Abstract
Purpose: The present study compares quality of life (QoL) after neoadjuvant radiochemotherapy with or without hyperthermia in patients with advanced rectal cancer.
Methods: Between April 1994 and May 1999, 137 patients were treated by neoadjuvant radiochemotherapy with (69 patients (50.4%)) or without (68 patients (49.6%)) hyperthermia. Forty-six patients (33.6%) filled-out a ‘Gastrointestinal Quality of Life Index’ (GIQLI) questionnaire at four time points (before and after neoadjuvant therapy, early after surgery and after long-term follow-up) and were included in the present study.
Results: There were no statistically significant differences in the global GIQLI index between patients treated with neoadjuvant radiochemotherapy with and without hyperthermia at any time point. The longitudinal analysis of GIQLI values in both treatment groups showed specific profiles that were identical in both treatment groups. Occurrence of severe toxicity during the neoadjuvant therapy in both arms lead to a significant temporary reduction of QoL scores at TP2 without any detrimental long-term effects. Patients with sphincter preservation and patients with sphincter resection reported similar QoL scores during long-term follow-up.
Conclusion: Neoadjuvant radiochemotherapy with and without hyperthermia has similar effects on the QoL of patients with locally advanced rectal cancer. The addition of hyperthermia during the neoadjuvant therapy with the potentially associated inconveniences has no negative effects on QoL.
Introduction
Until the late 1980s, disease was predominantly considered as a pathophysiologic dysfunction of the body. During the last two decades, however, considerable efforts were made to assess the patient's personal perception of his current physical, psychological and social well-being and to objectively measure the influence of disease and treatment on these categories. Quality of life (QoL) as a measurement category was developed to ‘prevent a devastating separation of a patients body from a patients biography during delivery of care’ Citation[1]. Moreover, QoL can assess an improvement of the patient's well-being that does not correlate with any objective biological response to treatment. While researching on QoL, a wide range of inventories designed to assess global or disease specific QoL was developed. By 1998, more than 300 different QoL scores had been published in the international literature Citation[2]. In chronic and sometimes incurable diseases, the positive or negative influence of treatment on QoL can be as essential as its efficiency in terms of classical biological measures. Even in the absence of quantifiable biological end-points, QoL can be an important end-point to assess the value of a therapeutic intervention Citation[3]. Therefore, the interest in QoL assessment increased considerably during the last decades. This is reflected by the fact that the American ‘Food and Drug Administration’ (FDA) accepts an improvement of QoL as a valid end-point of clinical studies to approve novel drugs for the palliative treatment of cancer patients.
The present study investigates the impact of a therapeutic protocol containing neoadjuvant radiochemotherapy with and without hyperthermia on the QoL of patients with locally advanced rectal cancer. In patients with advanced rectal cancer, locoregional recurrence remains a major problem and a major cause of mortality as well as a source of metastasis to distant organs Citation[4], Citation[5]. Considerable efforts have been made to further improve the treatment efficiency of classical treatment protocols by adding different neoadjuvant treatments, including different chemotherapeutic regimens Citation[6–8] as well as the application of local hyperthermia. The authors’ own group Citation[9–11] as well as other groups Citation[12–15] have shown the effectiveness of combining hyperthermia with radio- or radiochemotherapy.
The Gastrointestinal Quality-of-life Index (GIQLI) was developed especially to measure the QoL in patients suffering from malignant and benign diseases of the gastrointestinal tract Citation[16]. It has been widely used to monitor QoL of patients suffering from benign as well as from malignant diseases Citation[17–20]. The GIQLI measures the QoL in several domains: symptoms of disease, emotional well-being, social life, physical functions and medical treatment. An elevated GIQLI score means a higher quality of life. Symptoms and restrictions with negative influence on the subjective well-being of the patient result in a reduced GIQLI score. This instrument has been validated and tested for its reproducibility and sensitivity. The GIQLI shows a good correlation to the EORTC Quality of Life Core Questionnaire (EORCT-QLQ-C30) Citation[19].
Patients and methods
Patients
Patients with histologically proven, locally advanced non-metastatic rectal cancer were included in a phase II/III trial comparing neoadjuvant radiochemotherapy with and without hyperthermia Citation[11]. Tumour infiltration beyond the rectal wall was confirmed by endorectal ultrasound, CT or MRI scan. Tumour staging was done according to the UICC-TNM classification of malignant tumours. Patients with prior malignant disease or prior chemotherapy were excluded from the study. Informed consent was obtained from every patient before inclusion into the present study.
Between April 1994 and May 1999, 137 patients were assigned to receive either neoadjuvant radiochemotherapy with (68 patients (49.6%)) or without hyperthermia (69 patients (50.4%)) for locally advanced rectal cancer. Each patient was asked to answer the questions of a GIQLI questionnaire at four different time points (TP). Forty-six patients (33.6%) filled out a GIQLI questionnaire at every time point: before and after neoadjuvant therapy, early after surgery and after long-term follow-up. These patients were included in the present study. Fourteen patients (30.4%) were recruited during the phase II trial, 32 patients (69.6%) were randomized during the phase III trial. Twenty-seven patients (58.6%) were treated in the hyperthermia (HRCT) group, 19 patients (41.3%) were included in the group without hyperthermia (RCT). summarizes patient characteristics of both groups. There were no statistically significant differences between the two groups at a significance level of 95% (i.e. p < 0.05).
