Abstract
Purpose: It has been well established that inadequate blood supply combined with high metabolic rates of oxygen consumption results in areas of low oxygen tension (<1%) within malignant tumours and that elevating tumour temperatures above 39°C results in significant improvement in tumour oxygenation. Macrophages play a dual role in tumour initiation and progression having both pro-tumour and anti-tumour effects. However, the response of macrophages to heat within a hypoxic environment has not yet been clearly defined.
Methods: Raw 264.7 murine macrophages were incubated under normoxia and chronic hypoxia at temperatures ranging from 37–43°C. Under normoxia at 41°C, macrophages start to release significant levels of superoxide. The combination of heat with hypoxia constitutes an additional stimulus leading to increased respiratory burst of macrophages.
Results: The high levels of superoxide were found to be associated with changes in macrophage production of pro-angiogenic cytokines. While hypoxia alone (37°C) increased levels of hypoxia inducible factor-1α (HIF-1α) in macrophages, the combination of hypoxia and mild hyperthermia (39–41°C) induced a strong reduction in HIF-1α expression. The HIF-regulated vascular endothelial growth factor (VEGF) decreased simultaneously, revealing that heat inhibits both HIF-1α stabilization and transcriptional activity.
Conclusion: The data suggest that temperatures which are readily achievable in the clinic (39–41°C) might be optimal for maximizing hyperthermic response. At higher temperatures, these effects are reversed, thereby limiting the therapeutic benefits of more severe hyperthermic exposure.
Introduction
One of the major limitations to the success of radiation and chemotherapy is the intrinsic resistance of hypoxic cells to oxidative damage. The generation of reactive oxygen species (ROS) is the primary mechanism by which radiation therapy and some chemotherapeutic drugs achieve cancer control and cure. However, the efficiency of these conventional treatments is limited by low oxygen tension and poor perfusion. Previous observations have shown that mild hyperthermia (39–43°C) over 60 min can facilitate tumour reoxygenation in rodent and canine tumours Citation[1–5]. More importantly, human clinical trials have shown increased oxygenation following hyperthermia in breast cancers Citation[6], soft tissue sarcomas Citation[7] and rectal and neck cancers Citation[8]. Although it has been clearly shown that hyperthermia improves tumour response to radiation and chemotherapy, the precise mechanisms underlying heat mediated tumour reoxygenation have not been clearly defined. The effects of hypoxic environments have been predominantly studied in tumour cells. However, in addition to malignant cells, neoplasms are largely composed of tumour associated macrophages (TAM) Citation[9].
Within the tumour micro-environment, macrophages play a pivotal role in production of ROS through hypoxia-induced activation of the respiratory burst, which at moderate levels regulate signal transduction pathways Citation[10], Citation[11]. ROS release by macrophages stimulates these cells to have both pro-tumour and anti-tumour effects that include angiogenesis, neoplastic cell mitogenesis, antigen presentation, matrix degradation and cytotoxicity Citation[9], Citation[12–16].
In addition to being producers of oxidative stress within neoplasms, macrophages play a predominate role in tumour angiogenesis Citation[17], Citation[18]. In tumours, angiogenesis is an aberrant wound healing response orchestrated in part by macrophage production of VEGF in which newly formed vessels are generally immature and non-functional resulting in uneven red blood cell distribution, a decrease in oxygen bioavailability and treatment resistance Citation[9], Citation[13], Citation[19], Citation[20]. Transcriptional regulation of VEGF is controlled primarily by HIF-1α stabilization and binding to the hypoxia responsive element in the VEGF promoter Citation[18], Citation[21]. Although HIF-1α is present under normoxic conditions, stabilization is inhibited by von Hippel-Lindau (VHL)-dependent proteasomal degradation Citation[22]. Prolyl hydroxylases use oxygen to hydroxylate key residues on the HIF-1α protein, which permit binding of the HIF-1α protein to VHL. Under hypoxic conditions, the HIF prolyl hydroxylase cannot perform the hydroxylation, which prevents HIF degradation. Under these conditions, HIF-1α binds to its heterodimer, HIF-1β. Once heterodimerization occurs, the complex can bind to the hypoxia response element of the promoter region of VEGF and transcription proceeds Citation[22]. Histological analysis of human tumours Citation[20], Citation[23] has shown elevated levels of macrophages associated with hypoxic and perinecrotic regions which correlate with high micro-vessel density and prominent VEGF expression Citation[24], Citation[25].
