Abstract
Purpose: The goal of this study was to determine if reduced availability of the DNA repair protein, MRE11, for the repair of damaged DNA is a basis for thermal radiosensitization induced by moderate hyperthermia. To test this hypothesis, we measured the total amount of MRE11 DNA repair protein and its heat-induced alterations in four human tumor cell lines requiring different heating times at 41°C to induce measurable radiosensitization.
Materials and methods: Human colon adenocarcinoma cell lines (NSY42129, HT29 and HCT15) and HeLa cells were used as the test system. Cells were irradiated immediately after completion of hyperthermia. MRE11 levels in whole cell extract, nuclear extract and cytoplasmic extracts were measured by Western blotting. The nuclear and cytoplasmic extracts were separated by TX100 solubility. The subcellular localization of MRE11 was determined by immunofluorescence staining.
Results: The results show that for the human tumor cell lines studied, the larger the endogenous amount of MRE11 protein per cell, the longer the heating time at 41°C required for inducing measurable radiosensitization in that cell line. Further, the residual nuclear MRE11 protein level, measured in the nuclear extract and in the cytoplasmic extract as a function of heating time, both correlated with the thermal enhancement ratio (TER).
Conclusions: These observations are consistent with the possibility that delocalization of MRE11 from the nucleus is a critical step in the radiosensitization by moderate hyperthermia.
Introduction
While hyperthermia is one of the most potent cellular radiosensitizers known Citation[1] clinical trials targeting high (∼43°C) tumor temperatures achieved limited success. However, recent and ongoing clinical trails continue to support the conclusion that the radiosensitizing effects of hyperthermia can enhance the effectiveness of radiation therapy Citation[2–9]. Thus, there is a need for better understanding of the radiosensitizing effects of moderate (∼41°C) hyperthermia. It is commonly believed that heat effects on the proteins involved in DNA repair pathways are responsible for heat-induced radiosensitization. Therefore, we have undertaken studies to determine the effects of moderate heat shock on proteins involved in DNA repair.
The lethal effects of radiation exposure result from damage to DNA, specifically DNA double strand-breaks and clustered lesions involving double strand-breaks. Hyperthermia is known to alter protein folding and affect protein associations within the cell Citation[10], Citation[11]. Thus, it is commonly believed that heat effects on the proteins involved in DNA repair pathways are responsible for heat-induced radiosensitization. In recent years there have been major advances in our understanding of how cells respond to DNA damage from ionizing radiation. These advances include discovery and delineation of signaling, DNA repair and checkpoint pathways Citation[10–13]. Knowledge of additional pathways or additional steps in a pathway increases the number of plausible, alternative mechanisms for heat-induced radiosensitization, which increases the number of possible mechanisms that need to be tested. Fortunately these discoveries also provide the biological tools to investigate the relationship between modulation of a given pathway and cell survival following radiation exposure.
While most researchers have long agreed that heat effects on the repair of DNA double strand-breaks should be the cause of heat-induced radiosensitization, recent attempts to determine which DNA double-strand-break-repair pathway is involved have produced conflicting results Citation[1], Citation[14]. Specifically, studies with a variety of mutants in which the nonhomologous-end-joining (NHEJ) pathway was knocked out found that these cell lines could be radiosensitized Citation[1]. Similar results were found in mutants lacking the homologous recombination (HR) pathway Citation[14]. These results suggest that either the repair of DNA double strand-breaks is not an important target for heat-induced radiosensitization or such radiosensitization results from protein alterations that affect multiple DNA repair pathways. In order to test the latter possibility, we have focused on the potential role of MRE11 as a determinant for heat-induced radiosensitization. The DNA repair protein, MRE11, forms a complex with Nbs1 and RAD 50 Citation[15], Citation[16] the MRN complex is believed to be involved upstream of both the NHEJ and HR pathways. Therefore, the present study was undertaken to determine if heat effects on the DNA repair protein, MRE11, could play a role in radiosensitization by clinically achievable thermal doses.