Table I. Patient and treatment characteristics.
Study design
Patients were randomly assigned the standard treatment protocol consisting of neoadjuvant radiochemotherapy, followed by surgical resection and adjuvant chemotherapy or a treatment protocol adding hyperthermia to the standard neoadjuvant therapy.
Radiotherapy, neoadjuvant and adjuvant chemotherapy as well as surgery was performed as described by Rau et al. Citation[11].
Regional hyperthermia.
Regional hyperthermia was applied at weekly intervals using the BSD 2000s (BSD Medical Corporation, Salt Lake City, Utah) SIGMA 60-ring applicator. This system operates via radiofrequency at 90 MHz with a 20–40° phase delay on the pair of antennae at the dorsum and a 5–20° delay on the lateral pairs. Endoluminal thermometry catheters were placed in the rectum, bladder and, when possible, vagina. Beginning of the therapeutic period was defined as the moment when one tumour-related measurement point reached 42°C or 30 min after the power was turned on, whichever was earlier. Temperature position curves were recorded along the catheters at intervals of 5–10 min. Index temperatures (Tx) were determined and, during the therapeutic period, the mean Tx values were calculated with respect to the time. The time during which the index temperature of more than 90% of measurement points in contact with the tumour remained above the reference temperature of 40.5°C was determined and expressed as cumulative minutes (cum min T90). Of 27 HRCT patients, 12 (44.4%) received hyperthermia with a Tx superior to 40.5°C. In eight patients (29.6%), the index temperature remained superior to this value longer than 120 min. Patients receiving hyperthermia treatment with those parameters were shown to have a significantly better prognosis than patients treated with lower Tx and cum min T90 Citation[11]. Either four-to-five hyperthermia sessions were given per patient. The hyperthermia treatment was performed under supervision of a radiotherapist (JG, PW).
Radiotherapy.
The radiotherapy was performed using an open table-top device with the patient in a prone position 15–20 min after hyperthermia. A three-field technique with lateral wedge filters was employed. Individualized blockings were used to protect lateral field corners, dorsal soft tissue and, whenever necessary, cranial ventral parts of the small intestine. The upper field border was positioned at level L5–S1, dependent on the location of the primary tumour. The ventral border was chosen according to the location of the tumour and its infiltration in surrounding structures. Radiation was delivered 5 days a week with a fraction of 1.8 Gy to the reference point. Total dose to the reference point was 45 Gy, with a maximum dose of less than 50 Gy.
Chemotherapy.
Chemotherapy was administered in two cycles on days 1–5 and 22–26 before irradiation or during hyperthermia. Fifty milligrams of leucovorin was given by intravenous infusion over 30 min, followed by a 5-fluorouracil bolus (300 mg m−2 per day in the first course and 350 mg m−2 in the second). This regimen was also applied post-operatively.
Toxicity and post-operative complications
Toxicity attributable to chemotherapy was classified according to WHO Citation[21] and adverse reactions caused by radiotherapy were graded according to the LENT SOMA tables Citation[22]. Patients were re-evaluated at weekly intervals prior to continuation of the protocol and the dose of 5-FU was reduced by 25% when toxicity of >WHO grade 2 occurred during the preceding course or when toxicity of >WHO grade 1 was ongoing on the first day of the following cycle. Detailed information concerning frequency and severity of toxicity is listed in . Even if toxicity seems to occur slightly more frequently in the HRCT group, these differences did not reach statistical significance.
Table II. Toxicity during neoadjuvant therapy, classified according to the WHO toxicity scheme.
In the RCT group, one neoadjuvant radiochemotherapy (5.3%) was discontinued due to severe gastrointestinal side effects. In the HRCT group, hyperthermia had to be interrupted in four cases (14.8%) because of cutaneous or gastrointestinal side effects, in six further patients (20.2%) hyperthermia was definitively discontinued due to side effects or patient refusal.
Post-operative complications were documented by the treating physicians during the whole hospital stay and on follow-up visits. Post-operative complications occurred in 48.1% of patients with and 31.6% of patients without hyperthermia. Detailed complications are listed in .
Table III. Post-operative complications.
Follow-up
After completion of therapy, patients were followed-up by clinical and laboratory evaluation (including CEA serum levels) and abdominal ultrasound every 3 months during the first 2 years of follow-up and every 6 months thereafter. Evaluations where completed by endorectal ultrasound in patients who underwent anterior resection. Chest X-ray was performed every 6 and colonoscopy was performed every 12 months, as well as CT or MRI of pelvis and/or abdomen every 12 months. Imaging was advanced if patients presented with clinical signs of recurrence or progression. Local recurrence was defined as evidence of tumour within the lower pelvis (including anastomotic region) or the perineal scar. Distal recurrence was defined as evidence of tumour in any other area.