Although the effects of radiation on macrophage function have been reported previously, the impact of heat on macrophage activation and angiogenesis regulation in response to hypoxia has not been investigated. Herein, the degree to which macrophages were stimulated by hypoxia to undergo oxidative burst was analysed first. The extent of oxidative burst was detected by measuring superoxide production. Then, it was evaluated whether 1 h of hyperthermia in the clinically achievable range of 39–43°C could condition macrophages to alter production of -mediated pro-angiogenic factors, HIF-1α and VEGF.
Materials and methods
Cell culture
Raw 264.7 murine macrophages were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin in a 0.5% CO2 atmosphere at 37°C.
Normoxia and heat
Macrophages were seeded at 1 × 106 cells/T25 flask 2 h prior to treatment. Cell media was removed and cells were washed twice with PBS. Cells were then incubated for 80 min in 2 mL of phenol red-free Hanks Balanced Salt Solution (HBSS) containing 31.4 µL of equine ferricytocrome c (cyt c, Sigma, St. Louis, MO) with or without 250 µg unopsonized Zymosan A (Sigma, St. Louis, MO). Cells were then placed in a circulating water bath at 37°C for 20 min. Then, the water bath was heated to target temperature (37, 39, 41 or 43°C). Cells remained under hyperthermic conditions for 1 h. Cyt c reduction was measured in 100 µL aliquots of culture media sampled before and after treatment.
Hypoxia and heat
To determine whether macrophages were activated to produce under hypoxic conditions, 1 × 106 cells were plated in 100-mm diameter plates and incubated overnight with 1 µg mL−1 lipopolysaccharide (LPS, Sigma, St. Louis, MO). Cells were then incubated for 1 h in 2 ml of HBSS containing 31.4 µL cyt c under either 0.5% O2 (chronic hypoxia) or repeated 10-min cycles from 0.5% O2 to 20% O2 (to simulate intermittent hypoxia) in a hypoxia chamber. The temperature of the hypoxia chamber was preset to 37, 39, 41 or 43°C. Immediately following treatment, cell media were collected and
production was determined using the cyt c assay.
Superoxide assay
Superoxide release by macrophages was determined using the ferricytochrome c method by McCord and Fridovich Citation[26]. For the determination of levels, cells were incubated in a 2 mL phenol red free HBSS with 31.4 µL mL−1 of a 1 mM stock solution of equine ferricytochrome c (#7752 Sigma-Aldrich, St. Louis, MO). Briefly, cyt c reduction by
induces an increase in the absorbance at 550 nm which is measured from 100 µL aliquots before and after treatment. The change in cyt c absorbance upon reduction by
at 550 nm (ε550 = 21 000 M−1 cm−1) was used to calculate the levels of
Citation[27]. All experiments were run in triplicate.
Determination of HIF-1α levels
Twenty-two millimetre square glass cover slips (VWR, West Chester, PA) were placed on the bottom of 35 mm dishes in which 2.5 × 106 macrophages were plated overnight in the presence of 1 µg mL−1 LPS. Twenty-four hours later, cells were exposed to normoxia or chronic hypoxia as described above. Following treatment, cell media was disregarded and cells were washed twice in phosphate buffer saline (PBS). Cells were fixed in 1 mL of 4% paraformaldehyde for 30 min and stored in PBS at 4°C. Within 24 h of fixation, cells were blocked with 1% bovine serum albumin (BSA) in PBS for 30 min at 37°C, followed by 1 h incubation in a primary anti-HIF-1α rabbit polyclonal IgG antibody (5 µg mL−1, Santa Cruz Biotechnology, Santa Cruz, CA), washed three-times with PBS and incubated for an additional hour with the secondary donkey anti-rabbit Texas-red dye-conjugated antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA). Metamorph imaging software (Universal Imaging, Union City, CA) was used to analyse fluorescent intensity and ImageJ software (NIH) to determine the relative number of positively stained cells. Experiments were performed in duplicate.
