Abstract
Aim: In this study, we examined the efficacy of whole body hyperthermia (WBH) on obesity-induced insulin resistance in diabetic mice.
Methods: Male db/db mice were treated with WBH 3 times per week for 12 weeks. The rectal temperature of mice reached 38.0°C 5 min after heating, and was kept at 38.0°C for 30 min. At the end of each week, tail snip glucose levels were determined under fasting conditions. The GLUT-4 gene expression of muscle tissue was analyzed by real-time PCR.
Results: (1) WBH-treated db/db mice showed a significant decrease in fasting blood glucose level as compared with untreated db/db mice (p < 0.01). (2) Plasma insulin levels in untreated db/db mice at the age of 10 weeks were significantly increased compared with those of db/+ mice (p < 0.0001). On the other hand, the reduction (31%) in insulin levels in WBH-treated mice indicated improved insulin sensitivity. (3) The ability of WBH to increase insulin sensitivity was further established in glucose tolerance tests and insulin tolerance tests. (4) Urine albumin of db/db mice significantly increased compared with those of db/+ mice at 18 weeks of age (p < 0.001). This increase in urinary albumin was significantly inhibited by WBH (p < 0.01). (5) WBH up-regulated the expression of GLUT4 mRNA in skeletal muscle.
Conclusion: Although the mechanisms have not yet been completely investigated, WBH may provide a new therapeutic or preventive modality against obesity-related diseases such as T2DM and metabolic or insulin resistance syndrome.
| Abbreviations | ||
| WBH, | = | Whole body hyperthermia |
| T2DM, | = | Type 2 diabetes mellitus |
| FIR, | = | Far-infrared rays |
| GTT, | = | Glucose tolerance test |
| FFA, | = | Free fatty acid |
| PAS, | = | Periodic acid-Schiff stain |
| GLUT-4, | = | Glucose transport protein-4 |
Introduction
Obesity is strongly associated with the development of insulin resistance Citation[1]. Insulin resistance is a significant pathogenic factor in type 2 diabetes mellitus (T2DM) and metabolic syndrome. Physical exercise improves reduced insulin sensitivity in lifestyle-related diseases including T2DM. Exercise is recommended not only as an adjunct therapy to facilitate weight reduction, which has been shown to be an effective treatment to enhance insulin sensitivity Citation[2], but also as a beneficial therapy independent of weight reduction in preventing T2DM Citation[3]. The effects of exercise represented by in vivo improved insulin action are attributed chiefly to changes in muscular factors such as increased muscle volume Citation[4] and increased rate of blood flow to the exercising muscle Citation[5]. Blood flow is a critical parameter for obtaining satisfactory temperature distributions during clinical hyperthermia Citation[6]. In hyperthermia, as in exercise, muscle blood flow is greater in skeletal muscle in which higher temperatures are recorded. Thus, these facts raise a possible scenario that hyperthermia may improve reduced insulin sensitivity in T2DM by increasing muscle blood flow.
In this study, we treated db/db mice with whole body hyperthermia (WBH) (38°C of rectal temperature for 30 min) to investigate the efficacy of hyperthermia on insulin resistance in T2DM.
Materials and Methods
Animal care and treatment
Male C57BL/KsJ-Lepdb/db mice (db/db mice) and their lean littermates (C57BL/KsJ- Lepdb/+ mice, db/+ mice) were obtained at 5 weeks of age from Keari (Osaka, Japan). The animals were housed at 22°C in a controlled environment with 12 h of artificial light per day. They were allowed access to normal laboratory chow and water ad libitum. The db/db mice were used at 6 weeks of age after acclimation. The db/db mice and lean mice were weighed and tail snip glucose levels were determined by the glucose oxidase method (Terumo Co., Tokyo, Japan). The animals were separated into four groups (n = 5) on the basis of plasma glucose levels and body weight. The following method of whole body hyperthermia was employed. The WBH was performed in an insulated room lined with polyurethane. Far-infrared rays (FIR) panels were setted up on both the floor and ceiling, and controlled at 60°C for the first 5 min, then at 40°C for 30 min. The room temperature was then controlled at 36–38°C; the rectal temperature of mice reached 38.0°C 5 min after heating and was kept at 38.0°C for 30 min. Whole body hyperthermia was performed three times a week for five mice in WBH-treated db/db group from the age of 6 weeks to the age of 18 weeks.