Assessment of quality of life
The Gastrointestinal Quality-of-life Index was chosen to assess the QoL of both patient groups. This index is a system-specific index developed and validated to be employed in patients with benign and malignant diseases of the gastrointestinal tract Citation[16]. Briefly, it consists of 36 items that are grouped in five domains: symptoms, emotions, physical function, social life and medical treatment. Maximal number of obtainable points is 164, the results reported here are calculated as a percentage of this maximum. A higher GIQLI score corresponds to a higher quality of life. Symptoms and restrictions with negative influence on the subjective well-being of the patient result in a reduced GIQLI score. The ‘symptoms of disease’ domain contains 19 items and measures the effects of disease symptoms with high prevalence in the target population, including presence of frequent stools, diarrhoea, flatulence, etc. The ‘emotional well-being’ domain includes five questions and assesses the individual emotional reaction on the disease and the presence of frustration, satisfaction, anxiety, stress, etc. The ‘physical function’ domain contains seven questions and assesses global physical symptoms as in physical strength, fitness, endurance, fatigue, frequent nocturnal arousal and the perception of the own physical appearance. The ‘social function’ domain comprises four questions and assesses the influence of the disease state on social relations of the patient, the activities in the sexual life, professional life and the private life as well as contacts with close friends and relatives. Finally, the ‘medical treatment’ domain contains six questions and measures the perception of the therapeutic process by the patient. The GIQLI has been validated and tested for its reproducibility. Its sensitivity has been shown to be sufficient to assess the influence of routine therapeutic interventions.
Patients were asked to answer the questions of the Gastrointestinal Quality-of-life Index questionnaire at four time points: before the start of the neoadjuvant therapy (TP1; mean: 88 ± 2 days before surgery), after the end of the neoadjuvant therapy (TP2; mean: 9 ± 2 days before surgery), early after the surgical intervention (TP3; mean: 53 ± 4 days after surgery) and during long-term follow-up (TP4; mean: 849 ± 77 days after surgery). The GIQLI questionnaire was handed out to all patients at each time point. Participation of patients was voluntary. Incomplete series of answered questionnaires were excluded from the analysis.
Statistical analysis
To assess possible differences in the patient groups assigned to HRCT and RCT, characteristics of both groups were compared. For the comparison of categorical variables, Chi square tests were applied, for the continuous variables the t-test was used after verification of Gaussian distribution by the Kolmogorov-Smirnov-test. If the variables showed no Gaussian distribution, the Mann-Whitney test was applied.
GIQLI scores are reported in percentages to the maximally obtainable point number (144 points). If single responses were missing, the maximal point number was reduced by the corresponding point number and the percentage was calculated based on the new maximal point number. The mean number of responses obtained per domain and TP are summarized in . Comparison of GIQLI values between the two treatment groups was done by the non-parametric Mann-Whitney test. For longitudinal analysis of the GIQLI values within one treatment group, the Wilcoxon test was applied. All tests were two-tailed and statistical significance was defined as p < 0.05.
Table IV. Number of items assessed per domain of the GIQLI. The table depicts mean number and standard deviation of answered questions per domain of the GIQLI. TP1: before start of neoadjuvant therapy; TP2: after neoadjuvant therapy but before surgery; TP3: early after surgery; TP4: during long-term follow-up.
The SPSS 11.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Quality of life after HRCT vs. RCT
The global GIQLI scores for each time point are listed in and are depicted in . The global GIQLI scores in the HRCT and RCT group showed no statistically significant differences at any time point. When considering only those patients who completed the planned neoadjuvant treatment (HRCT: 21; RCT: 18), the global GIQLI scores remained without significant differences between both treatment groups. Within the HRCT group, no statistically significant differences of global GIQLI scores occurred between patients having received hyperthermia with a T90 below or above 40.5°C or a cum min T90 below or above 120 min.
Figure 1. Global GIQLI score of patients during the treatment of advanced rectal cancer. TP1: before start of neoadjuvant therapy; TP2: after neoadjuvant therapy but before surgery; TP3: early after surgery; TP4: during long-term follow-up.
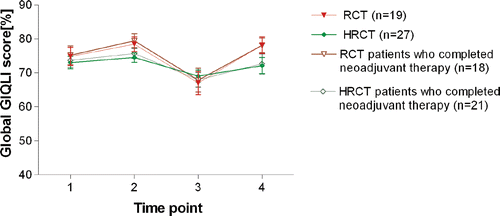
Table V. Global GIQLI score in uT3/uT4 rectum cancer patients before, during and after therapy. Reported scores are mean scores of the groups specified above.
Interestingly, single domains of the GIQLI showed differences at some time points (). Before the start of neoadjuvant therapy none of the domains of the GIQLI score (Symptoms of disease, Emotional features, Physical functionality, Social life, Medical treatment) differed significantly between the groups. After pre-operative therapy (TP2) HRCT patients reported lower scores in all domains with the exception of the domain ‘Emotions’. They complained more frequently about symptoms of disease than RCT patients and consequently had lower QoL values in the domain ‘Symptom’ (82.0% vs. 87.5%). There was also a tendency for lower ‘Social life’ and ‘Physical function’ scores in the HRCT group at TP2 (HRCT: 67.4% vs. RCT: 75.1%; and HRCT: 55.3% vs. RCT: 52.4%, respectively). Satisfaction with the medical treatment was lower in the HRCT group (HRCT: 63.9% vs. RCT: 73.6%). However, none of these differences were of statistical significance.
Figure 2. GIQLI domains compared between patients undergoing neoadjuvant therapy with vs. without hyperthermia. (a) ‘symptoms of disease’; (b) ‘emotion’; (c) ‘physical function’; (d) ‘social life’; (e) ‘medical treatment’.
