Abstract
Purpose: It has been known that the thermosensitivity of tumour cells can be increased by lowering intra-cellular pH (pHi) by inhibiting pHi control mechanisms. The pHi is partially controlled by transport of H+ from cytoplasm into endocytic and secretary systems in the cells mediated by vacuolar type H+ATPase and also by transport of H+ through plasma membrane.
Methods: This study investigated the effects the bafilomycine A1, an inhibitor of the vacuolar type H+ATPase and the EIPA, an inhibitor of the Na+/H+ exchanger in plasma membrane, on thermosensitivity of AsPC-1 cells, a human pancreatic cancer cell line. It also investigated the effects of combination of bafilomycine A1 and EIPA.
Results: The treatment of cancer cells with bafilomycine A1 or EIPA individually slightly lowered pHi of the cells in vitro and increased the thermosensitivity of the cells.
Conclusion: The combination of these two drugs significantly lowered pHi and increased thermosensitivity of cancer cells in vitro and enhanced the heat-induced the growth delay of AsPC-1 tumours grown s.c in the legs of BALB/cA nude mice.
Introduction
The intra-tumour environment is characterized by insufficient blood perfusion, poor nutritional supply, hypoxia and accumulation of acidic metabolites. These intra-tumour conditions, especially the low pH environment, are believed to enhance the response of tumours to hyperthermic treatment Citation[1–3]. It has been demonstrated that the intra-cellular acidity (pHi) but not the extra-cellular acidity (pHe) influences the thermosensitivity of cells Citation[4]. Song et al. Citation[5] previously reported that the thermosensitivity of tumour cells can be increased by inhibiting ion-channels at the plasma membrane, thereby lowering the pHi. For example by inhibitors of the Na+/H+ exchange and the Na+-dependent /Cl− exchange through the plasma membrane, major pHi regulatory mechanisms, sensitized cells to hyperthermia Citation[4], Citation[5].
The vacuolar type H+ ATPase transports cytoplasmic H+ ions into endocytic and secretory system such as endoplasmic reticulum and Golgi apparatus Citation[6]. Therefore, the pH of vacuolar system is maintained lower than that of cytoplasm. It has been reported that the acidic environment optimize various functions of vacuolar systems including synthesis of various enzymes such as a member of proteases and collagenases which are the arm for cancer to infiltrate into surrounding tissues. It may be expected that inhibition of transport of H+ ions from cytoplasm to vacuolar systems by inhibition of H+ ATPase would decrease pH of the cytoplasm. The present study investigated the effects of bafilomycin A1, an inhibitor of the vacuolar type H+ ATPase, on the thermosensitivity of a human pancreatic cancer cell line in vitro and in vivo. 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) is an inhibitor of the Na+/H+ exchange which also enhance thermosensitivity by decreasing pHi. Therefore, this study also investigated the thermosensitizing effect of a combination of bafilomycin A1 and EIPA.
Materials and methods
Cancer cell line and culture conditions
The AsPC-1 cells, a moderately differentiated human ductal pancreatic adenocarcinoma, were obtained from the American Type Culture Collection. Ohta et al. Citation[7], Citation[8] previously reported that the vacuolar type H+ ATPases are over-expressed in AsPC-1 cells. The cells were maintained routinely in RPMI 1640 medium containing L-glutamine, 10% foetal bovine serum (GIBCO BRL) and antibiotics at 37°C in a 5% CO2 incubator.
Chemicals
Bafilomycin A1, 15-membered ring macrolides isolated from Streptomyces griseus, was purchased from Wako Pure Chemical Industries (Tokyo, Japan). 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), an analogues of amiloride (a diuretic drug), was obtained from Sigma Chemical Co. These two inhibitors were dissolved in dimethyl sulphoxide (DMSO) and then diluted to appropriate concentrations with the culture medium. The final concentration of DMSO in the medium was 1% or less. The 1% DMSO alone did not significantly inhibit the proliferation of AsPC-1 cells (date not shown). For in vivo studies, the inhibitors were dissolved in ethanol and then diluted to appropriate concentrations with PBS. The final concentration of ethanol in the medium was 1% or less.