Serum chemistry and tissue analysis
Tail bleed samples (10–20 µL) were taken to determine the glucose levels under fasting conditions at the end of each week. Blood samples were taken from the animals by the right atriums at 10 weeks and 18 weeks of age. Plasma triglyceride and free fatty acid (FFA) levels were measured by enzymatic methods (GPO.HDAOS method, glycerol blanking method). Plasma insulin levels were determined with a commercial kit (Morinaga Institute of Biological Science, Yokohama, Japan). A gross estimation of food consumption was determined in db/db mice and lean mice at 9 weeks and 16 weeks of age as follows. Food was measured at 18:00 h each day and the difference between the starting and ending quantities was divided by the number of mice per cage for an index of estimated 24 h food consumption per mouse. Well-mixed 24 h urine (1.0–1.1 µL in case of db/m mice, 3.8–14.4 µL in case of untreated db/db mice, and 1.1–3.5 µL in case of WBH-treated db/db mice) was collected from mice at 16 weeks of age in metabolic cages. After the urine was centrifuged at 3000g for 10 min, 1 ml of supernatant was stored at −80°C until it was analyzed. Urinary albumin levels were measured by a competitive enzyme-linked immunosorbent assay (ELISA) (Albuwell M, Exocell Inc., Philadelphia, PA, USA) according to the manufacturer's instructions. For morphological analysis of the glomeruli, half of the middle portion of each left kidney was fixed with 10% buffered formalin and embedded in paraffin. Slices 4 µm thick were stained with periodic acid-Schiff (PAS) stain. The skeletal muscle were harvested and frozen immediately in liquid nitrogen for analysis of real-time polymerase chain reaction.
Glucose and insulin tolerance tests
An intraperitoneal glucose tolerance test (GTT) was performed at 1.0 g/kg (20% solution of glucose, Otsuka, Japan). After a 12 h fast beginning at 21:00 h, a base line 0 min sample was taken followed by an intraperitoneal injection of glucose. Tail bleed samples (10–20 µL) were taken at 30, 60, and 120 min following glucose injection. For the insulin tolerance test, an intraperitoneal injection of insulin (3.0 units/kg, Sigma) was given under 12 h fast conditions. Tail bleed samples were taken at 0, 30, 60, and 90 min for measurement of blood glucose.
Real-time-polymerase chain reaction
Total RNA was isolated by using an Isogen kit (Nippon Gene, Tokyo, Japan) according to the manufacturer's instruction. The concentration of RNA was determined from the absorbance at 260 nm in relation to the absorbance at 280 nm. RNA was stored at −70°C until reverse transcription was performed. An aliquot (1 µg) of extract RNA was reverse-transcribed into first-strand complementary DNA (cDNA) at 42°C for 40 min, using 100 U/ml reverse transcriptase (Takara Biochemicals, Shiga, Japan) and 0.1 µM of oligo (dT)-adapter primer (Takara) in a 50 µL reaction mixture. Real-time polymerase chain reaction (PCR) was carried out with a 7300 Real Time PCR system (Applied Biosystems, Foster City, CA, USA) using the DNA-binding dye SYBER Green I for the detection of PCR products. The reaction mixture (RT-PCR kit, Code RRO43A, Takara) contained 12.5 µL Premix Ex Taq, 2.5 µL SYBER Green I, custom-synthesized primers, ROX reference dye, and cDNA (equivalent to 20 ng total RNA) to give a final reaction volume of 25 µL. The specific primers used for PCR are shown as follows:
GLUT4 sense 5′-CATGGCTGTCGCTGGTTTCT-3′
antisense 5′- GCATCCGCAACATACTGGAA-3′
The PCR settings were as follows: Initial denaturation of 10 s at 95°C was followed by 40 cycle of amplification for 5 s at 95°C and 31 s at 60°C. The amplification products were quantified using standard DNA. The relative expression was then calculated as the density of the product of the respective target gene divided by that for β-actin from the same cDNA.
