Abstract
Hemorrhage control is a high priority task in advanced trauma care, because hemorrhagic shock can result in less than a minute in cases of severe injuries. Hemorrhage was found to be solely responsible for 40–50% of traumatic civilian and battlefield deaths in recent years. The majority of these deaths were due to abdominal and pelvic injuries with hidden and inaccessible bleeding of solid organs such as liver, spleen, and kidneys, as well as major blood vessels. High intensity focused ultrasound (HIFU) offers a promising method for hemorrhage control. An important advantage of HIFU is that it can deliver energy to deep regions of tissue where hemorrhage is occurring, allowing cauterization at depth of parenchymal tissues, or in difficult-to-access anatomical regions, while causing no or minimal biological effects in the intervening and surrounding tissues. Moreover, HIFU can cause both thermal and mechanical effects that are shown to work synergistically for rapid hemorrhage control. The major challenges of this method are in development of bleeding detection techniques for accurate localization of the injury sites, delivery of large HIFU doses for profuse bleeding cases, and ensuring safety when critical structures are in the vicinity of the injury. Future developments of acoustic hemostasis technology are anticipated to be for applications in peripheral vascular injuries where an acoustic window is usually available, and for applications in the operating room on exposed organs.
Introduction
Hemorrhage is an emergency medical problem, with the element of time playing the most important role. The human body has about 5 liters of blood that is circulated at a rate of approximately 1 l/min. Hemorrhagic shock occurs after the loss of 20% of the total blood volume, i.e. 1 liter, leaving only a minute or so before shock ensues after severe injuries Citation[1]. Therefore, a quick response is needed to stop profuse bleeding. The inability to provide timely care leads to high mortality and morbidity of the patients with severe injuries Citation[2], Citation[3].
Trauma due to accidents, assaults, terrorism, and war is a significant problem, accounting for an enormous loss of life and societal productivity Citation[4]. Intentional and unintentional injuries together are the leading cause of death for all age groups under 35 years of age, and the fifth-ranked cause of death overall in the United States, claiming the lives of more than 100 000 people in 2001 Citation[5]. Moreover, each year, about 2.6 million people are hospitalized in the United States as a result of acute injury Citation[6]. Many of these nonfatal injuries have far-reaching consequences in terms of reduced quality of life and high costs accrued to the health care system, employers, and society in general. The medical spending on management of injuries in 1987 in the USA was $64.7 billion (in 1993 dollars) Citation[7], and the total lifetime costs associated with both fatal and non-fatal injuries in 1995 were estimated to be over $260 billion Citation[8].
We are investigating the use of ultrasound for hemorrhage control. In this technology (also known as acoustic hemostasis), high-power ultrasound waves are focused to a small, roughly cylindrical volume (∼1 mm × 10 mm), resulting in a high intensity acoustic field (1000–10 000 W/cm2) at the focus. An important advantage of high intensity focused ultrasound (HIFU) is that it can deliver energy to deep regions of tissue where hemorrhage is occurring, allowing cauterization at depth in parenchymal tissues, or in difficult-to-access anatomical regions, while causing no or minimal biological effects in the intervening and surrounding tissues. The high rate of energy delivery to the tissue causes cautery and coagulative necrosis. HIFU cauterization is achieved by both thermal and mechanical effects of ultrasound Citation[9]. The thermal effects, due to ultrasound absorption, can lead to temperature increases in excess of 70°C in less than 1 s Citation[10]. The mechanical effects such as cavitation, i.e. activity of microbubbles Citation[11], are also important. The formation of microbubbles at a treatment site can enhance the heat deposition Citation[12] and result in faster treatm Citation[12], Citation[13]. Cavitation has also been shown to cause platelet activation which facilitates blood coagulation Citation[14], and produce tissue rupture and release of tissue factors that may stimulate coagulation resulting in the formation of a homogenized coagulum Citation[15]. This homogenized coagulum spreads quickly over the wound, and hardens within tens of seconds to produce a seal over the wound, effectively halting further bleeding Citation[15], similar to tissue glue.