Measurement of VEGF production
Macrophages were plated as described above and incubated under normoxic or chronic hypoxic conditions for 8 h. Then, medium was replaced with fresh medium for one additional hour under the same oxygen conditions at target temperature. Media before and after the 1-h treatments was collected and stored at −20°C. VEGF concentration was determined using a commercially obtained enzyme-linked immunosorbent assay murine VEGF kit (Biosource, Camarillo, CA), according to the manufacturer's instructions.
Statistical analysis
Data are shown as mean ± SEM. Statistical analyses were performed using unpaired Student's t-test. P < 0.05 was considered as statistically significant.
Results
Hypoxia enhances the superoxide production of macrophages
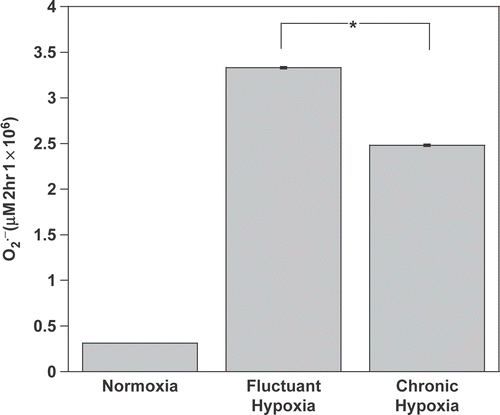
Tumour oxygenation is highly heterogeneous where well oxygenated regions, regions of severe chronic hypoxia and regions of fluctuant hypoxia are commonly found Citation[28]. In a model that reproduces the various micro-environment niches of TAM, this study first sought to determine whether changes in oxygenation could impact the oxidative activity of macrophages. At 37°C, both chronic (2 h 0.5% O2) and intermittent hypoxia (10 min cycles of 0.5%O2 vs. 20% O2 over 2 h) strongly stimulated the production of by non-activated macrophages. While
increase was marginal under normoxia, 2 h of chronic hypoxia or intermittent hypoxia significantly triggered an oxidative burst by these cells (, p < 0.001 vs. normoxia). Intermittent hypoxia was a stronger stimulus for the oxidative activity of macrophages than chronic hypoxia (p < 0.05).
Figure 1. Chronic and intermittent hypoxia stimulate oxidative activity of macrophages. production was determined from its ability to reduce cytochrome c. Non-activated macrophages were incubated for 2-h under normoxia (21% O2), chronic hypoxia (0.5% O2) or intermittent hypoxia (repeated cycles of 0.5–20% O2) at 37°C. *P < 0.05.
Mild hyperthermia further stimulates superoxide production of hypoxic macrophages
To identify whether heat influences macrophage activity, cells were exposed to temperatures that can be reached in tumours during clinical hyperthermia treatment. This study observed a significant increase in the release by normoxic macrophages when cells were heated for 1 h at 41°C and 43°C (, dashed bars, p < 0.01 vs. 37°C). These data indicate that at sufficiently high temperatures, hyperthermia alone stimulates the
production of macrophages.
Figure 2. The combination of hypoxia and hyperthermia fully activates macrophage oxidative activity. production was determined from its ability to reduce cytochrome c. Non-activated macrophages were incubated for 1-h under normoxia (21% O2) or chronic hypoxia (0.5%). *P < 0.05.