Early after surgery (TP3), HRCT patients presented with higher scores in the domain ‘Emotions’ than RCT patients (66.3% vs. 58.5%, respectively). This difference reached statistical significance (p = 0.047). The remaining domains showed no significant difference between the patient groups at TP3.
Although there was no difference in the global GIQLI score in the long-term follow-up, the ‘Symptoms’ score was slightly lower in the HRCT group (78.4% vs. 85.1%, p = 0.028). No significant difference in the remaining domains was detected at TP4.
Variations of GIQLI rates during the progress of therapy
During the progression of therapy, the global GIQLI score of patients in both groups showed a characteristic and similar evolution. In the HRCT as well as in the RCT group, global GIQLI score remained almost unchanged at TP2 compared to TP1. In contrast, a significant reduction of the GIQLI scores occurred in both groups early after the surgical therapy (TP2: 74.5% vs. TP3 69.1%, p = 0.018 in the HRCT group, TP2: 78.5% vs. TP3: 67.0%, p = 0.002% in the RCT group). In the long-term follow-up, both treatment groups reported GIQLI scores comparable to those before the initiation of neoadjuvant therapy.
The GIQLI domains showed a characteristic development as well. In the HRCT group, the ‘Symptom’ score () remained almost unchanged after neoadjuvant therapy (TP1: 80.3% vs. TP2: 82%, p = 0.241). In contrast, patients receiving RCT showed a tendency to improved ‘Symptom’ scores (TP1: 81.7% vs. TP2: 87.5%) However, this change did not reach statistical significance (p = 0.155). In both groups, the symptoms score dropped to similar levels early after surgery (HRCT: 79.0% and RCT: 77.9%). This reduction reached statistical significance only in the RCT group (p = 0.031). In the long-term follow-up, patients of both groups reached values comparable to those reported before the start of neoadjuvant treatment ().
The ‘Emotion’ score reached its minimum before the initiation of neoadjuvant therapy in both groups (, HRCT: 56.4%; RCT: 54.7%). During neoadjuvant therapy, the ‘emotion’ score improved in both treatment groups (HRCT: 63.9%; RCT: 64.2%), but this increase reached statistical significance only in the HRCT group (p = 0.027). Throughout the further treatment, no statistically significant changes in this domain occurred in either group. The value at TP4 was significantly higher than at the initial assessment at TP1 (HRCT 68.2%, p = 0.001; RCT: 69.7%, p = 0.038) in both groups.
Both groups presented with similar ‘Physical function’ scores at TP1 (HRCT: 72.0%, RCT: 73.4%, ). During the neoadjuvant therapy, HRCT patients experienced a large reduction of this domain (HRCT: 66.6%), whereas patients of the RCT group showed only a slight drop (RCT 71.2%). However, in neither group the observed difference reached statistical significance (p = 0.092). Early after surgery, the domain ‘Physical function’ reached its lowest level and this drop was significant in both treatment groups (HRCT: 55.3%, p = 0.002; RCT: 52.4%, p = 0.001). In contrast, at TP4 ‘Physical function’ scores increased significantly (HRCT: 63.4% and RCT: 69.7%) without reaching the initial values at TP1. This increase reached statistical significance in the RCT-group (p = 0.002).
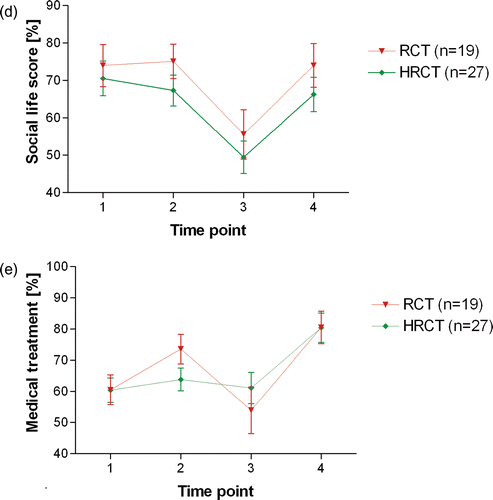
The domain ‘Social life’ reached highest values at TP1 (70.6%) in both groups (HRCT: 70.6%; RCT: 74.0%, ). This score remained stable during neoadjuvant therapy. Early after surgery, there was a highly significant reduction of this domain in both treatment groups (HRCT: 49.5%, p = 0.004; RCT: 55.6%, p = 0.002). However, after long-term follow-up, both treatment groups presented with ‘Social life’ scores only slightly inferior or similar to those at TP1 (HRCT: 66.3%, RCT: 74.1%). This represents a significant amelioration in both groups compared with TP3 (HRCT: p = 0.006; RCT: p = 0.023).
The domain ‘medical treatment’ behaved differently in both groups (). In the HRCT group, this domain showed almost no variation during the first three TP. Only at TP4 a significant amelioration of this domain did occur (HRCT: 80.4% vs. 60.4% at TP1, p = 0.013). In contrast, RCT patients showed a significant increase of this score at TP2 (73.6%) compared with TP1 (60.5%, p = 0.029) and a tendency to decreased values at TP3 compared to TP2 (53.9%). However, as in the HRCT group, RCT patients judged the medical treatment more positively at TP4 (80.6%, p = 0.009 compared to TP1).