Determination of pHi
The pHi of AsPC-1 cells in vitro was determined with the BCECF fluorescence spectroscopy method as previously described Citation[4], Citation[9] Citation[10]. Cultured AsPC-1 cells in exponential growth phase were dispersed to single cells with trypsin treatment, washed with Hank's solution and suspended in culture medium. Cell concentration was adjusted to 2 × 105 cells ml−1, 100 µl of the cell suspension were plated to each well in a 96-well flat-bottomed micro-titer plate (Coster, Cambridge, MA, USA) and incubated for 72 h at 37°C in a humidified 5% CO2 atmosphere. The cells were labelled with BCECF by adding 1 µl of 2',7'-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) in 1 mg ml−1 solution in DMSO and incubating at 37°C for 60 min. The cells were washed with Hank's solution to remove extra-cellular BCECF-AM and suspended in 100 µl of pH 7.4 or pH 6.8 HEPES-buffered RPMI 1640 medium containing 1 µM bafilomycin A1 or 10 µM EIPA. The plates were capped tightly and immersed in a 37°C water bath for 60 min. The fluorescence intensity of the cells was read at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using the Fluoroskan Ascent scanner. The ratios of the fluorescence intensities in the two wavelengths were converted to pHi using the calibration curve obtained in a separate study. The calibration curve was made from the date obtained from 24 measurements. The date was approved in every experiment.
Thermosensitizing effects
The thermosensitizing effect of bafilomycin A1 and EIPA on AsPC-1 cells was quantified using a MTT colorimetric assay Citation[11], Citation[12]. In brief, AsPC-1 cells in exponential growth phase were dispersed to single cells with trypsin treatment, washed with Hank's solution and resuspended in culture medium. Cell concentration was adjusted to 1 × 105 cells ml−1, 100 µl of the cell suspension was added to each well of a 96-well flat-bottomed micro-titer plate and the plate was incubated for 72 h at 37°C in a humidified 5% CO2 atmosphere. The culture medium was then removed and 100 µl of pH 6.8 or 7.4 HEPES-buffered RPMI 1640 medium containing 1 µM bafilomycin A1 and/or 10 µM EIPA were added to each well. The plates were capped tightly and immersed in a 37°C or 44°C water bath for varying lengths of time. After incubation for the pre-determined time, the plates were removed from the water bath and 100 µl of the culture medium was added to each well. The plates were then incubated for 48 h under a 5% CO2 atmosphere at 37°C. After incubation, 10 µl of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) in working solution (5 mg ml−1; Doujin chemical Industries) was added and incubated for 4 h. The solution was removed and 100 µl of DMSO was added to each well. The plates were shaken for 15 min to allow formazan dissolution and the absorbance was measured on an automatic micro-plate reader (Spectra Max 250) at 540 nm. Cell viability was calculated by the formula as follows:
Thermosensitivity of tumours in vivo
BALB/cA nude mice (Jcl-nu), ∼4 weeks old on arrival, were purchased from CLEA Japan, Tokyo and maintained under pathogen limited conditions in the animal experiments centre of Medical School, University of Fukui. AsPC-1 cells in exponential growth phase in culture were harvested with trypsin, washed and suspended in RPMI 1640 medium. About 4 × 105 cells in 0.05 ml of the medium without calf serum were injected s.c. into the right thigh of 6 week-old male mice. When tumours grew to ∼150 mm3, they were divided randomly into eight experimental groups: a control group, a bafilomycin A1 (1.0 mg kg−1) treated group, a EIPA (3.0 mg kg−1) treated group, a bafilomycin A1 (1.0 mg kg−1) and EIPA (3.0 mg kg−1) treated group, a heated-only group, a heated group treated with bafilomycin A1 (1.0 mg kg−1), a heated group treated with EIPA (3.0 mg kg−1), a heated group treated with bafilomycin A1 (1.0 mg kg−1) and EIPA (3.0 mg kg−1). The non-heated groups received i.p. injections of 1.0 mg kg−1 of bafilomycin A1 and/or 3.0 mg kg−1 of EIPA or PBS. The heated groups were injected IP with the drugs and tumours were heated beginning 60 min. For the tumour heating, the mice were anaesthetized with 10% pentobarbital sodium 1.3 ml per 100 g BW injected IP and mounted on plastic jigs and secured with adhesive tape. The tumour-bearing legs were gently extended along the leg holders attached to the jigs and one toe of the leg was secured with surgical silk to the leg holder. The jigs were placed on plastic supports over a water bath and the tumour-bearing legs were submerged into 43°C water. After heating the tumours for 60 min, the mice were freed from the jigs and returned to their cages. The growth of tumours was determined by measuring the tumour diameters with a caliper. The tumour volume was calculated with the formula V = a2b/2, where a and b are the short and long diameters of the tumours, respectively Citation[13].