Ethical considerations
The maintenance of the animals and the experimental procedures performed on them were carried out in accordance with the National Institute of Health guidelines for the use of experimental animals. All procedures were approved by the Animal Care Committee of Kyoto Prefectural University of Medicine (Kyoto, Japan).
Statistical analysis
The results are presented as means ± SEM. The data were compared using one-way analysis of variance (ANOVA); differences were considered significant if the P-value was <0.05 according to PLSD or Scheffe's F multiple comparison test. All analyses were performed using the Stat View™ 4.11-J software program (Abacus Concepts Inc., Berkeley, CA, USA) on a Macintosh computer.
Results
Concentrations of plasma glucose and insulin
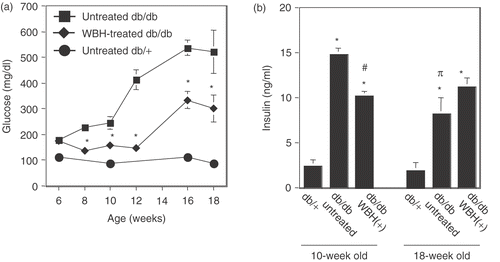
The db/db mice, a model for T2DM, exhibited hyperglycemia associated with obesity as compared with their nondiabetic db/+ littermates at 6 weeks of age. The food intake of db/db mice was 1.4–1.6-fold higher than that of db/+ mice. Throughout the experimental period (6–18 weeks of age), the food intake of WBH-treated mice was almost the same as that of untreated db/db mice. After 1 week of treatment with WBH, db/db mice showed a significant decrease in fasting blood glucose level as compared with untreated db/db mice (). Concomitant reduction in insulin levels in overnight-fasted WBH-treated mice at the age of 10 weeks (untreated db/db vs. WBH-treated db/db: 14.5 ± 1.37 ng/ml vs. 10.2 ± 1.04 ng/ml, decrease in 29.7%) indicated improved insulin sensitivity despite mild insulin resistance. On the other hand, the plasma insulin levels in overnight-fasted WBH-treated mice at the age of 18 weeks (untreated db/db vs. WBH-treated db/db: 8.21 ± 3.58 ng/ml vs. 11.9 ± 2.14 ng/ml, increase in 44.9%) were significantly increased compared with those of untreated db/db mice. Moreover, plasma insulin levels in overnight-fasted untreated db/db mice at the age of 18 weeks were significantly decreased compared with those of untreated db/db mice at the age of 10-weeks. Plasma insulin levels in the WBH-treated group showed no significant difference between that of 10-week-old mice and that of 18 week-old mice (). These data showed that chronic secretion of large amounts of insulin could lead to pancreatic β cell failure and WBH prevented this harmful phenomenon.
Figure 1. Effect of whole body hyperthermia on plasma glucose and insulin in db/db mice. Six-week-old db/db and db/+ mice were given free access to food and water. Whole body hyperthermia (38°C of rectal temperature for 30 min) was performed three times a week. In all panels, values are the means ± SEM of five mice, squares and solid lines represent the untreated db/db group, diamonds and solid lines represent the WBH-treated group, and solid circles and solid lines represent db/+ mice without whole body hyperthermia. (a) Fasting blood glucose concentrations in 6-week-old mice and 18-week-old mice. *p < 0.01 vs. untreated mice. (b) Fasting insulin concentrations in 10-week-old mice and 18-week-old mice. *p < 0.0001 vs. db/+ mice, #p < 0.001 vs. 10-week-old untreated mice, πp < 0.001 vs. 10-week-old untreated mice.