Influence of the surgical procedure on quality of life
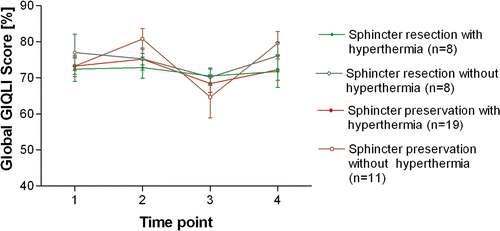
The comparison of the GIQLI scores of patients undergoing sphincter-preserving surgery with those of patients without sphincter preservation showed no differences neither in the early post-operative period nor in the long-term follow-up in both the HRCT and RCT group (). Analysis of single score items revealed no differences after surgery.
Influence of WHO grade 3 and 4 toxicity on quality of life
The occurrence of severe toxicity may be an important factor influencing the well-being. Patients having suffered WHO grade 3 and 4 toxicities were compared to those having experienced low grade (WHO 1 and 2) or no toxicity. There were significant differences in the global GIQLI score at TP2 (with toxicity: 71.7%, without toxicity: 78.1%; p = 0.027). However, at later time points, this difference between the global GIQLI indexes disappeared.
When patients, having experienced grade 3–4 toxicity, were compared with patients with grade 1–2 or without toxicity within the treatment groups, no significant differences in the global GIQLI indexes appeared neither in the HRCT nor in the RCT group.
Influence of peri-operative complications on quality of life
Surprisingly, patients having suffered from peri-operative complications had global GIQLI scores comparable to those scores of patients without peri-operative complications at any TP ().
Table VI. Influence of postoperative complications and histopathological response on global GIQLI scores in uT3/uT4 rectum cancer patients.
Influence of response in quality of life
Global GIQLI indexes from patients with a positive histopathological response (CR/PR) were not statistically different from those of patients with negative histopathological response (NC/PD) ().
When patients having experienced a positive histopathological response where compared with patients with negative histopathological response within the treatment groups, no significant differences in the global GIQLI indexes appeared neither in the HRCT nor in the RCT group (data not shown).
Influence of recurrence on quality of life at TP4
At TP4, 11 patients had developed distant metastasis or recurrence. While the global GIQLI scores of patients without recurrence or distant metastasis was 75.9 ± 12.3%, the scores of patients with recurrence or distant metastasis was lower, with 70.3 ± 10.0%. However, this difference was statistically not significant (p = 0.111).
Sexual life after HRCT vs. RCT
When the answers on the question assessing the impairment of the sexual life within the domain social life were considered individually, no statistically significant differences occurred between patients after RCT and HRCT (data not shown).
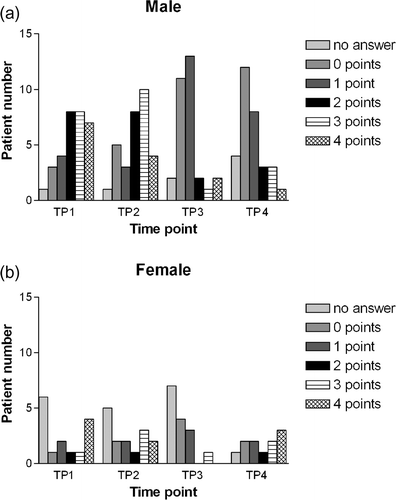
Interestingly, women refused to answer the question on the impairment of sexual functioning significantly more often than men at TP1 (men: 3.2%; women: 40%, p = 0.003), TP2 (men: 3.2%; women: 33.3%, p = 0.01) and TP3 (men: 6.5%; women: 46.7%, p = 0.003). On TP4, this difference was not statistically significant (men: 12.9%; women: 33.3%, p = 0.127). However, those men and women who answered on the question showed similar impairment of their sexual life at any TP of the current study ().
Discussion
In vivo and in vitro studies have reported synergistic effects of hyperthermia combined with radiotherapy alone or radiochemotherapy Citation[23–28] and a growing number of clinical trials shows the feasibility and efficiency of neoadjuvant hyperthermic radio- or radiochemotherapy Citation[13–15], Citation[29–32]. Although a considerable body of data concerning ‘objective’ outcome after neoadjuvant RCT and HRCT is available now, no data are available on the QoL of patients in these treatment groups.
Comparing QoL after neoadjuvant radiochemotherapy with and without hyperthermia revealed similar global GIQLI scores in patients of both treatment groups at any time assessed in this study. Although hyperthermia causes relatively few toxicities compared to radio- and chemotherapy alone, this treatment may give rise to non-negligible anxiety and general discomfort of the patient Citation[31]. Pain may occur due to the ‘hot-spot phenomenon’. These hot-spots, induced by heating at electrical interfaces in deep tissular layers Citation[33], cause acute to sub-acute side effects and may thus considerably reduce the patient's well-being Citation[34], Citation[35]. In spite of the risk of undesirable effects and inconvenience caused by hyperthermia induction, the application of hyperthermia during neoadjuvant therapy did not result in a significant reduction of QoL, neither concomitantly nor later in the therapeutic process. However, individual domains of the GIQLI index showed characteristic differences at TP2. The domains ‘Symptoms’, ‘Physical function’, ‘Social life’ and ‘Medical treatment’ showed a tendency towards reduced values at TP2 in the HRCT group. The domain ‘Symptom’ assesses the presence of gastrointestinal symptoms of the disease, e.g. defecation-related problems and nutritional problems. Local perineal skin irritation and gastrointestinal side effects were slightly more frequent in HRCT patients, possibly accounting for the reduced ‘Symptom’ score seen in this group of patients. However, this reduced score later returned to values comparable to those of the RCT group and is a temporary problem without negative long-term effects on the QoL of the HRCT group. Satisfaction with medical treatment may be affected by these unpleasant side effects of the therapy, resulting in the slight decrease in the domain ‘Medical treatment’ at TP2. In the literature, comparable studies in rectum cancer patients comparing neoadjuvant treatment regimes with and without hyperthermia are not available. However, in accordance with the results, van Vulpen et al. Citation[36] found no significant impact of hyperthermia on QoL in prostate cancer patients treated with radiotherapy with and without hyperthermia.