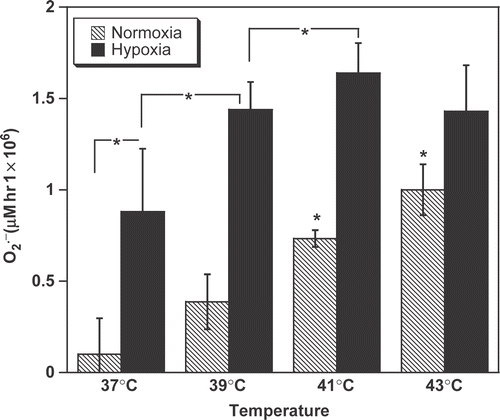
Interestingly, release by normoxic macrophages increased linearly between 37–41°C but did not reach a plateau at the highest temperature tested (). It was, therefore, envisaged that an additional stimulus might further enhance the oxidative burst. To test this hypothesis, cells were heated under hypoxic conditions. The combination of heat with hypoxia was much more efficient than heat alone (normoxia) at stimulating macrophages. Significantly higher
release was observed by the cells not only at 37°C, but also at 39 and 41°C (, plain bars, p < 0.02) vs. normoxic cells at the same temperature). Further heating of the cells to 43°C failed to enhance their
production, indicating that severely hypoxic macrophages reach their maximal oxidative activity (i.e. ∼1.5 pmol/cell per hour), between 41–43°C. The slight decrease in
levels at 43°C as compared to 41°C was further continued at 45°C (data not shown).
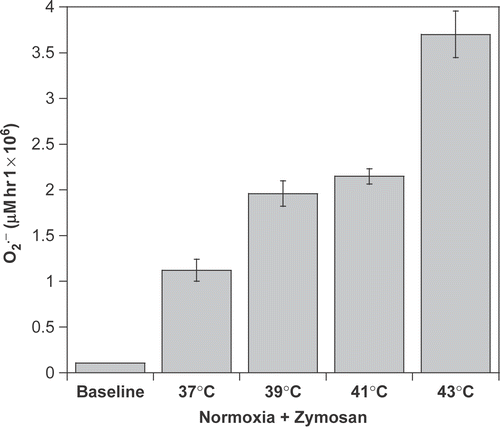
Zymosan is widely accepted to fully activate macrophages in experimental systems. The data suggest that hyperthermia could constitute an additional stimulus. To test this idea, macrophages were exposed to Zymosan as a positive control for macrophage activation of the respiratory burst. Macrophages were exposed to Zymosan alone or to a combination of Zymosan and heat. Hyperthermia did not depress production of macrophages in the presence of Zymosan (). Instead, heating Zymosan-treated cells at ≥39°C further enhanced
release above that seen with Zymosan alone (p < 0.001).
Mild hyperthermia inhibits HIF-1α activation by hypoxia in macrophages
Although high oxidative activity of macrophages is known to trigger tumour cell death, other adaptations of macrophages to extreme tumour micro-environment may promote angiogenesis and tumour cell survival Citation[11–13]. To investigate this possibility, it was examined whether heat could modulate HIF-1α activation by hypoxia in macrophages.
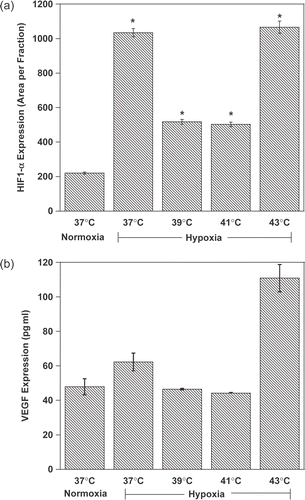
Using immunofluorescence, it was first observed that hypoxia alone induced a 5-fold increase in HIF-1α levels detected in macrophages at 37°C (, p < 0.001). Hypoxia-induced HIF-1α activation was greatly prevented when hypoxic macrophages were heated at 39 or 41°C (p < 0.05 vs. hypoxia at 37°C). In contrast, at higher temperatures (43°C) under hypoxic conditions, the destabilization of HIF-1α was reversed (p < 0.05 vs. normoxia and p > 0.05 vs. hypoxia at 37°C). Data shown in represent the return of HIF-1α levels at 43°C to levels observed at 37°C under hypoxia.
Figure 4. Mild hyperthermia represses HIF-1α expression and activity in macrophages. Macrophages were cultured for 1-h (HIF-1α) or 8 h (VEGF) under different conditions of oxygenation and heat, as indicated, and readily fixed with 4% paraformaldehyde. Layers were stained for HIF-1α (a) and positively labelled cells counted. VEGF (b) was determined by ELISA. *P < 0.05.