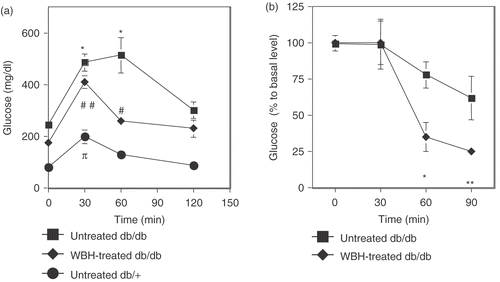
Glucose and insulin tolerance tests
According to the results shown in , the untreated db/db mice at the age of 10 weeks showed hyperglycemia and hyperinsulinemia, which indicated the status of insulin resistant. Therefore, the ability of WBH to increase insulin sensitivity was further established in glucose tolerance tests and insulin tolerance tests after the treatment with WBH for 4 weeks (from the age of 6 weeks to the age of 9 weeks, three times a week). These tests were performed 24 h after the last WBH. As shown in , db/db mice treated with WBH showed a remarkable improvement in plasma glucose control during the glucose challenge compared with untreated db/db mice. Intraperitoneal injection of insulin (3.0 units/kg) had no significant effect on blood glucose concentration in untreated db/db mice. However, the same insulin dose caused a decrease in blood glucose when given to WBH-treated mice ().
Figure 2. Effect of whole body hyperthermia on glucose and insulin tolerance tests in db/db mice. (a) For glucose tolerance tests, glucose (1.0 g/kg) was administered by intraperitoneal injection after an overnight fast. After administration of glucose, the plasma level of glucose was measured immediately before and 30, 60 120 min after administration of glucose. If insulin response against for administered glucose was appropriate, the plasma level of glucose, which was transiently increased, showed a speedy recovery. *p < 0.0001 vs. 0 min of untreated db/db mice, #p < 0.05, ##p < 0.0001 vs. 0 min of WBH-treated db/db mice. Pp < 0.05 vs. 0 min of untreated db/+ mice. (b) For insulin tolerance tests, an intraperitoneal injection of insulin (3.0 units/kg) was given after an overnight fast. After administration of insulin, the plasma level of glucose was measured immediately before and 30, 60, and 90 min after administration of insulin. If insulin sensitivity was kept appropriately, the plasma level of glucose decreased in response to administered insulin. Tail bleed samples were taken at 0, 30, 60, and 90 min for measurement of blood glucose concentrations. *p < 0.05, **p < 0.01 vs. 0 min of WBH-treated db/db mice.
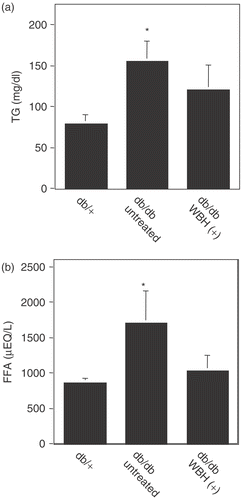
Concentrations of plasma triglyceride and free fatty acid
Blood glucose control might benefit from an improvement of insulin sensitivity, which may also result in a reduction of triglyceride and free fatty acid. In order to investigate the effects of WBH on serum levels of triglyceride and free fatty acid, we measured them at the age of 10 weeks. Untreated db/db mice showed a significant increase in triglyceride and free fatty acid levels as compared with db/+ mice (98% and 77% increase, respectively). Treatment with WBH induced the decrease of these plasma levels down to db/+ levels ().
