Abstract
Introduction: The growing interest and participation in multi-institutional trials involving deep hyperthermia treatment is an important step towards the further consolidation of hyperthermia as an oncological treatment modality. However, the differences in the clinical procedures of hyperthermia application also raises questions as how to compare the reported temperatures data obtained by the different institutes. In this study our recent developed approach, RHyThM (Rotterdam Hyperthermia Thermal Modulator), has been used for thermal data analysis to investigate the temperature dynamics behaviour of a series of deep hyperthermia treatments.
Patients and methods: All 22 patients (104 hyperthermia treatments) with locally advanced cervical carcinoma who participated in a feasibility study for treatment with a three-modality therapy were selected. The patients received mega-voltage external beam radiotherapy to the pelvis in daily fractions of 2 Gy five times a week to a total dose of 46 Gy and additional brachytherapy, at least four courses of weekly cisplatin (40 mg m−2) and five sessions of weekly loco regional deep hyperthermia treatments with the BSD2000-3D with the Sigma 60 or the Sigma-eye applicators at frequencies 70–120 MHz. Using RHyThM tissue type was defined along the insertion length, based on the CT scan information in radiotherapy position, for each single treatment. A step change in the slope of the profile of the first temperature map was identified to verify the insertion length of the thermometry catheter and precise location of the transition between in- and outside the body. Data analysis was performed based on the temperature readout provided by RHyThM.
Results: The temperature and RF-power data of 97 treatments could be analysed. The intra-vaginal temperature indices were slightly lower than those for bladder and rectum. The average T50 (median temperature) in all lumens, i.e. bladder, vagina and rectum, was 40.4 ± 0.6°C. The average vagina all lumen T50 was 40.0 ± 0.8°C. The average bladder and rectum all lumen T50 was 40.6 ± 0.7°C and 40.5 ± 0.6, respectively. When the analysis was restricted to the deepest 5 cm of the vagina lumen, the average T50 was 39.8 ± 0.9°C. Good correlation exists between the various temperature indices like T20, T50 and T90, for all lumen measurements in bladder, vagina and rectum. No correlation was found between temperature indices and treatment number. For the complete patient population, no relationship was found between T50 and net integrated RF-power applied. In an explorative analysis on individual patients a positive correlation coefficient or trend was found in 14 patients between normalized net integrated RF-power and vagina T50.
Conclusion: Average all lumen T50 for bladder, vagina and rectum differ less than 1°C, indicating that a large volume was heated relatively homogeneously. The vagina T50 value depends on how many measurement points are included for the analysis. In this group of patients the vagina T50 of the first treatment is not a good measure to discriminate between patients with ‘heatable’ and ‘non-heatable’ tumours. In order to compare temperature data reported by different institutes dealing with the same group of patients, one needs a strict and clear agreement on which temperature measurements or reference point(s) that should be included in the analysis.
Introduction
Well-controlled Phase III clinical trials have shown that hyperthermia in combination with radiotherapy results in better tumour response than radiotherapy alone Citation[1–5]. For advanced cervical cancer, van der Zee et al. Citation[6] and Harima et al. Citation[7] have demonstrated a significant benefit of adding hyperthermia to radiotherapy, i.e. an increase of the 3-year overall survival from 27% for radiotherapy-alone to 51% for radiotherapy plus hyperthermia and an increase in complete response rate from 50–80%, respectively; whereas Vasanthan et al. Citation[8] failed to demonstrate a benefit of the combined treatment. Van der Zee et al. indicate several reasons, which may explain why Vasanthan et al. were not able to demonstrate the benefit of a combination of radiotherapy and hyperthermia for their patients Citation[9]. For instance, the observed temperature distribution may not reflect the quality of the hyperthermia treatment due to a too low number of temperature probes Citation[9]. Although the observed temperature increase appears adequate, the data from limited thermometry is not representative for the whole target volume. The comment of van der Zee et al. addresses a major issue concerning the interpretation of hyperthermia results, that is: can one use thermometry or thermal dose data to compare clinical studies and/or explain strong variations in treatment outcome?
