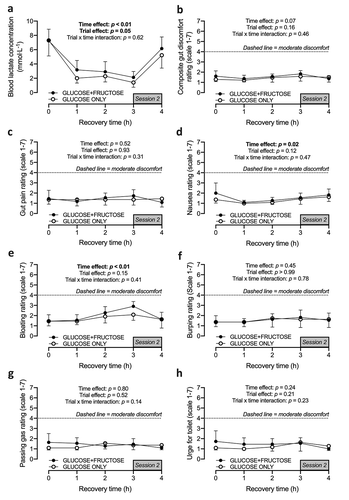
Blood lactate concentrations following the first training session did not differ between trials (); p = 0.94). Thereafter, a main effect of the trial was detected (p = 0.05), but no time × trial interaction effect. Similarly, no time × trial interaction effects were observed for any of the gut discomfort ratings (); all p > 0.05, all p-values displayed on the figure).
Figure 4. Blood lactate concentration (a), a composite gut rating (b), and the constituent gut rating scales (c-h) during recovery from a session of small-sided games, with ingestion of carbohydrate-protein drinks containing either glucose-based carbohydrates (GLUCOSE ONLY) or glucose-fructose mixtures (GLUCOSE+FRUCTOSE). Data are means ± 95%CI. n = 9 rugby players
Discussion
This is the first study to assess the effects of glucose-fructose co-ingestion on recovery and subsequent performance from intermittent exercise. These data suggest that glucose-fructose co-ingestion as part of a carbohydrate-protein recovery solution does not further enhance recovery of performance during an intensified day of rugby training, compared to when the carbohydrates provided are glucose-based. Therefore, in the context of the present study, at a dose of 0.8 g ⸱ kg BM ⸱ h−1, the type of carbohydrate ingested does not impact the short-term recovery from high-intensity intermittent exercise lasting roughly 60 min.
Restoration of muscle and liver glycogen stores is a key factor dictating recovery between intense bouts of endurance exercise (Gonzalez et al., Citation2017). The addition of fructose to glucose-based carbohydrates has been repeatedly shown to potently accelerate recovery of liver glycogen stores in the post-exercise period, when compared to isocaloric ingestion of glucose (polymers) alone (Decombaz et al., Citation2011; Fuchs et al., Citation2016; Gonzalez et al., Citation2017). Furthermore, this enhancement of liver glycogen restoration with glucose-fructose co-ingestion is not at the detriment of muscle glycogen recovery (Fuchs et al., Citation2016). Therefore, glucose-fructose co-ingestion can provide a potential metabolic advantage for subsequent exercise performance when carbohydrate availability is a limiting factor. Whilst this potential advantage for glycogen storage has been shown to translate into improvements in recovery and subsequent exercise capacity, this has only been demonstrated with continuous, endurance-type exercise (Gray et al., Citation2019; Maunder et al., Citation2018). The present study suggests there is no additional benefit of combining fructose with glucose-based carbohydrates in the 3-h recovery between two high-intensity rugby training sessions when the dose of carbohydrate is adequate to maintain performance in the second session. One limitation of the present study is that we did not obtain muscle biopsies to measure muscle glycogen concentrations. It should also be noted that these results are specific to the context of the dose of carbohydrate provided, and future work should determine whether higher carbohydrate doses may augment exercise performance. It is possible that a higher dose of carbohydrate may have resulted in differences between trials, especially as glucose-fructose mixtures can attenuate the gut discomfort associated with very high carbohydrate intakes (Fuchs et al., Citation2016; Trommelen et al., Citation2016).