The longitudinal analysis of the global GIQLI score showed almost constant values during neoadjuvant therapy. In contrast, a significant drop of the global GIQLI score occurred after surgery in both treatment groups. This seems evident, since surgery may generate a feeling of loss-of-control on decisions concerning the patient's own physical integrity. Surgery and subsequent post-operative therapy are profoundly invasive procedures and require hospitalization as well as the patient's exclusion from his social environment. Accordingly, the score of the domains ‘Symptom’, ‘Physical function’ and ‘Social life’ were highly reduced in both treatment groups, and the ‘Physical function’ and ‘Social life’ scores even reached their minimum. Consequently, satisfaction with medical treatment was also highly reduced. In the long-term follow-up, the GIQLI scores reached pre-therapeutical values in both treatment groups. In contrast to the domains ‘Symptom’, ‘Social life’ and ‘Physical function’, the domain ‘Emotions’ showed lowest values immediately after the diagnosis of cancer and before neoadjuvant therapy and reached highest values at TP4. This may reflect not only a result of the medical treatment, but also of the development of effective coping mechanisms by the patients who, after the initial shock of the diagnosis ‘cancer’, learn to live with their disease Citation[37]. As the domain ‘Emotion’, the domain ‘Medical Treatment’ reached highest values at TP4. However, due to the non-compulsory character of this study, it cannot be excluded that patients with effective coping mechanisms were more inclined to participate in the present survey, thus inducing a recruitment bias towards improved QoL scores.
Very interestingly, the QoL of patients with sphincter resection was not significantly different from that of patients with sphincter preservation, neither in the HRCT group nor in the RCT group. Especially during long-term follow-up, both groups had comparable GIQLI scores. The influence of sphincter preservation on QoL was subject of intense controversy. Most of the earlier studies did not employ standardized and validated QoL scores and tended to find advantages for anterior resection with preserved sphincter. In particular single items as distress, body image, single items of social functioning or sexual dysfunction were reported to be more problematic in patients with abdominoperineal resection Citation[38], Citation[39]. Other investigators found no differences between the two treatment groups Citation[40], Citation[41]. However, these observations described a multitude of single symptoms rather than the multi-dimensional and subjective feature ‘quality of life’. More recent trials using modern QoL scoring systems (e.g. the European Organization for research and Treatment of Cancer QLQ-C30, its colorectal module QLQ-CR38 and the Duke generic instrument) found no significant differences in the global QoL scores Citation[42–44]. In a recent study performed in the department, patients who underwent abdominoperineal extirpation had no poorer QoL than patients having undergone anterior resection Citation[45]. Patients after low anterior resection had even lower QoL scores than patients after abdominoperitoneal extirpation. The present results confirm the observation that loss of the natural sphincter does not necessarily lead to a reduced QoL.
Severe toxicity, occurring during neoadjuvant therapy, was shown to affect the QoL, but only very punctually and without any long-term effect. At TP3 and TP4, the global GIQLI score of patients with severe toxicity returned to levels comparable to those of patients who had not experienced significant toxicity. Surprisingly, the occurrence of peri-operative complications did not influence the global QoL scores in the patient group during the early post-operative period nor during long-term follow-up. Histopathological response did not correlate with the post-operative or the long-term follow-up GIQLI score. This observation underlines that the objectively measurable success of the treatment is a predictor of QoL of lower importance on a short-term basis than the side effects of the treatment.
Detrimental effects on sexuality is a recognized side effect of the therapy of rectal cancers. In the reported trial, the impairment of the sexual functioning was not significantly different in both treatment groups. Interestingly the question on sexual dysfunction was answered significantly less frequently by female patients. In contrast to reports of other investigators Citation[46], female patients experienced similar sexual impairment secondary to the disease and the treatment as did male patients.
The absence of a direct correlation between the GIQLI score and objective measurable factors, such as therapeutic success or severity of disease, emphasizes the fact that QoL is not the mere result of the medical treatment. It results from the difference between the present health status perceived by the individual patient, his expectations of his physical capacity, the anxiety when he faces the diagnosis of cancer and the necessity of highly invasive therapeutic procedures Citation[37], Citation[47]. The patient may also adapt to certain disabilities and thus improve its QoL. The ‘well-being-paradox’ describes the phenomenon that even very unfavourable living conditions or debilitating diseases have only very limited influence on the subjective QoL Citation[48]. However, even though the QoL reflects the result of a complex interaction of the patient's personality and experiences, his social life, the disease and the necessary therapy, it may be, from a patient's point of view, a parameter as important as the classical biological parameters commonly used to judge the quality of a therapeutic concept.