From a biological point of view the introduction of a thermal dose parameter is of great importance to predict treatment outcome. Reported data from human clinical trials illustrates that the temperature exceeded by 50% (T50) and 90% (T90) of monitored sites within the tumours may have prognostic significance and predictive values for response Citation[10], Citation[11]. Moreover, the concept of cumulative equivalent minutes at 43°C (CEM43°C), which was introduced by Sapareto and Dewey Citation[12] based on cell culture data, indicates the relation between different heating times and temperatures to one another. The study of Maguire et al. Citation[13] was a first attempt to test the concept of CEM43°CT90 as a prognostic and prescriptive thermal dose parameter. In this phase II trial study patients were first selected on the ‘heatability’ of their tumour and if the tumour was ‘heatable’ they received additional (n ≤ 10) hyperthermia treatments. Based on the thermal dose effect relationship for soft tissue sarcoma, as has been established by Oleson et al. Citation[14], a minimum thermal dose (CEM43°CT90 ≥ 10) was prescribed. Unfortunately, the complete response rate achieved (56%) was significantly below the projected complete response rate of 76%. The latter illustrates that translation of the thermal dose concept to a clinical prescriptive parameter is still complicated and hampered by limited knowledge of the biological processes involved in hyperthermia. In a recent letter to the editor, Dewhirst and Sneed Citation[15] elucidated on the rather complex and multi-functional character of the process involved in enhancing tumour cell kill by radiotherapy or chemotherapy in combination with hyperthermia, demonstrating the still un-mature state of this topic and the need for more research.
In the authors’ opinion, the above does not mean that one cannot exploit temperature data to improve treatment quality. If one limits the question to only address the day-to-day technical quality of a hyperthermia treatment within a single institute, the current thermometry can provide already valuable information. In case one wants to compare hyperthermia quality among various equipment, one needs to arrive at more uniform procedures of thermometry. For a technical evaluation of the hyperthermia treatment it was anticipated that it would suffice to focus the temperature analysis on T20, T50 and T90.
The current study concentrates on temperature dynamics of the treatment instead of a thermal dose effect analysis. In this study the most important reason to investigate treatment temperature dynamics is to evaluate reproducibility of the hyperthermia treatment, compare the hyperthermia quality in various institutes and compare the different equipment utilised for heating the same tumour area. This paper presents a temperature data analysis that is performed using a recently developed programme, RHyThM (Rotterdam Hyperthermia Thermal Modulator) Citation[16]. The thermal analyses of the data obtained for the patients with locally advanced cervical cancer, which were treated with radiotherapy plus hyperthermia and chemotherapy, has been primarily chosen with the intention to evaluate the performance of RHyThM. During this analysis it was noticed, however, that the analysis of the thermal data is also of high interest to identify potential discrepancies in analysis and reported ‘thermal dose’ among institutes participating in phase II/III clinical trials. When comparing the results with the data reported in other studies, we are confronted with the question whether the difference among the temperatures reported in these studies is a real difference or merely a reflection of a difference in the equipment and the protocols used. In the light of the currently active trials performed by different institutes, each with their own validated protocols and specific experience, we believe that there exist a clear need to arrive at consensus or new guidelines to make a valid assessment and comparison of the quality of the hyperthermia treatments performed in the various clinical studies and among the different institutes Citation[17].
Patients and methods
Patient population
The temperature data of all 22 patients (104 treatments) with advanced cervical carcinoma referred between May 2000 and June 2002 for hyperthermia treatment as part of a phase II study on the feasibility of three-modality cancer treatment, i.e. radiotherapy plus hyperthermia and chemotherapy, was used in this thermal data analysis. These patients are also included in the combined analysis of the three prospective phase II trials as were carried out in Norway, the USA and the Netherlands, of which the clinical results were recently published by Westermann et al. Citation[18]. Eligible patients were aged >18 years with previously untreated, histologically confirmed invasive cancer of the uterine cervix. Loco regional hyperthermia had to be technically feasible. Serious concomitant disease or active infection was not allowed and neither was previous malignancy that conceivably still could be active. Patients were aged 31–75 years (mean 44), all had a good general condition (WHO 0–1), squamous cell carcinoma FIGO (International Federation of Gynaecology and Obstetrics) stage IIB (n = 19), IIIA (n = 1) or IIIB (n = 2).