Several groups around the world have spent considerable effort on acoustic hemostasis and vessel occlusion projects. The team headed by Dr Cathignol reported the use of HIFU for generation of venous thrombosis in 1995 Citation[16], as a potential treatment for superficial varicose veins. In this study, rat femoral veins were occluded after 10–20 s of HIFU exposure (at a frequency of 7.3 MHz and intensity of ∼170 W/cm2). Dr Hynynen's group investigated the effects of HIFU on arteries i Citation[17], Citation[18]. This group showed that 1 s HIFU exposures at intensity levels above the threshold for transient cavitation (frequency of 1.5 MHz, intensities of 4400–8800 W/cm2) resulted in constriction of femoral arteries (in a rabbit model) Citation[17]. The constricted vessels relaxed to 50% of their diameter within several hours after the treatment, and completely recovered to their normal size within one week. Dr Hynynen's group also explored the potential usage of HIFU for non-invasive occlusion of arteries located deep inside the tissue. In this study, a branch of rabbit renal artery (diameter of 0.6 mm) was successfully occluded by utilizing a combination of thermal and mechanical effects of HIFU Citation[18]. Dr Ishikawa et al. reported in 2003 on their investigations of HIFU-induced functional and histologic changes in the arteries (in a rat model) Citation[19]. In these studies, 3.2 MHz HIFU applied for 25 s resulted in vessel constriction at intensities of 1080–2750 W/cm2, and complete blood flow occlusion at intensity of 4300 W/cm2. In addition, histologic changes were observed in the vessel wall (in the form of vacuolar degeneration and destruction of elastic fibers) at the intensities of 2750 W/cm2 and above. Dr ter Haar's group reported studies on vascular occlusion in 1999 and Citation[20], Citation[21], with the eventual goal of applying this method in fetal therapy (e.g., for occlusion of placental vessels mediating interfetal transfusion in monochorionic twins) and oncology (for destruction of vessels that provide tumor blood supply). Their experiments, performed in rat femoral arteries, indicated that, with a right choice of parameters, HIFU can produce reproducible vessel occlusion. This work was subsequently confirmed in human trials by Dr Wu's group in 2001 Citation[22]. In these trials, it was shown that extracorporeal HIFU could destroy small blood vessels that feed solid tumors, which led to secondary tumor cell death after the completion of HIFU treatment. The work by these investigators and others who have addressed various aspects of acoustically induced hemostasis represents a wealth of knowledge on the effects of therapeutic ultrasound on blood vessels.
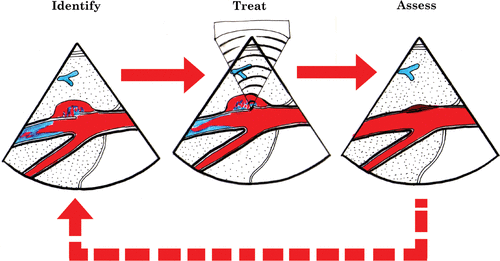
Our group at the University of Washington has focused on the utilization of HIFU therapy for hemostasis of active bleeding, i.e. hemorrhage control for traumatic injuries. Our goal has been to develop systems that have the capability of achieving hemostasis in a non-invasive manner, using ultrasound image-guided HIFU technology ().
Results and discussions
The initial work on acoustic hemostasis at the University of Washington (UW) started in 1996 with a United States Defense Advanced Research Projects Agency (DARPA) grant funded to UW under Principal Investigator Dr Lawrence Crum, Director of the Center for Industrial and Medical Ultrasound. The hemostasis work has continued at the UW with the most recent effort, also funded by DARPA, to develop an autonomous hemostasis cuff for peripheral vascular injuries. Here, we report a synopsis of our various projects at UW.
Hemostasis of visible wounds (intra-operative hemostasis)
This work had a fortuitous start. In 1996, when the hemostasis project was in its early stages at UW, several general and trauma surgeons were invited to attend a demonstration of HIFU technology. They all expressed their need for effective hemostasis tools in the operating room; this initiated our efforts to develop a portable intra-operative hemostasis system. This work was headed by Dr Roy Martin of UW Bioengineering.
The initial results demonstrated that HIFU was an effective method of hemorrhage control for visible wounds in solid organs such as liv Citation[23], Citation[24] and spleen Citation[25]. For example, in a model of intra-abdominal trauma Citation[26], grade III hepatic injury was induced (following a high-speed impact of an aluminum disk on the abdominal wall) and subsequently treated after laparotomy, using solid titanium-coupling cones as HIFU applicators Citation[23]. The animals were heparinized prior to injury, which resulted in a coagulopathic state to facilitate ongoing bleeding, permitting a more thorough evaluation of HIFU-induced hemostasis under hemodynamic conditions pertinent to trauma patients. HIFU was applied intra-operatively, using applicators operating at a frequency of 5.7 MHz and in situ average intensity of over 2000 W/cm2. Complete hemostasis was achieved within 15 ± 6 min of HIFU treatment time, and 54 ± 3 min of operating time. The HIFU-treated sites were hemostasis at re-laparotomy, performed 3 h post-treatment to assess the occurrence and extent of any re-bleeding. These results showed that HIFU treatment was effective in achieving hemostasis of injured parenchyma after blunt liver trauma Citation[23]. Similar results were obtained for spleen injuries Citation[25].
