Abstract
Background: Thermal treatments need verification of effectiveness. Invasive intra-tumoural thermometry was established as a standard method several years ago. However, in deep heating, invasive techniques have disadvantages. Therefore, alternatives have been suggested and are under development.
Methods: In three phase II studies treating rectal cancer, cervical cancer and prostate cancer, this study replaced invasive (intra-tumoural) thermometry by tumour-related reference points or catheter sections in the rectum, vagina or urethra. Index temperatures and thermal dose parameters were determined. Two recent studies treated patients with recurrent rectal cancer and soft tissue sarcoma using non-invasive MR-thermometry employing the SIGMA-Eye applicator. The proton resonance frequency shift (PRFS) method was employed to generate MR-temperature distributions during the entire heat treatment in 10 min intervals (via phase differences). Fat correction (nulling specified regions in the fat tissue) was utilized to calibrate the method, in particular with respect to the B0-drift.
Results: Statistically significant correlations were found between response (downstaging, WHO) and thermal parameters in rectal cancer (37 patients, rectum measurement, T90, cum min T90 ≥ 40.5°C) and cervical cancer (30 patients, vagina, mean temperature and equ min 43°C in a reference point). In prostate cancer (14 patients), a clear correlation was verified between long-term PSA control (≤1 ng ml−1) and urethral temperatures (T90, Tmax cum min T90 ≥ 40.5°C). The mean MR-temperature in the tumour at steady-state as well as the mean T90 were significantly correlated with response for recurrent rectal carcinoma regarding palliation and analgesia (15 patients) and with pathohistological regression rate in soft tissue sarcoma (nine patients).
Conclusions: For tumours in the pelvis and in the lower extremities, invasive measurements can be replaced by minimally-invasive or non-invasive techniques, which provide equivalent or even more complete information. Extending the application of these surveillance methods to abdominal tumours or liver metastases is a challenge, but strongly desirable for clinical reasons.
Introduction
For hyperthermia, international guidelines recommend invasive intra-tumoural temperature measurements, ideally along two-to-three orthogonal scanning lines Citation[1–3]. It is recommended to register temperature position scans every 5–10 min. Either index temperatures averaged over time or thermal dose parameters accumulated over time are derived from these data.
In common use are so-called index temperatures Tx. Tx is the temperature, which is exceeded by x% of the tumour-related temperature measurement points, either for a whole mapping matrix (time-averaged) or for every scanning time (to calculate a thermal dose parameter).
The thermal dose is typically presented in equivalent minutes to 43°C. It is assumed that 43°C represents the so-called ‘break’ point in the Arrhenius plot Citation[4], Citation[5]: every increase of temperature by 1°C above 43°C doubles the time in minutes equivalent to 43°C. Conversely, every decrease of temperature by 1°C below 43°C results in a reduction of equivalent time by a factor of ∼4 (range from 2–6). These rules are consistent with laboratory data, proven for a variety of cell lines with a wide range of temperature sensitivities.
These intra-tumoural measurements have been successfully correlated with clinical endpoints, in particular with response Citation[6–8].
Temperature-time curves provide additional valuable information about the quality of heat treatment Citation[9], Citation[10]. According to the formulae (right part of ) the technical quality (specific absorption rate: SAR in W kg−1) in the measurement point as well as the physiological conditions (perfusion coefficient w in ml per 100 g per min) are provided.
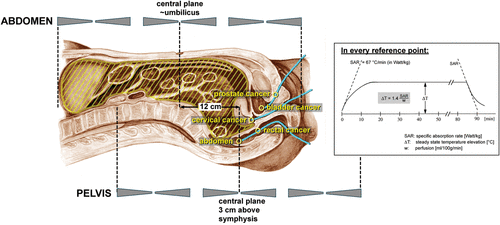
Figure 1. Specification of reference points for pelvic tumours and part body hyperthermia (PBHT), which are applied in disseminated diseases (peritoneal carcinosis). Every temperature-time curve is analysed according to the chart left below.