Radiotherapy and chemotherapy
All patients received megavoltage (≥10 MV) external-beam radiotherapy to the whole pelvis to a total of 46 Gy in five fractions of 2 Gy a week using a four-field box technique. Brachytherapy was administrated with high-dose rate brachytherapy (17 Gy, two fractions of 8.5 Gy). Radiotherapy was performed at different institutes (Rotterdam, Arnhem and Enschede). Chemotherapy consisted of at least four and maximum five courses of weekly cisplatin (40 mg m−2, i.v.) with standard hydration and anti-emetic pre-medication.
Hyperthermia
Regional whole pelvis hyperthermia was delivered once weekly during the period (5–6 weeks) of radiotherapy and chemotherapy administration, starting 1 to maximally 6 h after radiotherapy. Hyperthermia was delivered with the BSD2000-3D annular phased array system (BSD Medical Corporation, Salt Lake City, Utah) Citation[19] using the 12 channel Dodek solid state amplifier connected to the Sigma-60 or the Sigma-Eye applicator. Hyperthermia was carried out by the institutional protocol of the Erasmus MC Daniel den Hoed Cancer Center, in Rotterdam as follows. The frequencies used were in the range of 70–120 MHz. The initial RF-power was 400 W. RF-power output to the applicator was increased until the patient's tolerance threshold was reached. Hereafter, SAR-steering (SAR = specific absorption ratio) was applied by changing phase and amplitude settings with the aim to reduce power-limiting hot spots (i.e. normal tissue temperature >43°C or pain complaints of the patient) and to maintain or increase the temperature in the target volume. The hyperthermia treatment consisted of a heating phase of 30 min followed by 60 min therapeutic time. The temperature of the applicator's water bolus was maintained at 20°C. The increase in systemic temperature was limited by cooling measures: undressing, air-conditioning, wet towels, ice packs and cooling bolus placed in the neck.
Thermometry
For thermometry, closed-tip catheters (William Cook Europe, P5.0-CE-50-SFT-NS-0, Denmark) were placed in the urinary bladder lumen, rectum, vagina and at the perineal skin. These locations reflect tumour contact temperature, tumour indicative temperature or normal tissue temperature. Tumour contact is defined as close contact of the closed-tip thermometry catheter with the tumour; tumour indicative is defined as the position of the closed-tip thermometry catheter in the same transverse plane as the tumour, but not in contact Citation[20]. The hyperthermia physician decides on the assignment of the tissue type, which is deduced from the patient's CT-scan obtained in radiotherapy-position. Insertion depth of the thermometry catheter was measured manually using a standard calliper by the hyperthermia technician. Temperatures were measured using the Bowman Citation[21] probes of the BSD2000 system. Temperature mapping was performed along the length of the catheter in 1 cm increments with a maximum mapping length of 14 cm. Thermal mapping started just prior to the start of the hyperthermia treatment and repeated hereafter with 5-min intervals. Accuracy of the temperature measurement was ±0.1°C. Systemic (oral) temperature was measured with regular intervals, i.e. just before the start of the treatment, at 15, 30, 60 min, and at the end of the treatment session.
Data processing
In order to perform thermal data analysis, the BSD data of patients was transferred from PDOS to MSDOS format; using the BSD2000 computer console and empty MSDOS formatted 1.44 MB (2HD) disks. Then the MSDOS data was imported in RHyThM in MATLAB environment. Using RHyThM, tissue type was defined for each single treatment according to the tissue map trajectory per thermometry catheter as provided by the hyperthermia physician and for the insertion length as measured by the hyperthermia technician. A step drop of the temperature profile of the first thermal map was used to verify the location of the transition between in- and outside the body, as reported by the hyperthermia technician. If necessary the location of the transition was adapted to match better with the steep temperature gradient or to correct for a small movement of thermometry catheter during the whole treatment session. For a complete description of the RHyThM programme, this study refers to a previous publication Citation[16]. The data analysis is based on the temperature readout as provided by RHyThM. The following thermal dose parameters were calculated: Tmax, which is determined between start and end of the treatment, average of temperature (Tmean), T20, T50 and T90*, which are calculated between 30 min after treatment start time and end of the treatment (*: TX means the temperature which is exceeded by X% of all temperature readings). The temperature readout was also restricted to the deepest 5 cm of vagina lumen and repeated the data analysis. The purpose of this limitation was to exclude a potential effect of the cold water-cooling of the perineum tissue.