Diabetic nephropathy
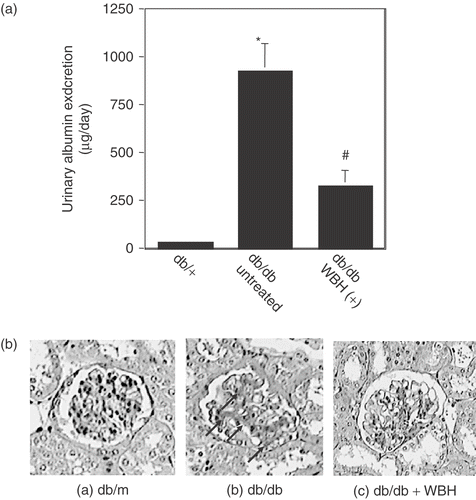
The db/db mouse exhibits clinical and histological features of diabetic nephropathy that track the human disease. Albumin levels in urine of db/db mice (923.8 ± 147.0 µg/day) significantly increased compared with those of db/+ mice (30.6 ± 11.7 µg/day) at 18 weeks of age. The increase in urinary albumin was significantly inhibited by WBH (332.1 ± 86.3 µg/day) (). There were no differences in the histology of renal tubes between db/+ and db/db mice. In contrast, the glomerular histology in db/db mice showed accelerated mesangial expansion characterized by an increase in periodic acid-Schiff stain (PAS)-positive mesangial matrix area relative to that observed in db/+ mice at 18 weeks of age. On the other hand, treatment with WBH partially reversed the mesangial matrix accumulation that had been established by 18 weeks of age ().
Figure 4. Effect of whole body hyperthermia on the induction of Diabetic nephropathy in db/db mice. (a) Effect of whole body hyperthermia on the urinary albumin excretion rate of db/db mice. A 24-h urine sample from each mouse was collected in metabolic cages 10 weeks after the start of this experiment. *p < 0.001 vs. db/+ mice and #p < 0.01 vs. untreated db/db mice. (b) Effect of whole body hyperthermia on accelerated mesangial expansion in db/db mice. Paraffin-embedded sections of the renal cortex were stained with periodic acid-Schiff stain. Representative light micrographs (magnification: 100 ×) from each of the groups are shown. (a) Normal glomerulus from a non-diabetic db/+ mouse at 18 weeks of age. (b) Glomerulus from an untreated db/db mouse at 18 weeks of age, showing hypertrophy and mesangial matrix expansion (arrows). (c) Glomerulus from a db/db mouse treated with 12 weeks of whole body hyperthermia until 18 weeks of age, depicting partial reversal of mesangial matrix expansion.
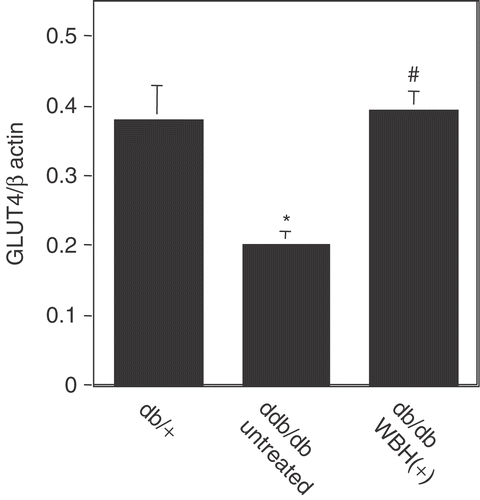
GLUT4 mRNA expression in mouse skeletal muscle
We further investigated the mechanisms by which WBH improved insulin resistance in db/db mice. Thirty to fifty percent of the insulin-mediated glucose disposal occurs in skeletal muscle, and decreased insulin-stimulated muscle glucose uptake is one of the mechanisms responsible for the development of insulin resistance. In this study, untreated db/db mice showed a significant decrease in GLUT4 mRNA level as compared with db/+ mice. However, WBH significantly increased the level of GLUT4 mRNA in WBH-treated skeletal muscle up to db/+ level ().
Figure 5. Real-time polymerase chain reaction analysis of GLUT4 mRNA expression in mouse skeletal muscle with or without whole body hyperthermia Total RNA was extracted from db/db or db/+ mice treated with or without whole body hyperthermia for 12 weeks and mRNA expression was analyzed by real-time polymerase chain reaction amplification. Actin was used as a control. Values are the means ± SEM of 4 mice. *p < 0.01 vs. db/+ mice and #p < 0.01 vs. untreated db/db mice.