An important finding during the preliminary work was that HIFU application could result in the production of a coagulum at the wound site. This coagulum hardened quickly to form an effective hemostatic seal for both parenchymal oozing injuries, and arterial and venous ruptures (of up to 2–3 mm in diameter). Hemorrhage control was shown to be achieved faster in the presence of coagulum, as compared to HIFU applications in which no coagulum was formed Citation[15]. The maximal temperature of the coagulum (measured using a thermocouple and infrared camera) was approximately 82°C, which was about 5° higher than the maximal temperature of the HIFU-treated wounds with no coagulum present. Mechanical effects of HIFU were also thought to be involved in the production of the coagulum. Using a dissecting microscope, in vivo, the coagulum was observed to be initially full of microbubbles (estimated to be on the order of 105 bubbles per cm3 with diameter of 50–100 µm). These microbubbles disappeared quickly as the coagulum cooled to body temperature. Histological analysis showed the coagulum to be a mixture of destroyed erythrocytes, neutrophils, lymphocytes, and other parts of whole blood, indicating the release of tissue and coagulation factors Citation[15].
In addition to solid organ hemostasis, HIFU has also been investigated for treatment of vascular bleeding, including punctures and longitudinal and cross-sectional lacerations in various arteries and veins Citation[27–29]. In these studies, HIFU showed a great success in achieving hemostasis of vessel injuries in a time shorter than what is required for conventional suture ligation. Furthermore, it was shown that the duration of HIFU exposure has a direct relationship with the final outcome. Use of a long HIFU duration to achieve hemostasis of an injured vessel resulted in total occlusion of the vessel Citation[29], an undesirable outcome as far as tissue ischemia and vascular repair is concerned. However, with accurate targeting of HIFU therapy, a minimal HIFU dose could be applied, resulting in hemostasis while maintaining patency of the vessel.
Hemostasis of hidden injuries (guided hemostasis)
The first guidance method (other than visual guidance) used for targeting of HIFU energy was Doppler ultrasound. Major porcine vessels (abdominal aorta, iliac, carotid, common femoral and superficial femoral arteries, and jugular vein) were surgically exposed, punctured, and subsequently treated using a HIFU device equipped with audio Doppler guidance Citation[27]. With Doppler guidance, complete hemostasis was achieved in an average of 13 s of HIFU application, as compared to 62 s when only visual guidance was used. The results were quite promising, indicating that Doppler guidance allows accurate targeting of vessel punctures.
The next step was to develop an image-guided system, in which we integrated real-time ultrasound imaging with HIFU. The integrated system was configured to have a HIFU transducer with a co-axial imaging probe. A major difficulty that had to be addressed was a significant noise produced in ultrasound images by the HIFU itself. This problem was solved by developing a method of time sharing between imaging and HIFU application Citation[30]. In this method, HIFU excitation (with 50–75% duty cycle) was synchronized with an ultrasound image frame rate, and an observation window was created between noise interference bands in the ultrasound image. This window could be adjusted to be located around the treatment region. Our method allowed the observation of a hyperechoic region (bright spot) in the B-mode images at the HIFU focal point during treatment, thus providing a valuable targeting aid Citation[31]. In addition, multiple ultrasound interrogation features, including Color/Power Doppler, Spectral Doppler, and Harmonic Imaging are currently available in our HIFU system integrated with full-featured ultrasound imaging systems.
An ultrasound image-guided HIFU system has been tested in various deep-tissue bleeding scenarios. In a pig model of penetrating pelvic bleeding, image-guided HIFU was used to treat a 4 cm deep partial transection injury (a 0.5 cm long laceration of the vessel wall) of the internal iliac artery Citation[30]. After induction of bleeding under ultrasound guidance with a penetrating scalpel, HIFU treatment was applied transcutaneously using a 1.1 MHz transducer. The bleeding was completely arrested within approximately 70 s of HIFU application, and the vessel was patent after the treatment. Color Doppler was used for identification of the bleeding site before the treatment and assessment of hemostasis and vessel patency after the treatment. These results provided important evidence for the feasibility of transcutaneous acoustic hemostasis of pelvic vascular injuries. Other studies, which further validated the feasibility of image-guided acoustic hemostasis, included occlusion of internal iliac vessels as a method of embolization for pelvic bleeding, and hemorrhage control in the posterior surface of the liver Citation[32]. Overall, these results showed that ultrasound-guided HIFU can provide an efficient tool for the treatment of hidden/inaccessible injuries.