In the 1990s, routinely performed invasive thermometry was questioned in some European centres Citation[11], Citation[12]. The low acceptance in patients and physicians was a limiting factor, when large patient numbers were to be heated according to randomized studies. Especially the problems of the time-consuming invasive placement of the catheters with the risk of haemorrhage or neurological complaints in form of acute side effects sometimes provoked refusal by the patients. Additional toxicity such as infections and a variety of discomforts were observed. Obliteration of catheters because of bending or deviations might finally hamper thermometry (e.g. thermal mapping). Furthermore, some clinicians had second thoughts to puncture tumours in the framework of curative treatment intent.
On the other hand, typical heat-related toxicity such as the occurrence of hot spots cannot be detected or prevented by invasive thermometry. These phenomena occur at certain structures (e.g. electrical boundaries), often far away from the actual target (where invasive thermometry has never been performed or recommended). Adequate treatment planning Citation[13], understanding of the underlying mechanisms, clinical experience and standardization of heat treatments are strategies to reduce such toxicity. On summary, to persist on invasive thermometry only for safety reasons is no longer indicated.
Two major issues remain as a rationale for invasive thermometry. First, intra-tumoural measurements could assist in finding an appropriate (or even the best) adjustment (e.g. phases of the channels or position of the applicator) for a heat treatment. The achieved SAR in the tumour (derived from the temperature increase per time according to ) is the main reason. It is known from modelling studies and clinical data Citation[11], Citation[13] that tumour-related measurement points, i.e. locations at the tumour boundary or close to the tumour, are also suitable to assess the SAR in the treatment region. Secondly, the invasive measurements are needed to consider the effectiveness of the heating because retrospective analyses verified correlations with response (see above). However, there might exist surrogate parameters for intra-tumoural temperatures or rises in temperature, and investigators have looked for such alternatives to invasive thermometry that also provide thermal data.
Two major principle strategies have been evaluated:
Registration of temperature in so-called tumour-related reference points, which are achievable by minimal-invasive techniques in hollow organs (rectum, vagina, bladder, urethra); and
Non-invasive thermography by heating in a magnetic-resonance-tomograph (MR).
Both substitutes are only suitable if they provide the same information as invasive techniques, i.e. differentiating between efficient and non-efficient heating sessions.
In the following, retrospective studies are described to validate alternative minimal-invasive or non-invasive techniques to replace invasive thermometry.
Studies to evaluate thermometry methods
Three phase II-studies were conducted on pelvic tumours using a substitution of invasive thermometry by minimally-invasive methods. A survey of reference points in hollow organs for the various indications is given in .
The first study applied pre-operative radiochemotherapy plus regional hyperthermia (RHT) in locally advanced rectal cancer uT3 or T4 cN ± M0 Citation[14]. During every RHT, a closed-end catheter was inserted in the rectum, the tip typically located 12–15 cm deep from the anal orifice. The (tumour-related) measurement points in contact to the tumour were specified by careful comparison of endorectal ultrasound examination, CT-images and the registered temperature-position curves. In correspondence to planning calculation Citation[15], typical curves were found with a peak of variable width at the tumour contact section and another peak across the anal sphincter. Index temperatures, time averaged for every single heat session and thermal parameters by analysing the index temperatures over time (as described above), were calculated. In addition, the time (in min) was determined when T90 exceeds a threshold temperature of 40.5°C (cumulative min T90 ≥ 40.5°C). This parameter is derived easily from the original data and proved useful to characterize the heating efficiency.
As a response parameter, pathohistological downstaging (pT) was utilized in comparison to the uT-category. In addition, the WHO (World Health Organization) response (tumour volume estimated by endoscopy and ultrasound in comparison to the intra-operative specimen) was documented.
A second study applied RHT with definitive external beam radiotherapy against locally advanced prostate cancer uT3 (proven by ultrasound) pN0. A hyperthermia catheter was inserted in the urethra (in some cases simultaneously with a bladder catheter), identified the intra-prostatic section of the urethra and determined index temperatures (T90, Tmax) as well as cummin T90 ≥ 40.5°C. Catheterization of the bladder or urethra was not demanded for every heat session due to patient refusal. To determine cumulative thermal doses, the measured temperatures were assumed typical and extrapolated to characterize the whole treatment course. Typical SAR and temperature distributions expected for RHT of prostate carcinoma have been displayed elsewhere Citation[13], Citation[15]. As a response parameter, PSA control was used after 5 years.