Results
Experiences from data analysis by RHyThM
Data transfer from PDOS to MSDOS was successful for 21 patients. For one patient the data of all four treatments was lost due to a reading failure of the diskette under PDOS. Additionally, it was not possible to transfer a single treatment for three other patients. Thus, of the 104 treatments performed 97 treatment data sets are available for analysis. Hereafter, the MSDOS data was imported in RHyThM to perform the data analysis. Overall, RHyThM performed satisfactory, was able to check integrity and validate the temperature data and performed the tissue type assignment and thermal analysis for all remaining 97 treatments; however, it was necessary to adapt the insertion length for 53 out of 291 thermal map trajectories (18%) or in 39 out of the 97 treatments (40%).
Results of temperature analysis
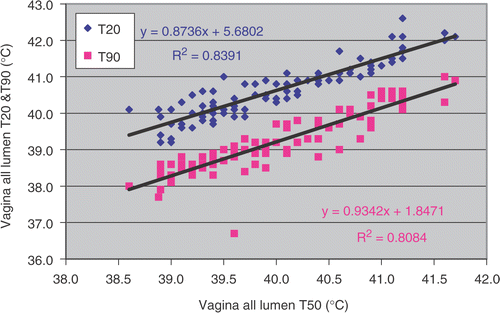
An overview of the temperature data analysed is presented in . Overall, the vagina temperatures were slightly lower than those for bladder and rectum. Median temperature (T50) in vagina tumour contact was 39.7 ± 0.8°C and vagina all lumen T50 was 40.0 ± 0.8°C. When the temperature analysis was restricted to the deepest 5 cm of the vagina lumen the T50 was 39.8 ± 0.9°C (). The bladder tumour indicative T50 was 40.8 ± 0.7°C vs 40.6 ± 0.7 for bladder all lumen T50. The rectum tumour indicative T50 and rectum all lumen T50 were 40.5 ± 0.7°C and 40.5 ± 0.6°C, respectively. If one considers all intra-luminal, i.e. all bladder, vagina and rectum, temperature measurements the T50 was 40.4 ± 0.6°C. More temperature indices, i.e. T20 and T90, are shown in . A strong correlation exists between T50 and T20 as well as T50 and T90 in vagina all lumen (and also for vagina tumour contact) (). The same results and similar figures were obtained for bladder and rectum temperature (not shown).
Table I. Average temperature (°C) in bladder, vagina and rectum obtained during deep hyperthermia treatment in 22 patients with locally advanced cervical carcinoma. Numbers in parentheses show 1 SD.
Table II. Average T20, T50 and T90 in different location of temperature measurements during deep hyperthermia treatment of patients with locally advanced cervical carcinoma treated with radiotherapy, hyperthermia and chemotherapy. Numbers in parentheses show 1 SD.
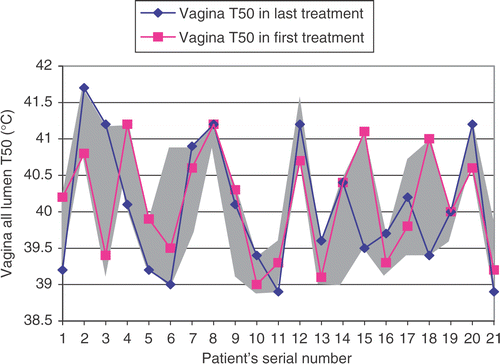
In general a typical temperature profile along the thermal mapping catheter inserted in the vagina starts with low temperatures at the transition between inside and outside, after which the temperature increases to a maximum within the first few centimetres and subsequently decreases again when reaching the deepest part of the vagina (). The average insertion depth in the vagina was 9.0 ± 2.1 cm. No correlation was found between T50 (or T20 or T90) and treatment number neither for tumour contact nor for all lumen. shows the T50 vagina all lumen as obtained during the first and last treatment as a function of patient number. Additionally, the grey area shows the range of T50 obtained during all treatments and it demonstrates that vagina T50 varies substantial from one treatment to another. In six out of 21 cases T50 was maximal in the first treatment, in seven cases T50 was maximal in the last treatment, in three cases T50 was maximal in the first and last treatments and, finally, for five cases T50 was maximal in the intermediate treatments. However, in one case maximal T50 was in treatments 1, 3, 4 and 5.
Figure 2. A typical temperature profile along the thermal mapping catheter inserted in the vagina. Position 0 cm represents the deepest point in tissue whereas the transition between inside and outside the lumen is at 11–12 cm.