The reasons for the lack of additional benefit from glucose-fructose co-ingestion for recovery of rugby performance could be due to numerous factors including, but not limited to, the mode, intensity, and duration of exercise, the relatively low carbohydrate ingestion rate employed, and/or the co-ingestion of protein with carbohydrate. Nutrition guidelines currently advise that, to optimise the recovery of muscle glycogen stores post-exercise, athletes should consume between 1.0 and 1.2 g carbohydrate ⸱ kg BM ⸱ h−1 for the first 4-h post-exercise. Since the present study utilised a lower carbohydrate intake than prior work, it could be questioned whether the total carbohydrate intake was insufficient to observe differences in performance. In the present study, players were provided with 0.8 g carbohydrate ⸱ kg BM ⸱ h−1, plus 0.3 g whey protein ⸱ kg BM ⸱ h−1. This dose was chosen to represent a practical scenario in which rugby players would normally consume protein following training coupled with the evidence that protein co-ingestion can accelerate muscle glycogen storage (Betts & Williams, Citation2010). Furthermore, the large body mass of rugby players resulted in a large absolute dose of carbohydrate (mean ±SD; 84.8 ± 8.8 g per hour, 254 ± 26.4 g throughout recovery). It is possible that the lower carbohydrate ingestion rate could have altered the liver glycogen response to glucose-fructose co-ingestion. However, this is unlikely because the effect size for liver glycogen appears to be consistent across a wide range of carbohydrate intake rates (Gonzalez et al., Citation2016, Citation2017). Furthermore, recent evidence has shown that glucose-fructose co-ingestion in recovery enhances subsequent cycling capacity compared to glucose-based carbohydrates alone, despite relatively low rates (<0.5 g ⸱ kg BM ⸱ h−1 when accounting for total recovery time) of carbohydrate intake (Gray et al., Citation2019). The effects of protein and carbohydrate co-ingestion on post-exercise liver glycogen repletion are currently unclear, and the higher glucagon response to protein co-ingestion (Van Hall et al., Citation1985) could counteract the effects of insulin on liver glycogen storage by maintaining liver glycogen breakdown/turnover (Roden et al., Citation1996). It is plausible that the addition of whey protein may have masked any effects of carbohydrate type as a confounding variable, since we did not include a condition without whey protein, which is a limitation of the present study. Further work is therefore needed to better understand the role of protein ingestion on liver glycogen recovery post-exercise. Future studies should aim to measure liver glycogen synthesis using non-invasive means like nuclear magnetic resonance imaging (Rothman et al., Citation1991). Furthermore, it is possible that the present study is underpowered to detect small effects because, due to participant dropout, the final analyses comprised n = 9 rather than the estimated n = 12 to observe an effect size of d = 1.00.
It was not possible to assess liver glycogen concentration in the present study design. The conclusions of previous studies measuring liver glycogen suggest that isocaloric glucose-fructose co-ingestion compared to glucose (polymers) ingestion alone leads to greater liver glycogen repletion at various modes, intensities, and durations (Decombaz et al., Citation2011; Fuchs et al., Citation2016; Gonzalez et al., Citation2017). Support for the assumption that the present nutritional intervention is likely to have produced the expected effect on liver glycogen is provided by the greater blood lactate concentrations in the glucose-fructose trial, suggesting the whole-body metabolic effects that have previously been observed were preserved in the present study (Fuchs et al., Citation2016). An increase in blood lactate concentrations indicates the release of lactate from the liver in response to the unregulated nature of hepatic fructose uptake (Bjorkman et al., Citation1989), suggesting the provision of fructose was sufficient to influence hepatic metabolism. The idea that liver glycogen is not limiting for the exercise employed is a possibility, as the duration of each individual session was ~60 min including work and rest, and therefore liver glycogen availability within each session may not have been limiting (Gonzalez et al., Citation2016). Nevertheless, the total session time across the day was 120 mins, which is ~50% longer than a typical rugby match whereby substantial (~40%) muscle glycogen depletion can occur (Bradley et al., Citation2016). Therefore, glycogen could be limiting across a full day of this form of training if insufficient nutrition is provided. Liver glycogen metabolism has never been assessed in an intermittent team-sport activity, and it is therefore difficult to speculate on the role of liver glycogen as a limiting factor for rugby performance.
Gastrointestinal distress is a common occurrence when attempting to adhere to novel or high-dose nutrition strategies, however when ingested in large quantities, glucose-fructose mixtures tend to produce less gastrointestinal distress than equivalent quantities of glucose-based carbohydrates (Fuchs et al., Citation2016; Gonzalez et al., Citation2015). This is likely due to fructose being absorbed by different intestinal transport proteins than glucose, thereby increasing the total capacity for intestinal carbohydrate absorption (Daniel & Zietek, Citation2015). Since gastrointestinal distress could directly impair the capacity to perform exercise, lower gastrointestinal distress with glucose-fructose mixtures could have contributed to some of the improvements in recovery of exercise capacity previously reported (Gray et al., Citation2019; Maunder et al., Citation2018). However, in the present study, we observed no difference in ratings of gastrointestinal discomfort between trials, and all gastrointestinal discomfort ratings were consistently below a level considered “moderate”. This is likely due to the relatively low rate of carbohydrate intake compared to previous studies. The addition of protein likely improved the tolerability of the carbohydrate dose (Betts & Williams, Citation2010).