A third study performed a pre-operative multi-modal treatment in non-resectable cervical cancer FIGO ≥ IIB (bulky). RHT was combined with external beam radiotherapy and (if possible) cisplatinum chemotherapy Citation[16]. Reference points were selected for temperature measurement in the vagina (maximum temperature near the tip of the catheter) and the rectum (maximum temperature behind the uterus). From these temperature-time curves, mean temperatures and thermal doses were extracted. As a response parameter, pathohistological downstaging and WHO response were utilized.
Two additional studies introduced non-invasive MR-thermography. A so-called hybrid system was employed, i.e. an annular-phased-array applicator (Sigma Eye applicator, BSD Corp., Salt Lake City, Utah, USA) integrated into an MR-tomograph (Magnetom Symphony, Siemens, Erlangen, Germany). The electronic requirements and the MR-acquisition techniques (including post-processing of the MR data) are described in Wust et al. Citation[17] and Gellermann et al. Citation[18]. Non-invasive MR-thermography has been evaluated for recurrent pre-sacral rectal cancer and soft tissue sarcomas. Well-defined tumour-related reference points in hollow organs do not exist for both tumour entities, thus requiring invasive thermometry according to the guidelines.
Three-dimensional MR-temperature distributions were successfully acquired in these tumour entities over time (typically in 10 min intervals) and were further evaluated with respect to index temperatures and thermal doses in the selected target volume. The MR-temperature increase was achieved relative to a basal distribution before turning the power on. Adding this increment to the systemic temperature (37–37.7°C) should provide the temperature in°C.
However, one has to consider that MR-temperature is also increased by changes in perfusion. Regions-of-interest were segmented in normal tissues such as muscle, fat and other organs (prostate, bladder) Citation[19], Citation[20] and a superposition of temperature and perfusion increase was found, especially in certain muscle regions.
Results
In each of the five studies, tumour-related thermal parameters (including non-invasive measurements) are retrospectively correlated with the available corresponding response parameters (, 6th row). However, the particular parameters can vary (, 3rd row).
Table I. Surrogate parameters for invasive temperature measurements, either acquired minimal-invasive or non-invasive.
For 37 patients with rectal cancer there was a statistically significant correlation between response (downstaging, WHO) thermal parameters T90 and cum min T90 ≥ 40.5°C Citation[13], Citation[21]. In 14 patients treated for locally advanced prostate cancer, a clear correlation was found between long-term PSA control (≤1 ng ml−1) and the temperatures measured minimal invasively in the urethra (T90 > 40.7°C; Tmax > 41.4°C and cum min T90 ≥ 40.5°C > 180 min) Citation[22]. In 30 patients with cervical cancer a correlation of the response (downstaging and volume reduction, WHO) with the mean temperature (>40.2°C) and equivalent min 43°C (>11 min) in a reference point in the proximal vagina Citation[16] was observed.
With non-invasive MR thermometry, statistically significant correlations were found with response criteria for recurrent rectal carcinoma regarding palliation and analgesia in 15 patients Citation[20]. For nine patients with soft tissue sarcoma there was a significant correlation of MR derives T90 (>41 MR°) and CEM 43°MR (>16 min) with pathohistological response (over 90% necrosis) Citation[23].
Discussion
Numerous studies have shown that higher tumour temperatures or thermal doses are retrospectively associated with improved clinical outcome. Because high index temperatures mostly cause large thermal doses, several parameters are typically useful. For pelvic tumours, specific reference points have been defined (), where temperature measurements can characterize the effectiveness of the heat treatment. This correlation has been validated for rectal cancer Citation[21], cervical cancer Citation[16], Citation[24] and prostate cancer Citation[22]. Possibly, an intra-vesical temperature measurement could be appropriate for bladder cancer.