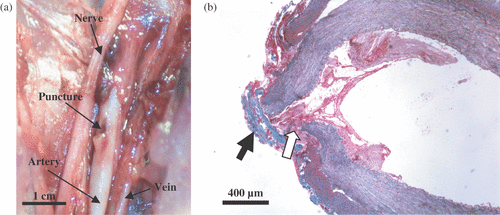
Research in the area of ultrasound-guided HIFU hemostasis has also been carried out in the commercial sector. For example, a Seattle-based company “Therus” was founded in 1998 to pursue the use of HIFU for catheter wound closure in patients, which has been to this date shown successful in three clinical trials on 162 patients. This method was originally developed at UW. In the preliminary feasibility studies, porcine femoral arteries (5–6 mm diameter), were catheterized (with a 9-French catheter) resulting in a 3 mm puncture wound in the artery (which was located at 1–1.5 cm below the skin surface). The catheter was kept in the artery for at least 30 min to ensure the occurrence of significant bleeding when the catheter was withdrawn. The injuries were treated transcutaneously using HIFU, sham treated, or served as control. The treatment time (HIFU or sham) was approximately one minute. When bleeding started upon removal of the catheter, HIFU was applied under Color Doppler guidance to the injured site using a concave, single element piezoelectric transducer (3.2 MHz, f number of 1, in situ average intensity of 3000 W/cm2, Sonic Concepts), integrated with an ultrasound-imaging probe (L10-5, Philips). Complete hemostasis was achieved in a majority of HIFU treatments (∼80%). The overall blood loss (in ml) was 19, 81, and 157 for HIFU, sham, and control groups, respectively. Both femoral arteries and veins were patent after the HIFU treatment, with some narrowing usually observed in the femoral artery at the treatment site. shows the gross appearance of the HIFU-sealed puncture in the femoral artery of a pig, which was sacrificed within several hours after the treatment. The hemostasis mechanism appeared to be a combination of the formation of a fibrous cap [black arrow, ], and coagulation of extravasated blood [white arrow, ].
Figure 2. Gross (a) and light microscopy (b) observations of a HIFU-sealed puncture site in the femoral artery. The puncture was sealed by coagulated blood (white arrow) and a fibrous cap (black arrow). The histological slide was stained with Masson's trichrome stain.
After establishing efficacy of the HIFU method, we performed a survival study to observe long-term safety of HIFU application in sealing of punctured arteries Citation[29]. In this study, femoral arteries of 25 adult rabbits were punctured and subsequently sealed with HIFU. The HIFU-treated vessels were observed at various time points (up to 60 days) utilizing ultrasound imaging and histological analysis. The vessels were patent after the treatment in a majority of cases, with a normal blood flow. No re-bleeding was observed. Immediately after the treatment, the HIFU-exposed area of the artery showed disorganized adventitia, and coagulation and thinning of tunica media. The vessels recovered to normal appearance within 28 days, with some thinning of the tunica media observed up to day 60. Further short- and long-term survival studies are needed to optimize the treatment parameters, and investigate the safety and efficacy of HIFU application. For example, the proximity of nerves to the HIFU-treated area is of particular concern, and the nerve function following the treatment has to be studied carefully.
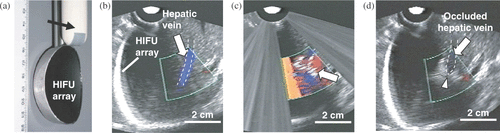
Injury sites in our initial studies (described earlier) were carefully selected to enable fixed-focus HIFU devices to be focused on the desired target. An adjustable-focus HIFU array is required to address bleeding scenarios in which injury occurs at various tissue depths. In our recent studies, an engineering prototype HIFU annular array Citation[33] [] was tested for selective occlusion of liver vessels, as a method equivalent to angioembolization used in trauma management. Adjustment of focal position of a HIFU beam was performed using LabView control, with pre-determined excitation phase angles of the transducer elements in the array. Twenty-five treatments were performed in five pigs in vivo. The HIFU array, designed in our laboratory, and manufactured by Imasonics (Besancon, France) had the following specifications: piezocomposite material; center frequency of 3 MHz; focal depth range of 3–6 cm; 11 elements: six complete annuli, five truncated annuli; in situ average intensity of up to 1000 W/cm2 Citation[33]. Color Doppler ultrasound imaging, synchronized with the HIFU system, allowed accurate targeting of the liver vessels [], treatment monitoring [], and assessment of the vessel patency after the treatment []. The results showed that blood vessels of up to 2 mm in diameter were occluded, while larger vessels were still patent after 30–60 s of HIFU treatment. Our goal is to develop annular HIFU arrays for hemostasis of large blood vessels (>5 mm) at tissue depths of up to 10 cm to be able to address high-grade liver injuries. The treatment of bleeding in deep regions of the body has remained a challenging problem due to difficulties including power delivery and bleeding detection. These topics are discussed subsequently.