The selection of patients having completed GIQLI questionnaires at all four TP for further analysis may have induced a bias of study results by choosing patients with a favourable course of disease or treatment results. However, characteristics from the patients having completed questionnaires at all four TP did not differ from those of patients not having completed all four TPs (age, gender, tumour characteristics, surgery, histopathological response). Particularly in regard to the occurrence of toxicity, complications or interruption of neoadjuvant treatment, no significant differences between those two groups could be observed (data not shown). Furthermore, the results of the present study may be biased by the fact that only 32 patients (69.6%) were treated in the phase III trial and the remaining 14 patients (30.4%) were included during the preceding phase II trial. However, the global QoL scores of phase II and phase III patients did not differ significantly in the present study, thereby excluding a significant effect of the partial randomization.
In summary, this is the first report on the quality of life in patients treated with neoadjuvant radiochemotherapy and hyperthermia for locally advanced rectal cancer. The results presented here show that adding hyperthermia to the neoadjuvant treatment regimen with its potential side effects does not result in a reduction of QoL. In the long-term follow-up, both groups report equally good QoL scores.
Acknowledgements
We thank Mrs Uta Goerling for critical review and discussion of the manuscript.
References
- Roy DJ. Language, meaning, and ethics. J Palliat Care 1992; 8: 3
- Sloan JA, Loprinzi CL, Kuross SA, Miser AW, Of JR, Mahoney MR, Heid IM, Bretscher ME, Vaught NL. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol 1998; 16: 3662–3673
- Cella D, Chang CH, Lai JS, Webster K. Advances in quality of life measurements in oncology patients. Semin Oncol 2002; 29: 60–68
- McDermott FT, Hughes ES, Pihl E, Johnson WR, Price AB. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg 1985; 72: 34–37
- Benotti P, Steele G, Jr. Patterns of recurrent colorectal cancer and recovery surgery. Cancer 1992; 70: 1409–1413
- Chari RS, Tyler DS, Anscher MS, Russell L, Clary BM, Mathorn J, Seigler HF. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995; 221: 778–786
- Rodel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol 2003; 21: 3098–3104
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittebind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740
- Rau B, Wust P, Riess H, Schlag PM. [Preoperative radiochemotherapy of rectal carcinoma. Current status]. Zentralbl Chir 2000; 125: 356–364
- Rau B, Gaestel M, Wust P, Stahl J, Mansman U, Schlag PM, Benndorf R. Preoperative treatment of rectal cancer with radiation, chemotherapy and hyperthermia: Analysis of treatment efficacy and heat-shock response. Radiat Res 1999; 151: 479–488
- Rau B, Wust P, Hohenberger P, Loffel J, Hunerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, Felix R, Schlag PM. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: A phase II clinical trial. Ann Surg 1998; 227: 380–389
- Takahashi T, Horie H, Kojima O, Itoh M. Preoperative combined treatment with radiation, intraluminal hyperthermia, and 5-fluorouracil suppositories for patients with rectal cancer. Surg Today 1993; 23: 1043–1048
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia 1990; 6: 881–890
- You QS, Wang RZ, Suen GQ, Yan FC, Gao YJ, Cui SR, Zhao JH, Zhao TZ, Ding L. Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: Long-term results of 44 patients. Int J Hyperthermia 1993; 9: 19–24
- Eypasch E, Wood-Dauphinee S, Williams JI, Ure B, Neugebauer E. [The Gastrointestinal Quality of Life Index. A clinical index for measuring patient status in gastroenterologic surgery]. Chirurg 1993; 64: 264–274
- Madisch A, Heymer P, Voss C, Wigginghaus B, Bastlein E, Bayerdorffer E, Meier E, Schimming W, Bethke B, Stolte M, Miehlke S. Oral budesonide therapy improves quality of life in patients with collagenous colitis. Int J Colorectal Dis 2005; 20: 312–316, Epub 2004 Nov 2011
- Nietert PJ, Mitchell HC, Bolster MB, Curran MY, Tilley BC, Silver RM. Correlates of depression, including overall and gastrointestinal functional status, among patients with systemic sclerosis. J Rheumatol 2005; 32: 51–57
- Schwenk W, Neudecker J, Haase O, Raue W, Strohm T, Muller JM. Comparison of EORTC quality of life core questionnaire (EORTC-QLQ-C30) and gastrointestinal quality of life index (GIQLI) in patients undergoing elective colorectal cancer resection. Int J Colorectal Dis 2004; 19: 554–560, Epub 2004 Jun 2016
- Maartense S, Dunker MS, Slors JF, Cuesta MA, Gouma DJ, van Deventer SJ, van Bodegraven AA, Bemelman WA. Hand-assisted laparoscopic versus open restorative proctocolectomy with ileal pouch anal anastomosis: A randomized trial. Ann Surg 2004; 240: 984–991
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–214