In previous studies using the human colon adenocarcinoma NSY cell line, we observed that 41°C hyperthermia induced the delocalization of MRE11 from the nucleus into the cytoplasm, a dissociation from one of its functional partners, Rad50, following heat exposures that radiosensitize NSY cells Citation[17]. Also we found: (i) that cells with reduced levels of MRE11 protein after siRNA knockdown were sensitive to radiation and no further thermal radiosensitization was induced by heating the cells at 41°C for 2 h Citation[18]; and (ii) that about 60% of the total amount of MRE11 protein was delocalized from nucleus and the 40% residual nuclear MRE11 protein formed extensive aggregation after heating for 2 h at 41°C and the MRE11 aggregates can be seen after 1 h at 41°C if cells are irradiated during heating Citation[19]. Based on these results, we proposed that the availability of the DNA repair protein, MRE11, for the repair of damaged DNA is a molecular mechanism for thermal radiosensitization induced by moderate hyperthermi Citation[17], Citation[18]. However, the question remains whether the heat effects on MRE11 observed in NSY cells are unique to that cell line or also true in cells with different thermal radiosensitivities. Therefore, in the present study, we determined the effects of 41°C hyperthermia on MRE11 localization in human tumor cells that required different thermal doses to induce radiosensitization.
Materials and methods
Cells and cell culture
The human colon adenocarcinoma cell lines HT29 and HCT15 were purchased from the American Tissue Culture Collection (ATCC). The NSY42129 (NSY) cell line was derived directly by one of us (MX) from a human colon adenocarcinoma Citation[20]. The HeLa S3 cell line was derived from a human cervical carcinoma and is routinely used in our lab. HeLa cells were cultured in DMEM medium. The other cells lines were cultured in RPMI 1640 medium. For all cell lines the culture media was supplemented with 10% fetal calf serum, 50 units/ml sodium penicillin G and 50 µg/ml streptomycin sulfate. Cell cultures were maintained at 37°C in a humidified incubator with 5% CO2.
Heat and radiation exposure
The heat and radiation exposures were carried out as previously described Citation[21]. Briefly, for the heat plus radiation experiment, exponentially growing cells in T25 flasks at 37°C were shifted to 41°C by placing the cultures in a water-jacketed CO2 incubator at that temperature Citation[21]. After the desired time at 41°C, cell cultures in T25 flasks were X-irradiated with a PANTAK pmc1000 X-ray machine (East Haven, CT, USA). At a dose rate of ∼0.9 Gy/min, the temperatures of the cell cultures were allowed to drop to 37°C and were irradiated within 10 min of the end of the heating time.
Colony formation assay
Exponentially growing cells were harvested by trypsinization (0.05% trypsin/53 mM EDTA) for ∼5 min at 37°C and the cell concentration was determined using a Coulter counter. An appropriate number of cells to produce approximately 50 colonies was seeded into T25-cm2 flasks and cultured at 37°C for 2 weeks. To correct for multiplicity, 500 particles containing one or a group of cells were counted microscopically from an aliquot of the culture. For each radiation dose, triplicate flasks were plated. Colonies were fixed with Carnoy's solution (3 parts methanol to 1 part acetic acid) and stained with crystal violet. Colonies containing more than 50 cells were scored. The surviving fraction was corrected for multiplicity and the numbers from the triplicate plates were averaged. The surviving fraction was corrected for any multiplicity and normalized for any cell killing by heat alone.