Statistical analysis
Data are expressed as mean ± SE. Statistical analysis of the pHi data was performed using the Bartlett's analysis and because the dispersion was unequal, the comparison between several groups was performed using 1-factor ANOVA. Statistical analysis of the tumour growth was performed using repeated measure ANOVA. The comparison between several groups was performed using Scheffe's F-test as a post-hoc test. A p-value less than 0.05 was considered to be significant.
Results
Changes in pHi
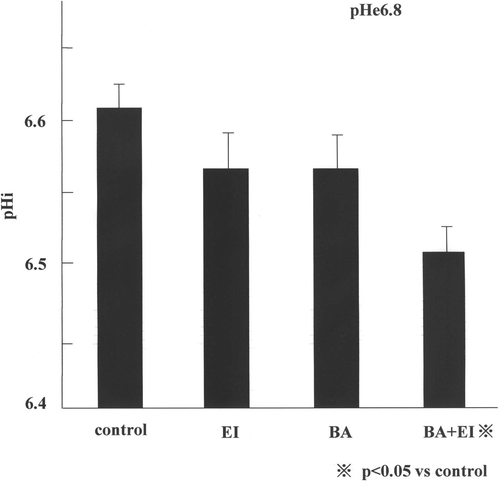
The results of the pHi study are shown in . When the AsPC-1 cells were exposed to the fresh pHe 7.4 medium for 60 min, the pHi was 6.70 ± 0.07. The pHi of the cells exposed to pHe 6.8 medium for 60 min was 6.6 0 ± 0.04 and exposure of cells to 10 µM EIPA alone and 1 µM bafilomycin A1 alone in pHe 6.8 medium caused a small decrease in pHi. However, the pHi of the cells exposed to the combination of 1 µM bafilomycin A1 and 10 µM EIPA in pHe 6.6 medium decreased to 6.50 ± 0.04, which was significantly lower than the pHi of other groups (p < 0.05).
Figure 1. The pHi of AsPC-1 cells incubated with bafilomycin A1 and/or EIPA at 37°C, 60 min. Shows the pHi in pHe 6.8 medium. Each data point is the mean ± 1 SE from 10 measurement. The pHi in the combination of the two drugs and in the pHe 6.8 medium was lower than any other experimental value and it was statistically significant (C; control, EI; 10 µM EIPA, BA; 1 µM bafilomycin A1, BA+ EI; 1 µM bafilomycin A1+ 10 µM EIPA).
The thermosensitizing effect of bafilomycin A1 or EIPA in vitro
The effects of 10 µM EIPA or 1 µM bafilomycin A1 alone or in combination on the viability of AsPC-1 cells heated/non-heated for varying length of time in pHe 7.4 medium are shown in . An incubation of cells with bafilomycin A1 and EIPA individually or in combination at 37°C did not significantly affect cell survival (). When heated at 44°C for 1 h without drug at pHe 7.4, the survival decreased significantly to 80% of the control value. When heated at pHe 7.4 in the presence of bafilomycin A1 and EIPA individually or in combination for 1 h, the survival decreased to ∼60% ().
Figure 2. Effect of bafilomycin A1 and/or EIPA on the thermosensitivity of AsPC-1 cells at pHe 7.4. (a) The bafilomycin A1 and/or EIPA effect at 37°C and (b) the drug effect after heating at 44°C. The data shown are mean from 15 measurements. The viability with/without the drugs or the heating in pHe 7.4 medium did not differ significantly from the control (C; control, EI; 10 µM EIPA, BA; 1 µM bafilomycin A1, BA+ EI; 1 µM bafilomycin A1+ 10 µM EIPA).