- EORTC. LENT SOMA tables. Radiother Oncol 1995; 35: 17–60
- Ohno S, Tomoda M, Tomisaki S, Kitamura K, Mori M, Maehara Y, Sugimachi K. Improved surgical results after combining preoperative hyperthermia with chemotherapy and radiotherapy for patients with carcinoma of the rectum. Dis Colon Rectum 1997; 40: 401–406
- Bisht KS, Uma Devi PU. Modification of radiation-induced chromosome damage and micronucleus induction in mouse bone marrow by misonidazole and hyperthermia. Acta Oncol 1995; 34: 913–918
- Nevaldine B, Longo JA, Hahn PJ. Hyperthermia inhibits the repair of DNA double-strand breaks induced by ionizing radiation as determined by pulsed-field gel electrophoresis. Int J Hyperthermia 1994; 10: 381–388
- Kido Y, Kuwano H, Maehara Y, Mori M, Matsuoka H, Sugimachi K. Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol 1991; 28: 251–254
- Tsumura M, Yoshiga K, Takada K. Enhancement of antitumor effects of 1-hexylcarbamoyl-5-fluorouracil combined with hyperthermia on Ehrlich ascites tumor in vivo and Nakahara-Fukuoka sarcoma cell in vitro. Cancer Res 1988; 48: 3977–3980
- Dewey WC. Interaction of heat with radiation and chemotherapy. Cancer Res 1984; 44: 4714s–4720s
- Korenaga D, Matsushima T, Adachi Y, Mori M, Matsuda H, Kuwano H, Sugimachi K. Preoperative hyperthermia combined with chemotherapy and radiotherapy for patients with rectal carcinoma may prevent early local pelvic recurrence. Int J Colorectal Dis 1992; 7: 206–209
- Furuta K, Konishi F, Kanazawa K, Saito K, Sugawara T. Synergistic effects of hyperthermia in preoperative radiochemotherapy for rectal carcinoma. Dis Colon Rectum 1997; 40: 1303–1312
- Rau B, Wust P, Gellermann J, Tilly W, Hunerbein M, Loffel J, Stahl H, Riess H, Budach V, Felix R, Schlag P. [Phase II study on preoperative radio-chemo-thermotherapy in locally advanced rectal carcinoma]. Strahlenther Onkol 1998; 174: 556–565
- Schaffer M, Krych M, Pachmann S, Abdel-Rahman S, Schaffer PM, Ertl-Wagner B, Dh E, Issels RD. Feasibility and morbidity of combined hyperthermia and radiochemotherapy in recurrent rectal cancer—preliminary results. Onkologie 2003; 26: 120–124
- Wust P, Stahl H, Loffel J, Seebass M, Riess H, Felix R. Clinical, physiological and anatomical determinants for radiofrequency hyperthermia. Int J Hyperthermia 1995; 11: 151–167
- Petrovich Z, Langholz B, Gibbs FA, Sapozink MD, Kapp DS, Stewart RJ, Emami B, Oleson J, Senzer N, Slater J, et al. Regional hyperthermia for advanced tumors: A clinical study of 353 patients. Int J Radiat Oncol Biol Phys 1989; 16: 601–607
- Feldmann HJ, Molls M, Adler S, Meyer-Schwickerath M, Sack H. Hyperthermia in eccentrically located pelvic tumors: Excessive heating of the perineal fat and normal tissue temperatures. Int J Radiat Oncol Biol Phys 1991; 20: 1017–1022
- Van Vulpen M, De Leeuw JR, Van Gellekom MP, Van Der Hoeven J, De Graeff A, Van Moorselaar RJ, Van Der Tweel I, Hofman P, Lagendijk JJ, Battermann JJ. A prospective quality of life study in patients with locally advanced prostate cancer, treated with radiotherapy with or without regional or interstitial hyperthermia. Int J Hyperthermia 2003; 19: 402–413
- Grumann M, Schlag PM. Assessment of quality of life in cancer patients: Complexity, criticism, challenges. Onkologie 2001; 24: 10–15
- Sprangers MA, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer. Stoma vs. nonstoma patients. Dis Colon Rectum 1995; 38: 361–369
- Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Quality of life in rectal cancer patients: A four-year prospective study. Ann Surg 2003; 238: 203–213
- Frigell A, Ottander M, Stenbeck H, Pahlman L. Quality of life of patients treated with abdominoperineal resection or anterior resection for rectal carcinoma. Ann Chir Gynaecol 1990; 79: 26–30
- Whynes DK, Neilson AR, Robinson MH, Hardcastle JD. Colorectal cancer screening and quality of life. Qual Life Res 1994; 3: 191–198
- Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. Quality of life among disease-free survivors of rectal cancer. J Clin Oncol 2004; 22: 354–360
- Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, Urban N. Quality of life in survivors of colorectal carcinoma. Cancer 2000; 88: 1294–1303
- Allal AS, Bieri S, Pelloni A, Spataro V, Anchisi S, Ambrosetti P, Sprangers MA, Kurtz JM, Gertsch P. Sphincter-sparing surgery after preoperative radiotherapy for low rectal cancers: Feasibility, oncologic results and quality of life outcomes. Br J Cancer 2000; 82: 1131–1137
- Grumann MM, Noack EM, Hoffmann IA, Schlag PM. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 2001; 233: 149–156
- Schmidt CE, Bestman B, Küchler T, Longo WE, Kremer B. Ten-year historic cohort of quality of life in patients with rectal cancer. Dis Colon Rectum 2005; 48: 483–492
- Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience?. BMJ 2001; 322: 1240–1243
- Herschbach P. [The ‘Well-being paradox’ in quality-of-life research]. Psychother Psychosom Med Psychol 2002; 52: 141–150