From a coaching perspective, it may be desirable to implement multiple high-intensity training sessions on the same day if a high-intensity level can be maintained in the subsequent training session. Interestingly, when the small-sided games were pooled and observed as whole sessions (am vs pm), the rate of accelerations (per minute) was marginally greater in the second session (pm) compared to the first session (am) and other performance outcomes were maintained in the second session (pm) compared to the first session (am). Whilst exploratory, these findings suggest that performance in the afternoon sessions was maintained compared with morning sessions. It is possible that the nutritional provision contributed to help support the physical demands of the afternoon sessions. Circadian variation cannot be ruled out as a factor explaining the maintenance of performance, as it is well established that muscle strength and oxidative capacity tend to peak in the evening (Brisswalter et al., Citation2007; Drust et al., Citation2005; Van Moorsel et al., Citation2016); however, duration between the sessions in the present study was relatively short (3 h) and sessions occurred between roughly 09:00, 14:00 hours. The performance measures in the present study were selected based on the goal of the training sessions, which was player physical conditioning, so it should be noted that it is unclear what effects intensified training days would have on motor skills and other aspects of rugby performance. Further work should explore the role of nutrition in supporting the first training sessions of the day for intermittent sports like rugby union and should investigate whether there is a benefit to providing nutrition support in the form such as that delivered in the present study (e.g., supplemental drinks), over and above the typical nutritional practices of rugby players. One limitation of the present study was the absence of a “control” trial where participants are either provided a mixed-macronutrient meal or allowed habitual nutrient intake between sessions; therefore, future studies should include a habitual arm if possible. Whilst some work has been done to characterise the nutritional intake of professional rugby players (Alaunyte et al., Citation2015; Lundy et al., Citation2006), it is likely that the nutrition practices vary between players and clubs. Our results provide a starting point by demonstrating one nutritional strategy that can be used to achieve effective performance for player conditioning in a double-session day.
In summary, the present data suggest that the addition of fructose to glucose and protein ingestion during recovery from rugby-specific training does not further enhance performance during training sessions performed later in the day when compared to glucose and protein ingestion alone. The maintenance of performance during the second session of the day raises the possibility that specific nutritional support over and above normal dietary practices of professional Rugby Union players may be of benefit. For intensified days of rugby training involving two high-intensity sessions of small-sided games, our data suggest that when ingesting 0.8 g ⸱ kg BM ⸱ h−1 of carbohydrates with protein, the type of carbohydrate ingested is not essential for the maintenance of performance in physical conditioning sessions.
Author contributions
JTG, JAB and GABR designed the research, AH, JW, HAS, RME, GABR and JTG conducted the research, JTG and GAVR analysed the data, JTG performed the statistical analysis, AH and JTG primarily wrote the paper, and all authors read and approved the final version of the manuscript.
Disclosure statement
J.T.G. has received research funding and/or has acted as a consultant for Arla Foods Ingredients, Lucozade Ribena Suntory, Kenniscentrum Suiker and Voeding, and PepsiCo. J.A.B. has received research funding and/or has acted as a consultant for GlaxoSmithKline, Lucozade Ribena Suntory, Kellogg’s, Nestlé and PepsiCo.