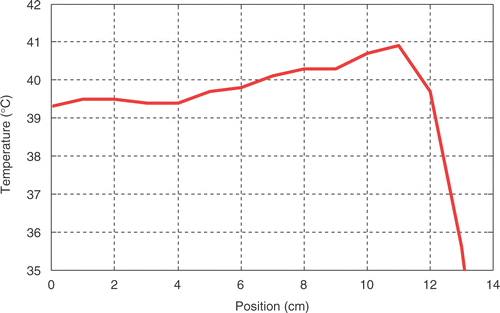
Figure 3. Vagina all lumen T50 in the first and last treatment plus range of maximum and minimum vagina T50 (grey area) during all of the treatments.
Another parameter investigated was the correlation between average net RF-power (J s−1) and average net integrated RF-power (kJ) on T50 for all lumen in vagina, bladder and rectum. For both parameters no correlation was found with T50. In an exploratory analysis by patient, i.e. when applied RF-power per treatment is normalized to the average RF-power over the whole series of treatments for a patient, a positive correlation coefficient or trend was seen in 14 patients. The average net input RF-power and net integrated RF-power (kJ) per patient body weight was 9.7 ± 2.0 J s−1 kg−1 and 46.4 ± 10.4 kJ kg−1, respectively. No correlation was found between the number of RF-power off-switches and T50 for all lumen.
Discussion
In our departments hyperthermia treatment of patients with a deep-seated tumour, e.g. uterine cervix is applied with the BSD2000 system using the Sigma-60 or the Sigma-Eye applicator. Our treatment strategy is to heat the target volume as homogenous and high as possible, which is achieved by SAR steering, i.e. changing phase and amplitude settings. From this exploratory analysis of the 22 patients treated with radiotherapy plus hyperthermia and chemotherapy it is found that the T50 temperatures for bladder (40.6 ± 0.7°C), vagina (40.0 ± 0.8°C) and rectum (40.5 ± 0.6°C) differ 0.6°C (), indicating that a large volume is heated relatively homogeneously.
The substantial and rather unpredictable large standard deviation (0.8°C) found in the vagina T50 among the different treatments for a patient indicates that, for this group of patients, the vagina T50 of the first treatment is not a good measure to discriminate between patients with a high average T50 and patients with a low average T50 or patients with ‘heatable’ and ‘non-heatable’ tumours ().
In the present study the average of all intra-luminal median temperature measurements, i.e. all bladder, vagina and rectum lumen T50 was 40.4 ± 0.6°C (). The T50 value as reported in several publications dealing with similar patients, i.e. locally advanced cervical carcinoma treated with the same treatment modality, i.e. radiotherapy plus hyperthermia and chemotherapy, are different from these results. For example, as reported in the compilation paper by Westermann et al. Citation[18], the T50 value was 41.0°C for the Dutch patients, 39.2°C for the USA patients and 41.1°C for the Norwegian patients. In a separate publication, Jones et al. Citation[22] reported a T50 of 39.4 ± 0.7°C for a similar group of patients and treatments. The question is whether these differences are real differences in achieved temperature distribution or merely a consequence of differences in thermometry procedures of the various departments. As reported by Westermann et al., the T50 value for the Dutch patients is an average of all intra-luminal and/or intra-tumoural temperatures measured, whereas the T50 reported for the USA patients reflected only the temperature in the most distal 5 cm of the cervical os and the data of Norway included intra-vaginal and tumour measurements Citation[18], Citation[22].
In an attempt to quantify the effect of the different manners of temperature analysis, this study has analysed the data using the same three manners. First, including all intra-luminal temperatures the T50 value was 40.4 ± 0.6°C. Secondly, if the data analysis was restricted to all intra-vaginal temperature measurements the T50 was 40.0 ± 0.8°C. Thirdly, when the analysis was limited to the deepest 5 cm of the vagina lumen the T50 was 39.8 ± 0.9°C. The typical temperature profile along the thermometry catheter in the vagina as plotted in is a relatively well-known phenomenon and may reflect the variation in permittivity and blood flow at this location. As a consequence of this typical profile, the vagina T50 is lower when the thermal analysis is restricted to the deepest 5 cm of the thermometry catheter in the vagina. At this moment it is difficult to conclude whether this difference has relevance for the clinical outcome, however, it does matter when the T50 is used for comparing the quality between patient treatments.