Preparations of the TX-100 soluble (cytoplasmic) and insoluble (nuclear) fractions
Exponentially growing cells were treated with heat and/or radiation using the protocols described above, or left cultured at 37°C as control. After treatment, cells were collected following trypsinization and washed two times with PBS. The pellet from the last PBS wash was re-suspended in 0.5 ml of 0.5% TX-100 solution (0.5% TX-100, 0.08M NaCl, 0.2 EDTA, pH 7.2) for HCT15 and HeLa cells and 0.5 ml of 1% TX-100 solution (1% TX-100, 0.08M NaCl, 0.2 EDTA, pH 7.2) for HT29 and NSY cells. (The different concentrations of TX100 used in this study are due to the different stabilities of nuclei, from the different cell lines, to the concentration of TX-100.) One criterion in selecting the TX100 concentrations used in this study was that the given concentration produced a 70% nuclear yield in a well-washed nuclear preparation. The suspension was passed through a 1-ml pipette three times and sedimented at 2000 rpm for 5 min. The supernatant, i.e. the TX-100 soluble fraction containing mainly cytoplasmic components, was removed from the tube and transferred to a 1.5-ml microcentrifuge tube containing 0.9 ml acetone for precipitation. After sedimentation at 10 000 rpm for 20 min, the supernatant was decanted and the pellet allowed to dry at room temperature for 1 h. The pellet was resuspended by homogenization in 70 λ TMNP solution (10 mM Tris base, 5 mM MgCl2, 10 mM NaCl, 0.1 mM phenylmethyl sulfony fluoride, pH 7.4) and a 10 λ aliquot was used for protein quantitation using the BioRad protein assay (BioRad Laboratories, Hercules, CA, USA). The pellet, i.e. the Triton X-100 insoluble fraction, containing mainly nuclear components, was resuspended in 70 λ TNMP solution and sonicated (two 10-s pulses on ice using a 60 sonic dismembrater (Fisher Scientific, St. Louis, MO, USA) at a setting of 100 W), and a 10 λ aliquot was removed for protein quantitation (see above). Then the samples were prepared for SDS-PAGE according to the method of Laemmli Citation[22]. An equal amount of protein was loaded onto each lane of a one-dimension SDS-PAGE gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes. After incubation of the membrane with blocking buffer (20% Gelatin, Sigma Company, St. Louis, MO, USA) overnight, the membrane was incubated with mouse anti-MRE11 monoclonal antibody (GeneTex, San Antonio, TX, USA, product number MRE11-12D7) at 1 : 4000 dilution and an anti-actin monoclonal antibody (MP Biotechnological Inc., clone 4) at 1 : 2000 dilution for 2 h. After extensive washing, the membranes were incubated with an alkaline phosphatase-conjugated goat anti-mouse antibody (Affinity Bioreagents, Neshanic Station, NJ) at a 1 : 800 dilution for 1 h. The blots were washed with PBS extensively and incubated with BCIP/NBT alkaline phosphatase substrate (Sigma Company, St. Louis, MO, USA, Lot 127H8200) on a rotator until the expected protein bands were visualized. The blots were scanned and quantitated using a Molecular Dynamic densitometer with ImageQuant software (Sunnyvale, CA, USA).
Immunofluorescence staining
Immunofluorescence staining was performed at room temperature unless otherwise noted. NSY cells were cultured at 37°C on coverslips for 48 h. Then the cells were washed three times with cold PBS and fixed with 3.7% formaldehyde in PBS containing 0.2% Triton X-100 for 20 min. The fixed cells were washed three times with PBS and permeabilized in cold acetone for 10 min. The permeabilized cells were washed three times with PBS and blocked with 10% goat serum for 30 min. The cells were incubated with mouse anti-MRE11 monoclonal antibody at a 1 : 80 dilution (GeneTex, San Antonio, TX, USA, product number MRE11-12D7) for 2 h. The cells were then washed with PBS at least three times and incubated with FITC-conjugated goat anti-mouse antibody at a dilution of 1 : 100 (Becton Dickinson, San Jose, CA, USA) at room temperature for 1 h. The stained cells were washed four times with 4°C PBS. The coverslips were mounted with Gelvatal (Monsanto Chemical Company, St. Louis, MO, USA) and observed using a Confocal microscope (Olympus Optical Company, Ltd, NY, USA) connected to a computer with Fluoview FV500 and a conventional microscope.
Results
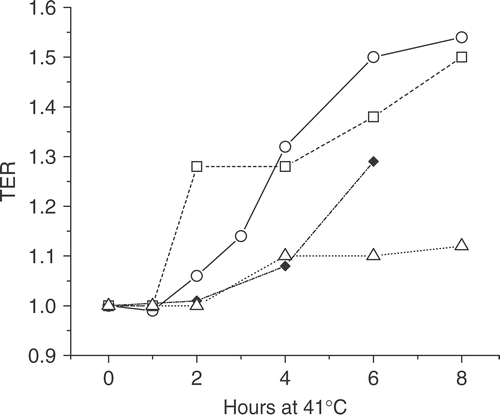
In order to test the relationship between MRE11 and radiosensitization we wanted to test cell lines with a variety of sensitivities to radiosensitization by 41°C hyperthermia. In other words, cell lines in which the time at 41°C required to increase the TER significantly. In general, complete survival curves were run (0–6 or 8 Gy) after heating times of up to 8 h. The TERs were calculated as the ratio of doses to achieve the 0.1 and those to achieve the 0.01 survival levels and averaged (see Citation[17]). shows that the time at 41°C required to produce a TER ≥ 1.2 was 6 h in HeLa cells; by contrast, for NSY cells the time required was 2 h. Intermediate between these two, HT29 cells required 4 h at 41°C to produce a TER ≥ 1.2. As an example of a cell line resistant to radiosensitization by 41°C hyperthermia, HCT15 cells showed a TER = 1.2 after 8 h at 41°C. In addition shows that the TER varied up to 1.54, providing a variety of values in between 1.0 and 1.5. Based on the variety of heating time intervals (2, 4 and 6 h) and TER values, as well as the presence of a negative example, we concluded that these four cell lines would be a reasonable system to test the relationship between MRE11 nuclear delocalization and radiosensitization induced by 41°C.