Figure 3. (a) HIFU annular array, integrated with an ultrasound imaging probe (arrow). (b) Before HIFU, patent hepatic vein (outlined by dashed lines) is visualized using Color Doppler (arrow). (c) During HIFU, hyperecho (arrow) formed at the position of the vessel. (d) After HIFU, the occluded vein (outlined by dashed lines) shows no flow (arrow). The site of treatment is seen as a hyperechoic region surrounding a vein that appears to be collapsed (arrowhead).
Power: A tough engineering challenge
Sufficient power delivery to deep tissue regions is a formidable challenge due to the tissue attenuation and presence of large blood vessels in the deep-seated regions. Injuries to these vessels usually cause profuse bleeding, requiring significant amounts of energy to achieve hemostasis. Such energy levels may not be achieved easily with the currently available HIFU transducers. The treatment can be even more difficult in overweight/obese patients, due to the additional attenuation caused by fat layers. For example, the profunda femoris artery could lie as deep as 15 cm in the thigh of someone of about 200 lbs weight. This blood vessel is about 7 mm in diameter. The bleeding rate can be estimated to be approximately 1 liter in the first minute after an injury that causes a 5 mm puncture hole in the artery. The high flow rates as well as deep location of this artery both impose demanding requirements for power/energy delivery.
We believe that the problem of depositing sufficient power for hemostasis at large tissue depths should be addressed using a multi-pronged approach. Decreasing the blood flow is an obvious physiological approach to facilitate hemostasis. We have recently tried manual compression of the injured blood vessels during hemostasis treatment, and this method appears to be effective. The mechanism is believed to be the reduction of the heat sink effect of blood flow, allowing cauterization of the injury site. It should be noted that in some locations in the body, such as the abdomen, compression may not be possible due to mobility of the organs. The second, mechanical, approach to addressing the high-power demands of hemostasis is to increase the acoustic aperture of HIFU transducer. In this approach, issues such as acoustic window, coupling to the tissue, and ergonomics are extremely important, and highly device-specific. The third, acoustic, approach to improve energy delivery to deep tissue regions is to boost energy conversion efficiency by using cavitatioCitation[12], Citation[13], Citation[34]. The mechanism of this approach is to produce microbubbles at the HIFU focus, which increase the equivalent attenuation coefficient thus improving energy transfer to the tissues Citation[34]. Cavitation appears to be a viable method for enhancement of HIFU treatment, as the pressure threshold for cavitation is lower at lower frequencies Citation[11] that inevitably have to be utilized for deep-tissue applications. The fourth and final approach is to utilize non-linear effects of HIFU Citation[35]. However, nonlinear effects are inherently associated with higher frequency components of the HIFU beam, which are bound to decay significantly before reaching the focus located at larger depths. A concerted and novel engineering effort appears to be needed to overcome the challenges of power requirements in deep-tissue hemostasis.
Bleeding detection: A necessary component of image-guided hemostasis
Development of hemorrhage control therapy for deep-tissue bleeding hinges on the availability of fast and accurate bleeding detection methods. Moreover, for severe bleeding cases, where every second counts, real-time methods are desirable that can be employed in the emergency or operating rooms or even outside the hospital setting. Currently, there are only a few methods available to detect and localize internal bleeding. The available modalities include peritoneal lavage, CT, MRI, exploratory laparoscopy, and physical examination Citation[36–38]. In general, these methods are invasive or time-consuming, and are not optimal for hemodynamically unstable patients in the emergency room. Ultrasound is usually present in the emergency and operating rooms, and could even be available outside the hospital. However, in its current state, conventional B-mode sonography is limited by its low sensitivity in detection and localization of bleedi Citation[39], Citation[40]. Ultrasound contrast agents have been used previously to improve sensitivity during B-mode ultrasound examinations of blunt hepatic trauma Citation[41–43], splenic injuries Citation[44], Citation[45] and renal parenchymal injuries Citation[46], Citation[47]. In these studies, damaged regions of tissues appeared hypoechoic as compared to surrounding undamaged parenchyma, thus allowing accurate visualization of the general area of the injury. Our recent data have also provided evidence of successful localization of bleeding sites using ultrasound-based methods (Spectral and Color Doppler, and contrast-enhanced Doppler and B-mode methods Citation[48]).