Mild hyperthermia and VEGF production in macrophages
Cells regulate HIF-1α activity by controlling proteosomal degradation of the HIF-1α sub-unit. Additional points of regulation include post-translational changes and various protein–protein interactions Citation[29], Citation[30]. To investigate the molecular relevancy of HIF-1α destabilization/inactivation by heat, this study measured the release of VEGF, a major HIF-regulated transcript, by macrophages. As expected, hypoxia stimulated the production and release of VEGF ( by macrophages at 37°C. However, at higher temperatures, VEGF levels fluctuated in parallel to HIF-1α expression. Heating hypoxic cells at 39 or 41°C prevented VEGF release, but the inhibitory effect was suppressed at 43°C. The results show that mild hyperthermia prevents hypoxia-induced HIF-1α stabilization and activation in macrophages.
Discussion
It is well established that inadequate blood supply combined with high metabolic rates of oxygen consumption results in areas of low oxygen tension (median pO2 < 10 mmHg) within malignant tumours Citation[28]. It has been shown that hypoxic cells are more radioresistant than their counterparts. Elevation of tumour temperatures above 39°C results in significant improvement in tumour oxygenation (median pO2 > 10 mmHg) and cell radiosensitivity Citation[6], Citation[31], Citation[32]. Published reports estimate that macrophages can compose between 50–80% of the total tumour mass Citation[9], Citation[14], Citation[33]. As regions of hypoxia develop in response to tumour growth and oxygen consumption, recruited macrophages aggregate adjacent to these poorly vascularized areas where they mount a pro-angiogenic response and increase their oxidative activity Citation[9], Citation[13–15], Citation[18]. Therefore, it is important to understand the physiological changes in macrophage function under hypoxic and normoxic conditions in response to heat therapy.
Oxygen tension within tumours is highly heterogeneous as a result of variations in tumour proliferation, oxygen consumption, angiogenesis and vascular remodelling Citation[34]. Instability in tumour oxygenation appears to be a dominant feature of oxygen transport in tumours Citation[35] and could greatly affect respiratory burst of macrophages. This study found that transient hypoxia stimulated a 68% increase in production of by macrophages in comparison with chronic hypoxia (). These results demonstrate the potency of fluctuations in oxygen availability as a stimulus for generating high levels of oxidative activity. The ability of hypoxia to stimulate oxidative activity in macrophages increases cellular oxygen consumption and could potentially contribute further to the hypoxic environment and radioresistance of solid tumours.
Oxidative stress is an important feature of the tumour micro-environment Citation[36]. Both and NO are produced by activated macrophages. These species cause damage by themselves but also through their reaction products such as peroxynitrite, carbonate radical, nitric dioxide and others Citation[37], Citation[38]. Although low levels of ROS participate in the promotion of tumour growth through signalling pathways and mutagenesis, these species also cause extensive damage to biomolecules and signalling proteins when produced at higher levels Citation[39]. This study observed that normoxia non-activated macrophages undergo respiratory burst without co-stimulation at temperatures above 37°C. Likewise, under hypoxia, which was in itself a stimulus for macrophage activation, an increase in temperatures up to 43°C mediated a further increase in superoxide production. Moreover, compared to drugs that activate macrophages (such as Zymosan) or to hypoxia alone (as found in tumours), it was shown that heat stimulates macrophages to produce much higher levels of ROS. It was further observed that, under mild hyperthermia, the increase in
levels parallelled the decrease in the expression and activity of HIF-1α at clinically relevant temperatures (39°C and 41°C). The decrease in HIF-1α levels is presumably due to the oxidation of the signalling protein/s. At T > 43°C the activity of macrophages seem to cease (the trend continues at 45°C), which resulted in increased HIF-1α activity. VEGF release by macrophages tightly reflects the fluctuation in HIF-1α with temperature.
As tumours expand beyond the limits of their vascularization to supply oxygen and nutrients, macrophages alter their phenotypic expression towards the promotion of angiogenesis via stabilization of HIF-1α and VEGF release Citation[9], Citation[12], Citation[14], Citation[18]. However, in tumours, new vasculature is characterized by multiple structural and functional abnormalities which compromise blood flow and result in tissue hypoxia Citation[40]. In addition, high micro-vascular density has been associated with a more aggressive tumour phenotype and increased metastasis Citation[40–43]. Jin et al. Citation[44] demonstrated anti-angiogenic compounds could function as radiosensitizers for hypoxic cells. Therefore, by repressing HIF-1α activity and reducing VEGF levels, mild hyperthermia has the potential to increase tumour oxygenation through down-regulation of pro-angiogenic responses in macrophages. The intrinsic effects of hyperthermia could, thus, be seen as a remodelling of the tumour micro-environment detrimental to tumour growth.