Additional information
Funding
References
- Alaunyte, I., Perry, J. L., & Aubrey, T. (2015). Nutritional knowledge and eating habits of professional rugby league players: Does knowledge translate into practice? Journal of the International Society of Sports Nutrition, 12:18. https://doi.org/10.1186/s12970-015-0082-y
- Austin, D., Gabbett, T., & Jenkins, D. (2011). Repeated high-intensity exercise in professional rugby union. Journal of Sports Sciences, 29(10), 1105–1112. https://doi.org/10.1080/02640414.2011.582508
- Betts, J. A., Gonzalez, J. T., Burke, L. M., Close, G. L., Garthe, I., James, L. J., Jeukendrup, A. E., Morton, J. P., Nieman, D. C., Peeling, P., Phillips, S. M., Stellingwerff, T., van Loon, L. J. C., Williams, C., Woolf, K., Maughan, R., & Atkinson, G. (2020). PRESENT 2020: Text Expanding on the Checklist for Proper Reporting of Evidence in Sport and Exercise Nutrition Trials. International Journal of Sport Nutrition and Exercise Metabolism, 30(1), 2–13. https://doi.org/10.1123/ijsnem.2019-0320
- Betts, J. A., & Williams, C. (2010). Short-term recovery from prolonged exercise: Exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Medicine, 40(11), 941–959. https://doi.org/10.2165/11536900-000000000-00000
- Bjorkman, O., Gunnarsson, R., Hagstrom, E., Felig, P., & Wahren, J. (1989). Splanchnic and renal exchange of infused fructose in insulin-deficient type 1 diabetic patients and healthy controls. The Journal of Clinical Investigation, 83(1), 52–59. https://doi.org/10.1172/JCI113884
- Bradley, W. J., Hannon, M. P., Benford, V., Morehen, J. C., Twist, C., Shepherd, S., Cocks, M., Impey, S. G., Cooper, R. G., Morton, J. P., & Close, G. L. (2017). Metabolic demands and replenishment of muscle glycogen after a rugby league match simulation protocol. Journal of Science and Medicine in Sport, 20(9), 878–883. https://doi.org/10.1016/j.jsams.2017.02.005
- Bradley, W. J., Morehen, J. C., Haigh, J., Clarke, J., Donovan, T. F., Twist, C., Cotton, C., Shepherd, S., Cocks, M., Sharma, A., Impey, S. G., Cooper, R. G., Maclaren, D. P., Morton, J. P., & Close, G. L. (2016). Muscle glycogen utilisation during Rugby match play: Effects of pre-game carbohydrate. Journal of Science and Medicine in Sport, 19(12), 1033–1038. https://doi.org/10.1016/j.jsams.2016.03.008
- Brisswalter, J., Bieuzen, F., Giacomoni, M., Tricot, V., & Falgairette, G. (2007). Morning-to-evening differences in oxygen uptake kinetics in short-duration cycling exercise. Chronobiology International, 24(3), 495–506. https://doi.org/10.1080/07420520701420691
- Daniel, H., & Zietek, T. (2015). Taste and move: Glucose and peptide transporters in the gastrointestinal tract. Experimental Physiology, 100(12), 1441–1450. https://doi.org/10.1113/EP085029
- Decombaz, J., Jentjens, R., Ith, M., Scheurer, E., Buehler, T., Jeukendrup, A., & Boesch, C. (2011). Fructose and galactose enhance postexercise human liver glycogen synthesis. Medicine and Science in Sports and Exercise, 43(10), 1964–1971. https://doi.org/10.1249/MSS.0b013e318218ca5a
- Drust, B., Waterhouse, J., Atkinson, G., Edwards, B., & Reilly, T. (2005). Circadian rhythms in sports performance–an update. Chronobiology International, 22(1), 21–44. https://doi.org/10.1081/CBI-200041039
- Foster, C., Florhaug, J. A., Franklin, J., Gottschall, L., Hrovatin, L. A., Parker, S., Doleshal, P., & Dodge, C. (2001). A new approach to monitoring exercise training. Journal Of Strength And Conditioning Research / National Strength & Conditioning Association, 15(1), 109–115. https://doi.org/10.1519/00124278-200102000-00019
- Fuchs, C. J., Gonzalez, J. T., Beelen, M., Cermak, N. M., Smith, F. E., Thelwall, P. E., Taylor, R., Trenell, M. I., Stevenson, E. J., & van Loon, L. J. (2016). Sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion compared with glucose ingestion in trained athletes. Journal of Applied Physiology, 120(11), 1328–1334. https://doi.org/10.1152/japplphysiol.01023.2015
- Fuchs, C. J., Gonzalez, J. T., & van Loon, L. J. C. (2019). Fructose co-ingestion to increase carbohydrate availability in athletes. Journal of Physiology, 597(14), 3549–3560. https://doi.org/10.1113/JP277116