Discussion
In this study, we indicated for the first time that WBH improves peripheral insulin action in the obesity and T2DM mouse model. We further demonstrate that WBH directly targets skeletal muscle, resulting in modifications in glucose transport through the induction of GLUT4. The decreased glucose uptake of skeletal muscle is one of the mechanisms responsible for the development of insulin resistance. Exercise stimulates the transport of glucose in skeletal muscle by causing movement of the GLUT4 isoform of the glucose transporter from intracellular sites to the cell surface, and induces an increase in GLUT4 protein in skeletal muscle Citation[5]. The abilities of glucose transport result from the quantity of GLUT4 protein present in the muscle. Holloszy et al. Citation[7] reported that the increases in cytosolic Ca2+ that occur in exercising muscle mediated the induction of GLUT4 protein in myocytes and skeletal muscle. The results of previous studies also showed that hyperthermia increases the cytosolic concentration of free Ca2+ Citation[8], Citation[9]. With previous reports, this study strongly suggested that WBH might induce the production of GLUT4 via an increase in the cytosolic concentration of free Ca2+. Thus, it is tempting to speculate that the improvement of insulin resistance by WBH might be at least partially mediated by increased blood flow, resulting in the increased delivery of both glucose and insulin to muscle, and by the induction of GLUT4 in skeletal muscle. This scenario is supported by the recent report from Horsman Citation[10], which suggests that the most important physiological parameter influencing tissue response to heat is blood flow, and is further supported by other studies that indicate that regional microwave heating generated a non-uniform increase of blood flow in normal skeletal muscle Citation[6]. Additional investigations are required to evaluate how WBH-mediated induction of GLUT4 could contribute to improve obesity-induced insulin resistance in diabetic mice and to study the other effects of WBH on insulin resistance in diabetic mice.
While exercise is a basic strategy for the treatment of T2DM Citation[2–5], many patients with T2DM, pre-T2DM, or metabolic syndrome are corpulent; therefore, it is difficult for them to practice exercise training. On the other hand, it may be easy for these patients to accept WBH. Many heat applicators for hyperthermia have already been developed. For example, radiofrequency heat and far-infrared ray heat applicators are utilized in the field of cancer therapies. Recently we have developed a far-infrared ray heat applicator for mild hyperthermia by making a refinement on the applicator for cancer treatment. It may be possible to apply this device in order to improve insulin sensitivity for corpulent patients. Although the mechanisms have not yet been completely addressed, we can conclude that WBH may provide a new therapeutic or preventive modality against obesity-related diseases such as T2DM and metabolic or insulin resistance syndrome.
References
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289: 76–79
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med 2002; 346: 393–403
- Horton E. Exercise and decreased risk of NIDDM. New Engl J Med 1991; 325: 196–198
- Yki-Järvinen H, Koivisto VA. Effect of body composition on insulin sensitivity. Diabetes 1983; 32: 965–969
- Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, Mueckler M, Sovijarvi A, Koivisto VA. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest 1993; 92: 1623–1631
- Akyurekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. Int J Hyperther 1997; 13: 481–496
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: Vidence for involvement of AMPK and Ca(2+). Am J Physiol-Endoc Metab 2002; 282: E1008–1013
- Kiang JG, McClain DE. N(omega)-nitro-L-arginine decreases resting cytosolic [Ca2+] and enhances heat stress-induced increase in cytosolic [Ca2+] in human colon carcinoma T84 cells. Chinese J Physiol 1999; 42: 153–159
- Kiang JG, McClain DE, Warke VG, Krishnan S, Tsokos GC. Constitutive NO synthase regulates the Na+/Ca2+ exchanger in human T cells: Role of [Ca2+]i and tyrosine phosphorylation. J Cell Biochem 2003; 89: 1030–1043
- Horsman MR. Tissue physiology and the response to heat. Int J Hyper 2006; 22: 197–203