Tumour hypoxia is associated with increased metastasis, a more aggressive malignant phenotype and increased angiogenesis Citation[6]. Moreover, it constitutes one of the primary barriers limiting the success of radiation and chemotherapy treatment. Therefore, targeting tumour reoxygenation may significantly improve the outcome in a variety of cancers Citation[6], Citation[32]. This study has shown hyperthermia in the range of 39–41°C to be optimal for altering macrophage function towards the promotion of high oxidative activity and mitigation of angiogenesis. Further studies are warranted to investigate the mechanisms of hyperthermic regulation of HIF-1α stabilization and transcriptional activity in macrophages. Studies have suggested ROS may participate indirectly in oxygen sensing and, therefore, regulate HIF-1α stabilization Citation[45]. However, HSP90 stabilization of HIF-1α at high temperatures may potentially compete with pVHL mediated HIF-1α degradation at temperatures above 41°C Citation[46]. In conclusion, changes in normal macrophage activity under hypoxic conditions due to elevated temperatures may provide an insight into the ability of hyperthermia to facilitate tumour reoxygenation and increase treatment success.
References
- Masunaga S, Takahashi A, Ohnishi K, Ohnishi T, Nagata K, Suzuki M, Kinashi Y, Ono K. Effects of mild temperature hyperthermia and p53 status on the size of hypoxic fractions in solid tumors, with reference to the effect in intratumor quiescent cell populations. Int J Radiat Oncol Biol Phys 2004; 60: 570–577
- Thews O, Li Y, Kelleher DK, Chance B, Vaupel P. Microcirculatory function, tissue oxygenation, microregional redox status and ATP distribution in tumors upon localized infrared-A-hyperthermia at 42 degrees C. Adv Exp Med Biol 2003; 530: 237–247
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, Rosner G, Azuma C, Poulson J, Pruitt AF, Stanley W, Hauck ML, Williams L, Hess P, Dewhirst MW. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res 2005; 11: 5206–5214
- Vujaskovic Z, Gillette SM, Powers BE, Stukel TA, Larue SM, Gillette EL, Borak TB, Scott RJ, Weiss J, Colacchio TA. Effects of intraoperative irradiation and intraoperative hyperthermia on canine sciatic nerve: Neurologic and electrophysiologic study. Int J Radiat Oncol Biol Phys 1996; 34: 125–131
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, MacFall JR, Brizel DM, Meyer RE, Prescott DM, Samulski TV, Dewhirst MW. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000; 46: 179–185
- Vujaskovic Z, Rosen EL, Blackwell KL, Jones EL, Brizel DM, Prosnitz LR, Samulski TV, Dewhirst MW. Ultrasound guided pO2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int J Hyperthermia 2003; 19: 498–506
- Dewhirst MW, Poulson JM, Yu D, Sanders L, Lora-Michiels M, Vujaskovic Z, Jones EL, Samulski TV, Powers BE, Brizel DM, Prosnitz LR, Charles HC. Relation between pO2, 31P magnetic resonance spectroscopy parameters and treatment outcome in patients with high-grade soft tissue sarcomas treated with thermoradiotherapy. Int J Radiat Oncol Biol Phys 2005; 61: 480–491
- Feldmann HJ, Molls M, Fuller J, Stuben G, Sack H. Changes in oxygenation patterns of locally advanced recurrent tumors under thermoradiotherapy. Adv Exp Med Biol 1994; 345: 479–483
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004; 104: 2224–2234
- Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol 2001; 280: H2357–2363
- Clark RA, Valente AJ. Nuclear factor kappa B activation by NADPH oxidases. Mech Ageing Dev 2004; 125: 799–810
- Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: The double-edged sword in cancer progression. J Clin Oncol 2005; 23: 953–964
- Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 2002; 7: 177–189
- Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: Relevance to disease mechanisms. J Leukoc Biol 1999; 66: 889–900
- Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: Role in tumour progression. Eur J Cancer 2004; 40: 1660–1667
- Tomasovic SP, Klostergaard J. Hyperthermic modulation of macrophage-tumor cell interactions. Cancer Metastasis Rev 1989; 8: 215–229
- Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macsrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res 2002; 62: 1326–1329
- Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol 2001; 70: 478–490
- Dewhirst MW, Kimura H, Rehmus SW, Braun RD, Papahadjopoulos D, Hong K, Secomb TW. Microvascular studies on the origins of perfusion-limited hypoxia. Br J Cancer Suppl 1996; 27: S247–S251
- Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 2000; 192: 150–158
- Hagg M, Wennstrom S. Activation of hypoxia-induced transcription in normoxia. Exp Cell Res 2005; 306: 180–191
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005; 8: 143–153
- Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol 1997; 150: 1723–1734
- Hemmerlein B, Johanns U, Halbfass J, Bottcher T, Heuser M, Radzun HJ, Thelen P. The balance between MMP-2/-9 and TIMP-1/-2 is shifted towards MMP in renal cell carcinomas and can be further disturbed by hydrogen peroxide. Int J Oncol 2004; 24: 1069–1076
- Konig JE, Tolnay E, Wiethege T, Muller KM. Expression of vascular endothelial growth factor in diffuse malignant pleural mesothelioma. Virchows Arch 1999; 435: 8–12
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969; 244: 6049–6055
- Kirby TW, Fridovich I. A picomolar spectrophotometric assay for superoxide dismutase. Anal Biochem 1982; 127: 435–440
- Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol 1998; 8: 143–150
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004; 19: 176–182
- Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 2002; 64: 993–998
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, Samulski TV, Prosnitz LR, Dewhirst MW. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996; 56: 5347–5350
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol 2005; 98: 715–721
- Moeller BJ, Cao Y, Vujaskovic Z, Li CY, Haroon ZA, Dewhirst MW. The relationship between hypoxia and angiogenesis. Semin Radiat Oncol 2004; 14: 215–221
- Cardenas-Navia LI, Yu D, Braun RD, Brizel DM, Secomb TW, Dewhirst MW. Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res 2004; 64: 6010–6017
- Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 2002; 62: 307–312
- Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA 1997; 94: 6954–6958
- Linares E, Giorgio S, Mortara RA, Santos CX, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Radic Biol Med 2001; 30: 1234–1242
- Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett 1996; 381: 82–86
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005; 15: 102–111
- Alexandrakis MG, Passam FJ, Ganotakis E, Dafnis E, Dambaki C, Konsolas J, Kyriakou DS, Stathopouls E. Bone marrow microvascular density and angiogenic growth factors in multiple myeloma. Clin Chem Lab Med 2004; 42: 1122–1126
- Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microveessel density determined by anti-CD105/endoglin monoclonal antibody in astrocytic tumors: Comparison with an anti-CD31 monoclonal antibody. Neuropathology 2005; 25: 201–206
- Sun XY, Wu ZD, Liao XF, Yuan JY. Tumor angiogenesis and its significance in pediatric malignant liver tumor. World J Gastsroenterol 2005; 11: 741–743
- Jin CZ, Nagasawa H, Shimamura M, Uto Y, Inayama S, Takeuchi Y, Kirk KL, Hori H. Angiogenesis inhibitor TX-1898: synthesis of the enantiomers of sterically diverse haloacetylcarbamoyl-2-nitroimidazole hypoxic cell radiosensitizers. Bioorg Med Chem 2004; 12: 4917–4927
- Wenger RH. Mitochondria: oxygen sinks rather than sensors?. Med Hypotheses 2006; 66: 380–383
- Katschinski DM, Le L, Schindler SG, Thomas T, Voss AK, Wenger RH. Interaction of the PAS B domain with HSP90 accelerates hypoxia-inducible factor-1alpha stabilization. Cell Physiol Biochem 2004; 14: 351–360