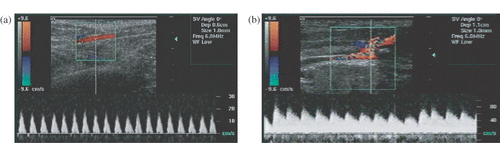
In our bleeding detection studies, puncture injuries were produced in rabbit and pig arteries (femoral, axillary, splenic, and abdominal aorta). Doppler interrogation was performed at and around the puncture sites. The maximal blood flow velocity at the puncture site, determined using pulsed Doppler, increased 2–3 times after the injury was produced (). The results showed that the flow exiting the injured artery at the puncture site was present even during diastole, resulting in increased spectral background levels. This constant flow was not observed in normal arteries. Furthermore, spectral broadening of >50% was detected in the systolic period for the injured arteries. The spectrum in the hematoma surrounding the injury showed short-duration, bi-directional flow patterns of lower velocity and duration. In Color Doppler images, the injury site showed a distinctive checkered pattern, an indication of flow turbulence. Signals described here were observed for all injuries with only minor differences among the various arteries tested. Therefore, the Color- and Pulsed-Doppler patterns of arterial injuries appeared to provide valuable signatures of bleeding. These characteristics are currently being used to develop bleeding detection algorithms and protocols for our project to develop an acoustic hemostasis cuff, which should be able to automatically find the bleeding site using ultrasound-based methods and seal the injury using HIFU.
Figure 4. Pulsed Doppler spectrum obtained from the femoral artery before and after the injury was produced. (a) Normal arterial blood flow before the injury. (b) Bleeding artery shows a spectrum with increased baseline and elevated peak amplitude.
We have also investigated other methods of bleeding detection including the use of ultrasound contrast agents and harmonic imaging Citation[48]. These methods were primarily investigated due to the challenge presented by detection of slow bleeding, such as solid organ parenchymal oozing and venous bleeding. For example, triangular wedge incisions (depth of 0.5 cm, length of 2 cm, width of 0.4 cm) were produced in the posterior surface of the liver and kidneys in 10 rabbits, resulting in a bleeding rate of ∼0.1 ml/s. Ultrasound interrogation (Color Doppler or harmonic imaging) was performed from the anterior surface on the organs. Ultrasound contrast agent (Optison at a dose of 0.1 ml/kg) was injected into the ear vein, and imaging was continued for up to 2 min after injection. Without contrast agent, no bleeding could usually be detected at the incision site. However, after Optison was injected, the incisions would turn bright indicating an active bleeding site. Contrast enhancement at the bleeding site appeared within 15 s after the Optison injection, and lasted for 40–50 s, after which the images returned to pre-contrast appearance. Therefore, our results indicated that ultrasound contrast agents can be used to improve localization of injuries with low-bleeding rates Citation[48].
Future directions
The future of acoustic hemostasis technology depends on the degree of its success in providing an alternative solution for hemorrhage control in scenarios for which no other methods are either available or equally successful. A typical example of this scenario is in trauma, in which: the patients can be outside of a hospital setting, the time window for the application of therapy is short, the power requirements for cauterization energy can be very demanding due to high bleeding rates, the device needs to be carried to a potentially remote location, the training requirements cannot be stringent if first responders are to apply the treatment, the work area is not clean, and the method has to be safe and effective with high sensitivity and specificity. There is currently no solution for hemorrhage control in such a scenario, except using tourniquets, which are only useful for peripheral vascular injuries, are often used incorrectly, and carry the risk of causing ischemia when used for long periods of time (e.g. during transportation to the hospital). Overall, the challenges of acoustic hemostasis include: (1) bleeding detection for slow bleeds, (2) hemorrhage control for fast bleeds, (3) detection and treatment of bleeding vessels located deep in the tissue, (4) avoiding collateral damage to nearby nerves and blood vessels, (5) achieving coupling to skin/tissue, with minimal preparation in terms of hair removal and degassing, (6) diverse body habitus of fat, bone, and air contents, (7) anatomy that is rapidly changing with persistent bleeding, (8) potential presence of bones, gas, and foreign body fragments in the tissue, and (9) automation of the bleeding detection and application of hemostasis treatment. It is by meeting these challenges that acoustic hemostasis can provide a valuable tool for advanced trauma care.