The range of endoluminal temperatures is different from intra-tumoural temperatures as outlined by Wust et al. Citation[12]. Nevertheless, the temperature levels itself are predictive for the efficiency, i.e. the group of patients with lower index temperatures has a worse response than patients with higher index temperatures. In particular, estimations of the SAR in the beginning of the treatment and derived from temperature changes during treatment are helpful to improve the treatment adjustments. No regulation procedure has been developed until now using only directly measured temperatures.
It is not clear whether or not intra-tumoural temperatures are more strongly correlated with response than temperatures achieved minimal-invasively. However, avoiding invasive measurements has many advantages. Most important is the increased acceptance of hyperthermia for patients and physicians. This is important to conduct studies with larger patient numbers.
There are tumour locations where minimally invasive temperature measurements are difficult or impossible. In particular, in the abdomen only one single point in the proximal rectum (>15 cm deep from the anal orifice) could be defined ().
In case of soft tissue sarcomas of the extremities and for pre-sacral recurrent rectal cancer, minimal invasive thermometry is also difficult. Therefore, especially for these tumours non-invasive thermography using a hybrid-system has been implemented and studied Citation[18], Citation[19].
MR-temperature distributions in the target region are even better correlated with response than parameters provided by minimally-invasive thermometry. Time averaged index temperatures or thermal dose parameters derived from the three-dimensional MR-temperature data sets proved useful.
MR-temperature distributions are a superposition of actual temperature change (in °C) and perfusion changes. Typically, the perfusions in the tumours are low and the resulting perfusion changes seldom disturb the temperature measurement. This has been validated by comparison of direct temperature measurements with MR-temperature measurements Citation[23]. In normal tissues such as muscles, perfusion changes can be significant. Nevertheless, non-invasive MR-temperature distributions offer a lot of valuable information about the tumour and normal tissues and strategies to separate the actual temperature increase in °C and the perfusion increase from the MR-temperature are under development. Clearly, information obtained by MR-thermography is more complete, including information on the tumour itself, various muscle regions and organs like the prostate and the uterus. Other regions such as fat could be added if T1 measurements are utilized. This information is useful to regulate and optimize the temperature distribution in the tumour usually (better than when using direct temperature measurements), the MR temperature or even the actual temperature in °C. Such regulation tools, which employ the planning software on a patient-specific model, are under development.
Non-invasive thermometry in the abdomen is an exceptional challenge. Here, the physiological movement of the patient (due to pulsation and breathing) has proven to be a significant hindrance to apply the PRFS method. Alternative MR-acquisition methods are under study to extract temperature information in the abdomen or liver and to realize a regulation.
In summary, strategies to replace invasive thermometry have been successful. Therefore, under specific conditions it is possible to avoid intra-tumoural temperature measurements, which are otherwise regarded as standard. According to ESHO-guidelines Citation[3], suitable tumour-related measurements are sufficient to control and characterize the heat sessions.
Acknowledgements
This work has been supported by a grant of the Lieselotte Beutel Stiftung (Project ‘Prostate Center’). We gratefully thank them for the support.