- Gonzalez, J. T., Fuchs, C. J., Betts, J. A., & van Loon, L. J. (2016). Liver glycogen metabolism during and after prolonged endurance-type exercise. American Journal of Physiology. Endocrinology and Metabolism, 311(3), E543–553. https://doi.org/10.1152/ajpendo.00232.2016
- Gonzalez, J. T., Fuchs, C. J., Betts, J. A., & van Loon, L. J. (2017). Glucose plus fructose ingestion for post-exercise recovery-greater than the sum of its parts? Nutrients, 9(4). https://doi.org/10.3390/nu9040344
- Gonzalez, J. T., Fuchs, C. J., Smith, F. E., Thelwall, P. E., Taylor, R., Stevenson, E. J., Trenell, M. I., Cermak, N. M., & van Loon, L. J. (2015). Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. American Journal of Physiology-Endocrinology and Metabolism, 309(12), E1032–1039. https://doi.org/10.1152/ajpendo.00376.2015
- Gray, E. A., Green, T. A., Betts, J. A., & Gonzalez, J. T. (2019). Post-exercise glucose-fructose co-ingestion augments cycling capacity during short-term and overnight recovery from exhaustive exercise, compared to isocaloric glucose. International Journal of Sport Nutrition and Exercise Metabolism, 30(1), 54–61. https://doi.org/10.1123/ijsnem.2019-0211
- Hart, S., Drevets, K., Alford, M., Salacinski, A., & Hunt, B. E. (2013). A method-comparison study regarding the validity and reliability of the Lactate Plus analyzer. BMJ Open, 3. https://doi.org/10.1136/bmjopen-2012-001899
- Lundy, B., O’Connor, H., Pelly, F., & Caterson, I. (2006). Anthropometric characteristics and competition dietary intakes of professional rugby league players. International Journal of Sport Nutrition and Exercise Metabolism, 16(2), 199–213. https://doi.org/10.1123/ijsnem.16.2.199
- Maunder, E., Podlogar, T., & Wallis, G. A. (2018). Postexercise fructose-maltodextrin ingestion enhances subsequent endurance capacity. Medicine and Science in Sports and Exercise, 50(5), 1039–1045. https://doi.org/10.1249/MSS.0000000000001516
- McLaren, S. J., Weston, M., Smith, A., Cramb, R., & Portas, M. D. (2016). Variability of physical performance and player match loads in professional rugby union. Journal of Science and Medicine in Sport, 19(6), 493–497. https://doi.org/10.1016/j.jsams.2015.05.010
- Read, D. B., Jones, B., Williams, S., Phibbs, P. J., Darrall-Jones, J. D., Roe, G. A. B., Weakley, J. J. S., Rock, A., & Till, K. (2018). The physical characteristics of specific phases of play during rugby union match play. International Journal of Sports Physiology and Performance, 13(10), 1331–1336. https://doi.org/10.1123/ijspp.2017-0625
- Revicki, D. A., Wood, M., Wiklund, I., & Crawley, J. (1997). Research Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Quality of Life, 7(1), 75–83. https://doi.org/10.1023/A:1008841022998
- Roden, M., Perseghin, G., Petersen, K. F., Hwang, J. H., Cline, G. W., Gerow, K., Rothman, D. L., & Shulman, G. I. (1996). The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. Journal of Clinical Investigation, 97(3), 642–648. https://doi.org/10.1172/JCI118460
- Rothman, D. L., Shulman, R. G., & Shulman, G. I. (1991). N.m.r. studies of muscle glycogen synthesis in normal and non-insulin-dependent diabetic subjects. Biochemical Society Transactions, 93(11), 992–994. https://doi.org/10.1073/pnas.93.11.5329
- Thomas, D. T., Erdman, K. A., & Burke, L. M. (2016). American college of sports medicine joint position statement. Nutrition and athletic performance. Medicine and Science in Sports and Exercise, 48(3), 543–568. https://doi.org/10.1249/MSS.0000000000000852
- Trommelen, J., Beelen, M., Pinckaers, P. J., Senden, J. M., Cermak, N. M., & Van Loon, L. J. (2016). Fructose coingestion does not accelerate postexercise muscle glycogen repletion. Medicine and Science in Sports and Exercise, 48(5), 907–912. https://doi.org/10.1249/MSS.0000000000000829
- van Hall, G., Shirreffs, S. M., & Calbet, J. A. (1985). Muscle glycogen resynthesis during recovery from cycle exercise: No effect of additional protein ingestion. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 2000(88), 1631–1636. https://doi.org/10.1152/jappl.2000.88.5.1631
- van Moorsel, D., Hansen, J., Havekes, B., Scheer, F., Jorgensen, J. A., Hoeks, J., Schrauwen-Hinderling, V. B., Duez, H., Lefebvre, P., Schaper, N. C., Hesselink, M. K. C., Staels, B., & Schrauwen, P. (2016). Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Molecular Metabolism, 5(8), 635–645. https://doi.org/10.1016/j.molmet.2016.06.012