Figure 1. The thermal enhancement ratio (TER) as a function of time at 41°C. The time at 41°C required to increase the TER significantly varies with different tumor cell lines. The TER is shown for HeLa (solid diamonds), NSY (open squares), HT29 (open circles) and HCT15 (open triangles) cells after various time intervals of heating at 41°C. The TER values were calculated as the average of the dose ratios at 0.1 and 0.01 survival levels after X-irradiation without and with prior hyperthermia.
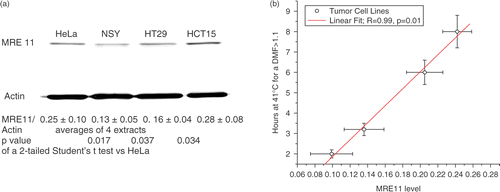
Prior to quantifying the effects of hyperthermia on the nuclear cytoplasmic distribution of MRE11, we first measured the amount of MRE11 in whole cell extracts relative to actin. The initial experiments suggested the amount of MRE11 appeared to vary slightly between the cell lines. To confirm that these cell lines had different levels of MRE11 we repeated the analysis on four separate extracts and performed a paired Student's t-test of the values, which showed that these slight differences in MRE11 levels were significant. To obtain a preliminary indication that heat effects on MRE11 might contribute to heat-induced radiosensitization, the average MRE11/actin values ( were plotted against the time at 41°C required for the first detectable TER (i.e. ≥ 1.12 which in the case of HCT15, was the highest average TER measured). The correlation () was linear with a p-value of 0.01 consistent with the possibility that heat effects on MRE11 do contribute to heat-induced radiosensitization.
Figure 2. Whole cell MRE11 levels relate to the time at 41°C needed to induce a measurable TER. The MRE11/actin ratios as measured by western blots of whole cell lysates are significantly different between four human tumor cell lines by a Student's t-test (a). The ratios are the average of four blots of separate cell lysates. The p-values are the results of a Student's t-test vs. the ratios observed in HeLa cells. Regression analysis (b) suggests that MRE11 levels within a certain range affect the thermal dose required to induce measurable heat-induced radiosensitization (TER > 1.1) by moderate hyperthermia.
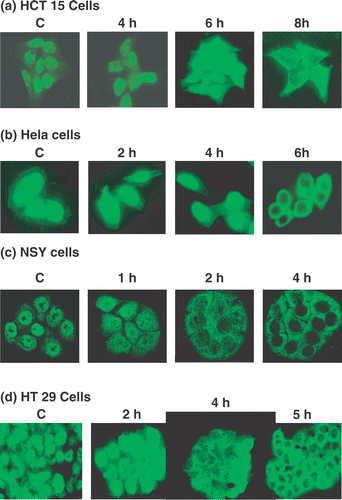
We next measured the subcellular distribution of MRE11 by in situ fluorescence immunostaining (). In HCT15 cells there was minimal MRE11 delocalization from nucleus into cytoplasm for up to 8 h of heating at 41°C (). In HeLa cells, after 6 h of heating a significant reduction in nuclear MRE11 could be observed (). By contrast, only 2 h at 41°C was required to significantly reduce nuclear MRE11 staining in NSY cells with a clear absence of such staining at 4 h (). HT29 cells appeared to be intermediate between HeLa and NSY cells with delocalization of MRE11 being detectable after 4 h and clear after 5 h at 41°C (). At 8 h, delocalization was apparent but its extent was not as great as that in the other cell lines (see below), consistent with the observation that the TER in HT29 cells only reached 1.12. Thus, the time at 41°C required to delocalize MRE11 from the nucleus corresponded approximately to the time required to increase the TER to 1.12 or more. To test this apparent relationship more quantitatively we conducted the subcellular fractionation experiments described below.