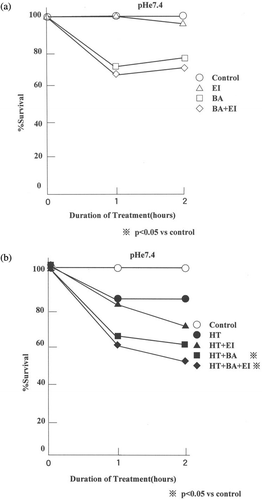
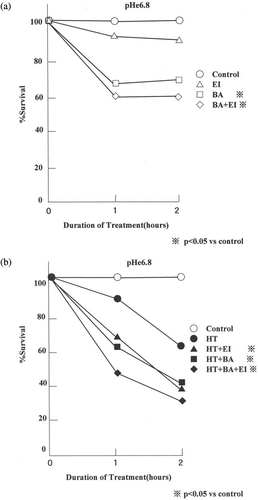
The effects of 10 µM EIPA or 1 µM bafilomycin A1 alone or in combination on the viability of AsPC-1 cells heated/non-heated for varying length of time in pHe 6.8 medium are shown in . shows the effect of bafilomycin A1 and/or EIPA on the viability of cells at 37°C at pHe 6.8 and shows the drug effect on the viability of cells treated at 44°C at pHe 6.8. The viability of cells decreased to 89.4% and 62.4% when heated in pHe 6.8 medium without the drugs for 1 and 2 h, respectively. The viability of cells heated with EIPA in pHe 6.8 medium decreased to 67.2% by 1 h and 38.5% by 2 h. The viability of cells heated with bafilomycin A1 in pHe 6.8 medium decreased to 61.4% by 1 h and 40.3% by 2 h. The viability of cells heated with the combination of two drugs in pHe 6.8 medium decreased to 46.6% by 1 h and 29.7% by 2 h. The viability of cells heated within the presence of both drugs in pHe 6.8 medium was lowest and was significantly different from others (p < 0.05).
Figure 3. Effect of bafilomycin A1 and/or EIPA on the thermosensitivity of AsPC-1 cells at pHe 6.8. (a) The bafilomycin A1 and/or EIPA effect after incubation at 37°C and (b) the drug effect after heating at 44°C. The data shown are mean from 15 measurements. The viability of heated cells in the combination of the two drugs was lower than any other viability (p < 0.05) (C; control, EI; 10 µM EIPA, BA; 1 µM bafilomycin A1, BA+ EI; 1 µM bafilomycin A1+ 10 µM EIPA).
The thermosensitizing effect of bafilomycin A1 or EIPA on tumour growth in vivo
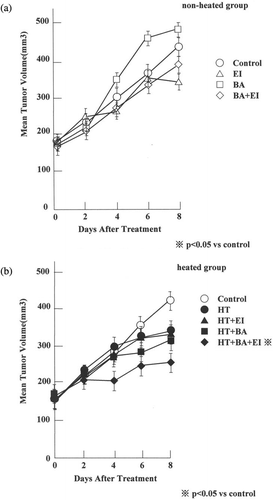
The effects of the drugs alone on the growth of AsPC-1 tumours are shown in . There were no significant differences between the growth rates of tumour treated with different drugs. The effect of heating at 43°C for 60 min on the growth of AsPC-1 tumours with or without prior injection of the drugs into the tumour-bearing mice are shown in . In 8 days, the control tumour volumes increased from ∼150 mm3 to 407.6 ± 21.2 mm3. During the same 8 days period, the volume of the tumour heated increased to 325.2 ± 52.8 mm3 while the tumour volume increased to 328.7 ± 34.0 mm3 when heated after an injection of EIPA, to 300.5 ± 40.4 mm3 when heated after an injection of bafilomycin A1 and to 217.2 ± 28.9 mm3 when heated after an injection of both EIPA and bafilomycin A1. The tumour volumes after heating alone or heating after an injection of EIPA or heating after an injection of bafilomycin A1 did not differ significantly from the control. However, heating after an injection of both bafilomycin A1 and EIPA inhibited the tumour growth significantly (p = 0.0071).
Figure 4. Effect of bafilomycin A1 and/or EIPA on the growth of AsPC-1 tumours. (a) The mean tumour volume of the non-heated group and (b) the mean tumour volume of the heated group. The mean value of eight tumours/groups are shown. Only heating after an injection of both bafilomycin A1 and EIPA ± 1 SE inhibited the tumour growth significantly, compared with controls (p = 0.0071) (C; control, EI; 3.0 mg kg−1 EIPA, BA; 1.0 mg kg−1 bafilomycin A1, BA+ EI; 1.0 mg kg−1 bafilomycin A1+ 3.0 mg kg−1 EIPA).