In addition to the uncertainty introduced by variations in the exact location of the temperature measurements comes a still unknown uncertainty associated with differences in the used probes, e.g. Bowman probes (the BSD users) or thermocouples (Amsterdam Hyperthermia Group) and differences in mapping intervals, e.g. 0.5 or 1 cm. There are a multitude of reasons why the T50 data for the various institutions are different. Without detailed knowledge of SAR patterns, patient characteristics, use of analgesics, etc. it will be impossible to determine whether such differences are real, particularly when there was no a priori temperature goal set for these studies.
The reported differences illustrate the need to reach consensus on new guidelines to allow a valid assessment and reliable comparison of the quality of the hyperthermia treatments performed in different clinical studies and among the various institutes. Introduction of reference points similar to radiotherapy may constitute a good solution to enable a more rigid comparison of the quality of the hyperthermia treatments delivered in different institutes. From a QA point of view one of the issues of thermal data analysis is to compare the ‘quality’ of the hyperthermia treatments administered by different hyperthermia groups. Given the fact that at present a standardized thermal dosimetry system is still lacking Citation[15], Citation[23], we consider flexibility as an essential requirement to easily enable a specific and reliable comparison of the quality of the hyperthermia treatment applied by different hyperthermia groups based on an identical thermal dose parameter definition.
In the clinical study reported here we had to correct the insertion depth of the thermal catheters for 53 (of 291) thermal map trajectories. Hence, in 82% the tissue assignment used in the data analysis is valid for the entire treatment duration and for the remaining 18% the tissue assignment used resembles only the tissue distribution valid at the end of the treatment. As we question whether this approach to correct for changes of the insertion depth is appropriate, we are currently investigating the need for dynamic tissue assignment based on a varying insertion depth of the thermometry catheter. Another factor associated with assigning the correct insertion depth is the cooling effect of the coldwater bolus against the skin, especially at the perineum. Due to thermal conduction, the temperatures measured at points near the transition of the perineum tissue to the water bolus (13°C) are substantially below normal tissue temperature (<37°C) and thus appears to be outside the vaginal or rectal lumen. For longer preparation/installation times the cold front will be moving further into the tissue, making it more difficult to identify the correct insertion depth. The solution to this problem is difficult as the manual measurement of the insertion depth has a poor accuracy.
The availability of the full details of the RF-power applied to the patient provides the opportunity for new interesting analyses between RF-power, temperatures achieved and pain complaints. The correlation observed in the exploratory analysis between normalized net integrated RF-power and vagina T50 is an experimental support for the optimization of the SAR distribution as is being advocated for long by several theoretical studies Citation[24], Citation[25]. This finding is a stimulus for rigid investigation (including the statistical power) of this potential relation in a data set of a larger patient population. Our hyperthermia treatment protocol prescribes maximization of the applied RF-power during a deep hyperthermia treatment through continuous increase of the RF-power until the patient complains about discomfort. Next phase- and amplitude steering is applied to reduce the discomfort and, if successful, RF-power is increased again until the next complaint of the patient.
Conclusion
The exploratory analysis performed for 22 patients with advanced cervical carcinoma, treated with radiotherapy plus hyperthermia and chemotherapy, demonstrated that RHyThM operates satisfactorily. The analysis also indicates some areas for further improvement. In the present thermal data analysis the intra-vaginal temperature indices were found to be slightly lower than those for bladder and rectum temperatures. Average all lumen T50 for bladder, vagina and rectum differ 0.6°C, indicating that a large volume was heated relatively homogeneously. In this group of patients the vagina T50 of the first treatment is not a good measure to predict ‘heatablity’ and ‘non-heatablity’ tumours. When we compare our thermometry results with the data reported from other studies we are confronted with the question whether the differences among the reported temperatures are real or merely reflect differences in the equipment and the protocols used. Hence, these differences demonstrate the necessity to reach consensus on new guidelines allowing a valid comparison of the quality of the hyperthermia treatments performed in different institutes. Such guidelines are also mandatory for the quantitative evaluation of major improvements in hyperthermia technology.