Figure 3. The subcellular distribution of MRE11 during 41°C hyperthermia. MRE11 in tumor cells was localized by immunofluorescence staining after the indicated time intervals at 41°C. (a) HCT15 cells, (b) HeLa cells, (c) NSY cells, (d) HT29 cells. In all panels ‘C’ indicates cells that were not heated.
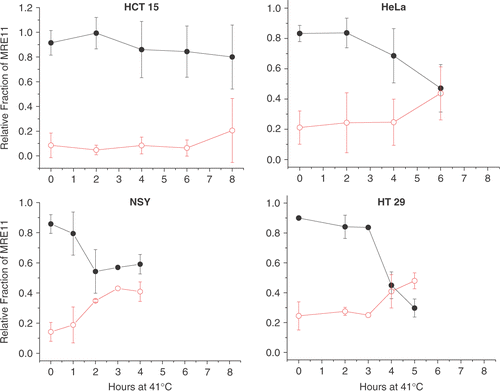
To quantify the relative levels of nuclear and cytoplasmic MRE11 levels we used a one-step fractionation procedure. Tumor cells were lysed in 1% Triton X100 and separated into soluble (SF) and insoluble fractions (ISF) by sedimentation as described in the Methods. While the nuclear fraction is not completely purified, the one-step separation has the advantage that little of the MRE11 is lost. By contrast, purification of nuclei would require washing steps that can result in the loss of some nuclear proteins. Western blots, using anti-MRE11 and anti-actin, of the SF (Cytoplasm) and ISF (nuclei with some perinuclear contamination) show () that with time at 41°C the amount of MRE11 in the cytoplasmic fraction increased with a corresponding decrease in the nuclear fraction. Further, the data from the four human tumor cell lines suggested that different time intervals at 41°C were required for a significant redistribution of MRE11 from the nuclear fraction to the cytoplasmic fraction. To quantify this effect, the density of the bands was measured and the amount in each fraction was computed as a fraction of the total. Actin levels served as loading controls. Total MRE11 was held as being constant because hyperthermia did not alter the whole cell MRE11 levels (data not shown). However, any normalization for loading or constant MRE11 level was minimal. In HCT15 cells (, upper left panel) there were no significant changes in the fraction of MRE11 in either the ISF or the SF during 8 h at 41°C. However, there was a trend showing a slight increase (∼10%) in the average MRE11 level in the SF and 10% decreased in that for the ISF over the 8-h heating interval. In HeLa cells (, upper right panel) there was no change in the fractions of MRE11 in either the ISF or SF during the first 2 h at 41°C. After approximately 4 h at 41°C, the fraction of MRE11 in the ISF was observed to decrease, while that in the SF increased, until at 6 h about 49% of MRE11 was in the ISF and 48% in the SF. In NSY cells (, lower left panel) after only 1 h at 41°C we observed a significant decrease in the fraction of MRE11 in the ISF with a corresponding increase in the SF. By 2 h the fraction of MRE11 in the ISF had decreased to ∼50%. In HT29 cells (, lower right panel) there was no significant change in the fractions of MRE11 in the ISF and SF fractions for 3 h at 41°C. After 4 h at 41°C, the fraction of MRE11 in the ISF had decreased to 43%. By 5 h the fraction of MRE11 in the SF had increased to 50%. These data show that there is significant variability between the cell lines in terms of the time at 41°C required to induce a significant reduction in the amount of MRE11 in the nucleus and a corresponding increase in the cytoplasm.
Figure 4. Western blots of the TX100 soluble (SF) and insoluble (ISF) fractions from four human tumor cell lines heated at 41°C. The loss of MRE11 from the ISF and increase in the SF represents the delocalization of MRE11 from the nucleus into the cytoplasm. The indicated tumor cells were heated at 41°C for the indicated time and the TX100 SF and ISF were separated as described in the text. The blots were probed with antibody against MRE11 and actin. Results from a typical experiment are shown.