Discussion
Pancreatic cancer is a devastating disease with a poor prognosis. In some clinical trials, hyperthermia was demonstrated to be an effective treatment for pancreatic cancer. It has also been reported that a number of different drugs could enhance thermosensitivity of the tumour cells Citation[5], Citation[9], Citation[14], Citation[15]. Song et al. Citation[5], Citation[9], Citation[14] reported that the thermosensitivity of SCK mammary adenocarcinoma cells of A/J mice could be markedly enhanced when the pHi was lowered by simultaneously blocking the Na+/H+ exchange and the Na+-dependent /Cl− exchange with amiloride and DIDS, respectively. The authors have observed that an acidic environment kills tumour cells by apoptosis or necrosis depending on the degree of acidity. The present study investigated the effect of vacuolar type H+ ATPase on the thermosensitivity of human pancreatic cancer cells. It has been reported that the vacuolar type H+ ATPase, identified in organelles belonging to the central vacuolar system, is the most probable candidate for maintaining the intra-vacuolar system more acidic relative to the acidity of cytoplasm for optimal synthesis of various proteases and collagenase within the vacuolar system Citation[6]. Therefore, inhibitor of H+ ATPases is expected to retard the transfer of H+ ion to vacuolar system, thereby acidifying the cytoplasm. Ohta et al. Citation[16] reported that the vacuolar type H+ATPase is expressed in almost all human pancreatic ductal cancer tissues and many human pancreatic cancer cell lines. It has been known that intra-cellular acidity is maintained near neutral even in acidic environment by virtue of the presence of strong pHi control mechanisms Citation[17]. However, in the Aspc-1cells, the pHi was 6.6 when the cells were exposed to pHe 6.8 medium (). It is conceivable that the pHi control mechanism in Aspc-1 cells is unusual so that pHi is decreased to values lower than pHe when cells are exposed to an acidic environment.
The present study investigated the efficacy of bafilomycin A1, a vacuolar type H+ ATPase inhibitor, in lowering the pHi and sensitizing a pancreatic cancer cell line to hyperthermia. In addition, it investigated the effect of a combination of bafilomycin A1 and EIPA, an inhibitor of the Na+/H+ exchange through cell membrane. As shown in , pHi decreased in the presence of one of those drugs and decreased most significantly in the presence of both drugs at pHe 6.8 but not at pHe 7.4. It has been reported that acidic environment causes apoptosis in cells Citation[17]. However, in this study, as shown in and , little change occured in the viability of AsPC-1 cell when exposed to pH 7.4 and pH 6.8 medium for 2 h.
As shown in , bafilomycin A1 alone or EIPA alone was not effective enough to decrease the pHi of AsPC-1 cells in the pHe 6.8 in vitro. However, the decline in pHi by the combination of the two drugs at pHe 6.8 was statistically significant. These findings suggest that pHi was lowered most when the compensatory pHi regulatory mechanisms was lost by the inhibition of those two ion-channels, the Na+/H+ exchange in the plasma membrane and the vacuolar type H+ ATPase in organelles belonging to the central vacuolar system.
As shown in and , cell viability, as determined with MTT colourimetric method, was significantly lower after heating with EIPA or bafilomycin A1 or with the combination of two drugs than that after heating without the drugs. The decline in cell viability after heating in the combination of the two drugs was most pronounced in an acidic environment (). These results were interpreted to mean that the significant decrease in pHi by inhibiting the Na+/H+ exchange through the cell membrane and inhibiting the H+ import to vacuolar system sensitized the cells to hyperthermia treatment.
The in vivo study also demonstrated that an administration of EIPA alone or bafilomycin A1 alone had little effect on the response of tumour to hyperthermia. However, the combination of bafilomycin A1 and EIPA enhanced the heat-induced suppression of tumour growth (). Rhee et al. Citation[18] and Jayasundar et al. Citation[19] reported the change in pHe of tumour tissue by hyperthermia. Arakawa Citation[20] reported that chronic treatment with bafilomycin A1 at 1 mg kg−1 per day did not affect the growth of nude mice for up to 28 days and that no abnormality was histologically observed in liver, pancreas or kidney of the bafilomycin A1-treated mice.