Acknowledgements
This work was supported by DARPA Deep Bleeder Acoustic Coagulation grant W81XWH-06-C-0025, US Army Remote Acoustic Hemostasis grant DAMD17-02-2-0014, National Institutes of Health grant R01 EB00292, and National Space Biomedical Research Institute grant through NASA NCC 9-58.
References
- Greenfield L. Surgery: Scientific principles and practice. Lippincott Williams & Wilkins. 2005
- Bonnie RJ, Fulco C, Liverman CT. Institute of medicine (US). Committee on injury prevention and control. Reducing the burden of injury: Advancing prevention and treatment. National Academy Press, Washington, DC 1999
- Starnes BW, Beekley AC, Sebesta JA, Andersen CA, Rush RM, Jr. Extremity vascular injuries on the battlefield: Tips for surgeons deploying to war. J Trauma 2006; 60: 432–442
- Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, et al. The epidemiology of traumatic death. A population-based analysis. Arch Surg 1993; 128: 571–5
- Anderson RN. Deaths: Leading causes for 2000. Natl Vital Stat Rep 2002; 50: 1–85
- MacKenzie E, Fowler C. Epidemiology. Trauma, KFD Mattox, EE Moore. McGraw Hill, New York, NY 2000; 21–40
- Miller TR, Lestina DC. Patterns in US medical expenditures and utilization for injury, 1987. Am J Public Health 1996; 86: 89–93
- Rice DP, Max W. The high cost of injuries in the United States. Am J Public Health 1996; 86: 14–15
- Vaezy S, Andrew M, Kaczkowski P, Crum L. Image-guided acoustic therapy. Annu Rev Biomed Eng 2001; 3: 375–390
- Hill CR. Optimum acoustic frequency for focused ultrasound surgery. Ultrasound Med Biol 1994; 20: 271–277
- Leighton T. The acoustic bubble. Academic Press, San Diego 1994
- Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 2001; 27: 1399–1412
- Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 2003; 48: 223–241
- Poliachik SL, Chandler WL, Mourad PD, Ollos RJ, Crum LA. Activation, aggregation and adhesion of platelets exposed to high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1567–1576
- Vaezy S, Vaezy S, Starr F, Chi E, Cornejo C, Crum L, Martin RW. Intra-operative acoustic hemostasis of liver: Production of a homogenate for effective treatment. Ultrasonics 2005; 43: 265–269
- Delon-Martin C, Vogt C, Chignier E, Guers C, Chapelon JY, Cathignol D. Venous thrombosis generation by means of high-intensity focused ultrasound. Ultrasound Med Biol 1995; 21: 113–119
- Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol 1996; 22: 193–201
- Hynynen K, Colucci V, Chung A, Jolesz F. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med Biol 1996; 22: 1071–1077
- Ishikawa T, Okai T, Sasaki K, Umemura S, Fujiwara R, Kushima M, et al. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med Biol 2003; 29: 1471–1477
- Denbow ML, Rivens IH, Rowland IJ, Leach MO, Fisk NM, ter Haar GR. Preclinical development of noninvasive vascular occlusion with focused ultrasonic surgery for fetal therapy. Am J Obstet Gynecol 2000; 182: 387–392
- Rivens IH, Rowland IJ, Denbow M, Fisk NM, ter Haar GR, Leach MO. Vascular occlusion using focused ultrasound surgery for use in fetal medicine. Eur J Ultrasound 1999; 9: 89–97
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol 2002; 28: 535–542
- Cornejo CJ, Vaezy S, Jurkovich GJ, Paun M, Sharar SR, Martin RW. High-intensity ultrasound treatment of blunt abdominal solid organ injury: An animal model. J Trauma 2004; 57: 152–156
- Vaezy S, Noble ML, Keshavarzi A, Paun M, Prokop AF, Cornejo C, et al. Liver hemostasis with high-intensity ultrasound: Repair and healing. J Ultrasound Med 2004; 23: 217–225
- Noble ML, Vaezy S, Keshavarzi A, Paun M, Prokop AF, Chi EY, et al. Spleen hemostasis using high-intensity ultrasound: Survival and healing. J Trauma 2002; 53: 1115–1120
- Cohn SM, Cross JH, Ivy ME, Feinstein AJ, Samotowka MA. Fibrin glue terminates massive bleeding after complex hepatic injury. J Trauma 1998; 45: 666–672