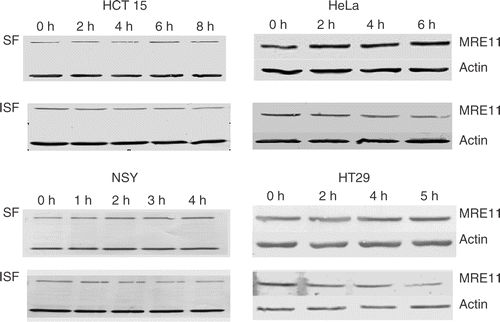
Figure 5. The fractions of MRE11 in the ISF and SF as a function of time at 41°C. Results from three to four separate experiments as shown in were quantified by densitometry as described in the text and averaged. The means, ± 1 SD, are plotted. The fraction of the total cellular MRE11 in the ISF is plotted with closed circles and that in the SF is plotted with open circles.
To determine how the redistribution of MRE11 from the nucleus into the cytoplasm might relate to radiation sensitivity, we determined if the fraction of MRE11 in either the ISF or the SF after a given heat shock correlated with the TER induced by that heat shock regardless of cell line. This analysis suggested that when the amount of MRE11 in the nucleus (ISF) decreased and/or increased in the cytoplasm (SF), then the TER would increase (). The underlying hypothesis was that the residual amount of MRE11 in the nucleus would be a critical factor. Therefore, we included a point from a previous study that reduced nuclear MRE11 levels using a siRNA Citation[18]. The plotted data () fell on a straight line, as determined by regression analysis, consistent with the TER being related to the residual levels of MRE11 in the nucleus. We found that the fit was better (i.e. a lower p-value if the data were fit with a slight threshold (i.e. 0.85) as shown in .
Figure 6. TER as a function of the subcellular localization of MRE11. The TER values from Table I are plotted as a function of the fraction of MRE11 in the ISF (nuclear) in (left panel) and as function of that in the SF (cytoplasmic) (right panel). Data from all four cell lines were included in this analysis. The point marked siRNA was replotted from Xu et al. Citation[18]. The slopes were obtained by linear regression analysis. The data for ISF fractions above 0.85 and SF fractions below 0.15 were excluded from the regression analysis (see text for further discussion).
![Figure 6. TER as a function of the subcellular localization of MRE11. The TER values from Table I are plotted as a function of the fraction of MRE11 in the ISF (nuclear) in Figure 4 (left panel) and as function of that in the SF (cytoplasmic) (right panel). Data from all four cell lines were included in this analysis. The point marked siRNA was replotted from Xu et al. Citation[18]. The slopes were obtained by linear regression analysis. The data for ISF fractions above 0.85 and SF fractions below 0.15 were excluded from the regression analysis (see text for further discussion).](/cms/asset/cc0a45e5-b917-4bcf-ab6a-8cd8d2165b29/ihyt_a_238189_f0006_b.gif)
Discussion
Although hyperthermia is one of the most potent known radiosensitizers, producing TERs of up to 3 or more Citation[1], the mechanism for heat-induced radiosensitization remains unclear. While conventional wisdom would suggest that inhibition of the repair of DNA double strand-breaks must be involved in heat-induced radiosensitization, the literature shows that the situation is not so simple Citation[1], Citation[14], Citation[23], Citation[24]. Cells defective in the non-homologous end-joining (NHEJ) pathway show essentially the same TERs as their isogenic wild-type cells Citation[1] even though the mutated cells were more radiosensitive than their wild-type counterparts. Similarly, cells defective in homologous recombination (HR) show essentially the same TERs as their isogenic wild type cells Citation[14]. The investigators interpreted these results to mean that neither of these pathways is a critical target for heat-induced radiosensitization. Recent studies of DNA repair underscore the concept that a step to sense DNA damage and a transduction step in which a complex of proteins is assembled at the site of DNA damage occur prior to the actual DNA repair process Citation[25]. The MRN complex appears to function in the sensing-transduction steps because MRE11 is one of the first proteins detected at the site of DSB Citation[26], the MRN complex is believed to align the ends of the DSB Citation[27] is required for NHEJ Citation[27] and HR Citation[28], and appears to modulate which of the pathways actually repair the double strand-break Citation[29]. Thus, heat effects on MRE11 availability as reviewed in Dynlacht et al. Citation[19], and described in this paper would represent such an upstream target and subsequently the ability of the MRN complex to function could affect both the NHEJ and the HR pathways equally, consistent with the previously published reports Citation[1], Citation[14].