In conclusion, the thermosensitivity of AsPC-1 cells in vitro could be increased by decreasing the intra-cellular pH with combination of two drugs, bafilomycin A1, the inhibitor of the vacuolar type H+ ATPase and EIPA, inhibitor of the Na+/H+ exchange. The combination of the two drugs markedly enhanced the effect of heating to suppress growth of AsPC-1 tumour zenografts in nude mice.
References
- Wike JL, Hooley W, Haveman J, Reinhold HS. The relevamce of tumour pH to the treatment of malignant disease. Radiothera Oncol 1984; 2: 343–366
- Song CW. Effect of local hyperthermia on blood flow and micro-environment: A review. Cancer Res 1984; 44: 4721s–4730s
- Song CW, Lyons JC, Luo Y. Intra- and extracellular pH in solid tumor: Influence of therapeutic response. Mech Drug Resist Oncol 1993; 25–52, Marcel Dekker, New York
- Lyons JC, Kim GE, Song CW. Modification of intracellular pH and thermosensitivity. Radiat Res 1992; 29: 79–87
- Song CW, Lyons JC, Griffin RJ, Makepeace CM, Cragoe EJ, Jr. Increase in thermosensitivity of tumor cells by lowering intracellular pH. Cancer Res 1993; 53: 1599–1601
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacular-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 1991; 266: 17707–17712
- Ohta T, Nakagawara H, Arakawa H, Futagami F, Tsukioka Y, Kitagawa H, Kyagaua M, Nagakawa T, Miyazaki I. A new strategy for the therapy of pancreatic cancer invasion and metastasis by protease inhibitor and proton pump inhibitor agents. Jpn J Gastroenterol Surg 1996; 29: 888–892
- Ohta T, Numura M, Yagishita H, Futagami F, Tsukioka Y, Kitagawa H, Kayahara M, Nagakawa T, Miyazaki I, Yamamoto M, et al. Expression of 16 kilodalton proteolipid of vacuolar type H+ATPase in human pancreatic cancer. Br J Cancer 1996; 73: 1511–1517
- Kim GE, Lyons JC, Song CW. Effects of amiloride on intracellular pH and thermosensitivity. Int J Radiat Oncol Biol Phys 1991; 20: 541–549
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 1979; 18: 2210–2219
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 1983; 65: 55–63
- Charmichael J, Degraff WG, Gadzar AF, Minna JD, Mitchell JB. Evaluation of tetrazolium-based semi-automated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 1987; 47: 936–941
- Song CW, Lyons JC, Griffin RJ, Makepeace CM. Thermosensitization by lowering intracellular pH with 5-(N-ethyl-N-isopropyl) amiloride. Radiother Oncol 1993; 27: 252–258
- Song CW, Lyons JC, Makepeace CM, Griffin RJ, Cragoe EJ, Jr. Effects of HMA, an analogs of amiloride, on the thermosensiivity of tumors in vivo. Int J Radiat Oncol Biol Phys 1994; 30: 133–139
- Asaumi J, Kawasaki S, Nishikawa K, Kuroda M, Hiraki Y. Effects of amiloride derivatives on the thermosensitivity of an adriamycin-resistant strain of Ehrlich ascites tumor cells. Anticancer Res 1994; 14: 805–808
- Ohta T, Arakawa H, Futagawa F, Fushida S, Kitagawa H, Kayahara M, Nagakawa T, Miwa K, Kurashima K, Numata M, Kitamura Y, Terada T, Ohkuma S. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line capan-1. J Pathol 1998; 85: 324–330
- Song CW, Park HJ, Ross MD. Intra- and extracellular pH in solid tumor. Antiangiogenic Agents Cancer Ther (Cancer Drug Disc Dev) 1999; 51–64, Humana Press, Totowa, NJ
- Rhee JG, Kim TH, Levitt SH, Song CW. Changes in acidity of mouse tumor by hyperthermia. Int J Radiat Oncol Biol Phys 1984; 10: 393–399
- Jayasundar R, Honess D, Hall LD, Bleehen NM. Simultaneous evaluation of the effects of RF hyperthermia on the intra- and extracellular tumor pH. Magn Reson Med 2000; 43: 1–8
- Arakawa H. Inhibitory effect of bafilomycin A1 on growth of human pancreatic ductal cancer cell lines. Juzen Med Soc 1999; 108: 419–428