Acknowledgements
This work was supported by the Dutch Cancer Society, grant DDHK 2003-2884. The first author was financially supported by the Shahrekord University of Medical Sciences (related to the Iranian Ministry of Health, Treatment and Medical Education). The authors would like to thank L. Verloop and A. Ameziane for their technical assistance.
References
- Overgaard J, Gonzalez Gonzalez D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996; 12: 3–20
- Valdagni R, Liu FF, Kapp DS. Important prognostic factors influencing outcome of combined radiation and hyperthermia. Int J Radiat Oncol Biol Phys 1988; 15: 959–972
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymphnodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1993; 28: 163–169
- Vernon CC, Hand JW, Field SB, Machine D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, Liu FF, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, Gutin PH. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- van der Zee J, Gonzalez GD, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y, Hiraoka M. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys 2005; 61: 145–153
- van der Zee J, van Rhoon GC, Wust P. Letters to editor. Int J Radiat Oncol Biol Phys 2005; 62: 940–941
- Leopold KA, Dewhirst M, Samulski T, Harrelson J, Tucker JA, George SL, Dodge RK, Grant W, Clegg ST, Prosnitz LR. Oleson JR. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1992; 22: 989–998
- Leopold KA, Dewhirst MW, Samulski TV, Dodge RK, George SL, Blivin JL, Prosnitz LR, Oleson JR. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1993; 25: 841–847
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Maguire PD, Samulski TV, Prosnitz LR, Jones EL, Rosner GL, Powers B, Layfield LW, Brizel DM, Scully SP, Harrelson JM, Dewhirst MW. A phase II trial testing the thermal dose parameter CEM43°CT90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia 2001; 17: 283–290
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Dewhirst KM, Sneed PK. Those in gene therapy should pay closer attention to lessons from hyperthermia. Int J Radiat Oncol Biol Phys 2003; 57: 597–599
- Fatehi D, de Bruijne M, van der Zee J, van Rhoon GC. RHyThM, a tool for analysis of PDOS formatted hyperthermia treatment data generated by the BSD2000/3D system. Int J Hyperthermia 2006; 22: 173–184
- van Rhoon GC, Fatehi D, van der Wal E, van der Zee J. Analysis of thermal data of deep hyperthermia. Do we need a reference point?. ESHO 2005 Book of abstracts 22nd Annual Meeting of the European Society for Hyperthermic Oncology. 8–11 June, 2005, 93
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, Uitterhoeve ALJ, de Wit R, van der Velden J, Burger C, van der Wilt CL, Dahl O, Prosnitz LR, van der Zee J. First results of triple modality treatment by combining radiotherapy, chemotherapy and hyperthermia for treatment of stage IIB-III-IVA cervical cancer. Cancer 2005; 104: 763–770
- Turner PF, Tumeh A, Schaefermeyer T. BSD-2000 approach for deep local and regional hyperthermia: Physics and technology. Strahlenther Onkol 1989; 165: 738–741
- Wielheesen DHM, de Bruijne M, Graveland WJ, van Rhoon GC, van der Zee J. Leg coverage with towels during regional deep hyperhermia treatment and its effect on pelvic temperature and temperature distribution. Int J Hyperthermia 2005; 21: 77–87
- Bowman R. A probe for measuring temperature in radio frequency heated material. IEEE Trans MTT 1976; 24: 43–45
- Jones EL, Samulski TV, Dewhirst MW, Secord AA, Berchuck A, Pearson DC, Havrilesky LJ, Soper J, Prosnitz LR. A pilot phase II trial of concurrent radiotherapy, chemotherapy, and hyperthermia for locally advanced cervical carcinoma. Cancer 2003; 98: 277–282
- Dewhirst MW, Griffin TW, Smith AR, Paker RG, Hanks GE, Brady LW. Intersociety Council on Radiation Oncology essay on the introduction of new medical treatments into practice. J Natl Cancer Inst 1993; 85: 951–957
- Paulsen K, Geimer S, Tang J, Boyse W. Optimization of pelvic heating rate distributions with electromagnetic phased arrays. Int J Hyperthermia 1999; 15: 157–187
- Wust P, Seebass M, Nadobny J, Felix R. Electromagnetic deep heating technology. Principles and practice of thermoradiotherapy and thermochemotherapy, MH Seegenschmiedt, P Fessenden, CC Vernon. Springer Verlag, Berlin 1995; Vol I: 219–251