- Martin RW, Vaezy S, Kaczkowski P, Keilman G, Carter S, Caps M, et al. Hemostasis of punctured vessels using Doppler-guided high-intensity ultrasound. Ultrasound Med Biol 1999; 25: 985–990
- Vaezy S, Martin R, Yaziji H, Kaczkowski P, Keilman G, Carter S, et al. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med Biol 1998; 24: 903–910
- Zderic V, Keshavarzi A, Noble ML, Paun M, Sharar SR, Crum LA, et al. Hemorrhage control in arteries using high-intensity focused ultrasound: A survival study. Ultrasonics 2006; 44: 46–53
- Vaezy S, Shi X, Martin RW, Chi E, Nelson PI, Bailey MR, et al. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol 2001; 27(1)33–42
- Rabkin BA, Zderic V, Vaezy S. Hyperecho in ultrasound images of HIFU therapy: Involvement of cavitation. Ultrasound Med Biol 2005; 31: 947–956
- Burgess S, Zderic V, Vaezy S. Image-guided acoustic hemostasis for treatment of hidden liver injuries. 2007; 33: 113–119, Ultrasound Med Biol
- Held RT, Zderic V, Nguyen TN, Vaezy S. Annular phased-array high-intensity focused ultrasound device for image-guided therapy of uterine fibroids. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: 335–348
- Melodelima D, Chapelon JY, Theillere Y, Cathignol D. Combination of thermal and cavitation effects to generate deep lesions with an endocavitary applicator using a plane transducer: Ex vivo studies. Ultrasound Med Biol 2004; 30: 103–111
- Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum LA. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. J Acoust Soc Am 2006; 119: 1834–1848
- Balci NC, Sirvanci M, Tufek I, Onat L, Duran C. Spontaneous retroperitoneal hemorrhage secondary to subcapsular renal hematoma: MRI findings. Magn Reson Imaging 2001; 19: 1145–1148
- Miller FH, Kline MJ, Vanagunas AD. Detection of bleeding due to small bowel cholesterol emboli using helical CT examination in gastrointestinal bleeding of obscure origin. Am J Gastroenterol 1999; 94: 3623–3625
- Sato T, Hirose Y, Saito H, Yamamoto M, Katayanagi N, Otani T, et al. Diagnostic peritoneal lavage for diagnosing blunt hollow visceral injury: The accuracy of two different criteria and their combination. Surg Today 2005; 35: 935–939
- McGahan PJ, Richards JR, Bair AE, Rose JS. Ultrasound detection of blunt urological trauma: A 6-year study. Injury 2005; 36: 762–770
- Poletti PA, Kinkel K, Vermeulen B, Irmay F, Unger PF, Terrier F. Blunt abdominal trauma: Should US be used to detect both free fluid and organ injuries?. Radiology 2003; 227: 95–103
- Catalano O, Lobianco R, Raso MM, Siani A. Blunt hepatic trauma: Evaluation with contrast-enhanced sonography: Sonographic findings and clinical application. J Ultrasound Med 2005; 24: 299–310
- Catalano O, Nunziata A, Lobianco R, Siani A. Real-time harmonic contrast material-specific US of focal liver lesions. Radiographics 2005; 25: 333–349
- Miele V, Buffa V, Stasolla A, Regine G, Atzori M, Ialongo P, et al. Contrast enhanced ultrasound with second generation contrast agent in traumatic liver lesions. Radiol Med (Torino) 2004; 108: 82–91
- Catalano O, Lobianco R, Sandomenico F, Siani A. Splenic trauma: Evaluation with contrast-specific sonography and a second-generation contrast medium: Preliminary experience. J Ultrasound Med 2003; 22: 467–477
- Glen P, MacQuarrie J, Imrie CW, Leen E. A novel application of ultrasound contrast: Demonstration of splenic arterial bleeding. Br J Radiol 2004; 77: 333–334
- Liu JB, Merton DA, Goldberg BB, Rawool NM, Shi WT, Forsberg F. Contrast-enhanced two- and three-dimensional sonography for evaluation of intra-abdominal hemorrhage. J Ultrasound Med 2002; 21: 161–169
- Schmiedl UP, Carter S, Martin RW, Eubank W, Winter T, Chang PP, et al. Sonographic detection of acute parenchymal injury in an experimental porcine model of renal hemorrhage: Gray-scale imaging using a sonographic contrast agent. AJR Am J Roentgenol 1999; 173: 1289–1294
- Luo W, Zderic V, Carter S, Crum L, Vaezy S. Detection of bleeding in injured femoral arteries with contrast-enhanced sonography. J Ultrasound Med 2006; 25: 1169–1177