References
- Dewhirst M. Thermal dosimetry: Thermo-radiotherapy and thermochemotherapy. Springer-Verlag, Berlin 1995; 123–136
- Hand JW, Lagendijk JJW, Andersen JB, Bolomey JC. Quality assurance guidelines for ESHO protocols. Int J Hyperthermia 1989; 5: 421–428
- Lagendijk JG, van Rhoon G, Hornsleth S, Wust P, de Leeuw A, van Dijk J, van der Zee C, van Heek R, Rahman S, Schneider C, Gromoll C. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998; 14: 125–133
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Dewhirst MW, LaRue SM, Gerweck L. Tumor physiology and cell kinetics. Semin Vet Med Surg 1995; 10: 148–157
- Issels RD, Prenninger SW, Nagele A, Boehm E, Sauer H, Jauch KW, Denecke H, Berger H, Peter K, Willmann W. lfosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: A phase II study. J Clin Oncol 1990; 8: 1818–1829
- Leopold KA, Dewhirst MW, Samulsi TV, Dodge RK, George SL, Blivin JL, Prosnitz LR, Oleson JR. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1993; 25: 841–847
- Oleson JR, Dewhirst MW, Harrelson JM, Leopold KA, Samulski TV, Tso CY. Tumor temperature distributions predict hyperthermia effect. Int J Radiat Oncol Biol Phys 1989; 16: 559–570
- Wust P, Stahl H, Löffel J, Seebass M, Riess H, Felix R. Clinical, physiological and anatomical determinants for radiofrequency hyperthermia. Int J Hyperthermia 1995; 11: 151–167
- Tilly W, Wust P, Harder C, Gellermann J, Schlag P, Budach V, Felix R. Temperature data and specific absorption rates in pelvic tumors: Predictive factors and correlations. Int J Hyperthermia 2001; 17: 172–188
- Van der Zee J, Peer-Valstar JN, Rietveld PJ, de Graaf-Strukowska I, van Rhoon C. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys 1998; 40: 1205–1212
- Wust P, Gellermann J, Harder C, Tilly W, Rau B, Dinges S, Schlag P, Budach V, Felix R. Rationale for using invasive thermometry for regional hyperthermia of pelvic tumors. Int J Radiat Oncol Biol Phys 1998; 41: 1129–1137
- Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Deuflhard P, Felix R, Wust P. Clinical application of the hyperthermia treatment planning system HyperPlan—comparison of algorithms and clinical observables. Int J Radiat Oncol Biol Phys 2003; 55: 407–419
- Rau B, Wust P, Hohenberger P, Löffel J, Hünerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, Felix R, Schlag PM. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer. A phase II clinical trial. Ann Surg 1998; 227: 380–389
- Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, Beier J, Hege HC, Deuflhard P, Felix R. Clinical evaluation and verification of the hyperthermia treatment planning system HyperPlan. Int J Radiat Oncol Biol Phys 2000; 47: 1145–1156
- Sreenivasa G, Hildebrandt B, Jungnickel K, Kümmel S, Cho CH, Tilly W, Böhmer D, Budach V, Felix R, Wust P. Radiochemotherapy combined with regional hyperthermia induces high response and resectability rates in patients with non-resectable cervical cancer ≥ IIB bulky. Int J Radiat Oncol Biol Phys 2006, in review
- Wust P, Gellermann J, Seebass M, Fähling H, Turner P, Wlodarczyk W, Nadobny J, Rau B, Hildebrandt B, Oppelt A, Schlag PM, Felix R. Teilkörper-Hyperthermie mit einem Radiofrequenz-Multiantennen-Applikator unter on-line Kontrolle in einem 1,5 T MR-Tomographen. Fortschr Röntgenstr 2004; 176: 363–374
- Gellermann J, Wlodarczyk W, Feussner A, Fähling H, Nadobny J, Hildebrandt B, Felix R, Wust P. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia 2005; 21: 497–513
- Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fähling H, Seebass M, Felix R, Wust P. A practical approach to perform the thermography in a hyperthermia/MR hybrid system—validation in an anthropomorphous phantom. Int J Radiat Oncol Biol Phys 2005; 61: 267–277
- Gellermann J, Wlodarczyk W, Hildebrandt B, Ganter H, Tilly W, Fähling H, Nadobny J, Felix R, Wust P. Non-invasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res 2005; 13: 1–9
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, Budach V, Felix R, Schlag PM. Preoperative radio-chemotherapy in locally advanced recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000; 48: 381–391
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weißbach L, Budach V, Felix R, Wust P. Regional hyperthermia in conjunction with definitve radiotherapy against recurrent or locally advanced prostate cancer T2 pN0 M0. Strahlenther Onkol 2005; 181: 35–41
- Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Nadobny J, Budach V, Felix R, Wust P. Non-invasive MR-thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006, in review
- Dinges S, Harder C, Wurm R, Buchali A, Blohmer J, Gellermann J, Wust P, Randow H, Budach V. Combined treatment of inoperable carcinomas of the uterine cervix with radiotherapy and regional hyperthermia. Strahlenth Onkol 1998; 174: 517–521