Previous studies showed that moderate (41°C) and acute (43–45.5°C) hyperthermia both caused a delocalization of MRE11from the nucleus into the cytoplasm Citation[30], Citation[31]. In addition, moderate hyperthermia caused an aggregation of the nuclear matrix associated MRE11 and increased the association between MRE11 and HSP70 Citation[19]. These results show that moderate hyperthermia has significant alterations in the subcellular distribution of MRE11 and in its molecular associations. The results presented in this paper provide further evidence that a heat-induced reduction in nuclear MRE11 contributes to radiosensitization by moderate hyperthermia. Although all of the four cell lines require different thermal doses to induce measurable radiosensitization, the same correlation between residual nuclear MRE11 and the TER was the same for these cell lines. Similarly the fraction of MRE11 in the cytoplasm also correlated with TER. Differences in the heat dose required for increasing sensitivity to radiation appeared to be related to small differences in the amount of MRE11 per cell. These parameters would be interrelated through a mechanism, in which heat effects on MRE11 play a role in radiosensitization by moderate hyperthermia. Further siRNA knockdown studies produced a dose-modifying factor of ∼1.4, similar to the TER induced by hyperthermia when the levels of MRE11 had been reduced to the same extent by both treatments Citation[18]. Thus, the results of this paper, combined with published results provide strong evidence that thermal effects reducing the availability of MRE11 for DNA repair contributes to an increased radiation sensitivity of the heat shocked cells.
However, it appears that thermal effects on MRE11 cannot be the only effect of hyperthermia that contributes to radiosensitization, especially for TERs above 2. Our results show that the thermal effects on MRE11 cannot contribute to TERs equal to or above 2 because the regression line extrapolates to a TER of 1.75 at zero nuclear MRE11. While extrapolation of the data to zero MRE11 gives a TER of 1.75, the actual limit to the TER produced by heat effects on MRE11 may, in fact, be 1.5. Two reasons for this speculation are: (i) the TER in NSY cells did not increase above 1.5 when the time at 41°C was extended to 28 h Citation[21]; and (ii) when the NSY cells are radiosensitized, the residual nuclear MRE11 is aggregated with the nuclear matrix Citation[19] and may not be functional. While this conclusion could be solidified by molecular approaches, there are confounding issues with expressing MRE11 constructs. Experiments to increase expression of MRE11 are compromised by the fact that the much of the additional MRE11 is in the cytoplasm and not available for DNA repair. Further full knockdowns appear to be lethal. Thus, it will be difficult to investigate the effects of very low MRE11 levels. Interestingly, the ability of hyperthermia to redistribute all of the nuclear MRE11 may be limited, owing to the aggregation of MRE11 with the nuclear matrix.
One alternative mechanism that could conceivably apply to radiosensitization by moderate hyperthermia is cell cycle redistribution. However, there are two arguments against this possibility. One is that moderate hyperthermia causes an accumulation of cells in S-phas Citation[31], Citation[32]. Second, TERs of ∼1.3 are achieved in NSY cells prior to any significant cell cycle redistribution Citation[21]. Thus, it is unlikely that cell cycle redistribution plays a role in radiosensitization by moderate hyperthermia for heating times of one to a few hours. After 8 h at 41°C, we find evidence for cell cycle rearrangements that can contribute to radiosensitization Citation[21].
In conclusion, different thermal doses are required to induce radiosensitization in different tumor cell lines. Further, the thermal dose required to induce significant redistribution of MRE11 from the nucleus to the cytoplasm in these cell lines also varies among the cell lines. However, the relationship between residual nuclear MRE11 and the TER fell on a common regression line for all four cell lines, consistent with the hypothesis that thermal effects on MRE11 contribute to the increased radiation sensitivity induced by moderate hyperthermia.
Acknowledgement
This work was supported by our Program Project grant (PO1 CA104457-03) from the National Cancer Institute of the US Department of Health and Human Services.
References
- Kampinga H, Dikomey E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int J Radiat Biol 2001; 77: 399–408
- Jones E, Thaddeus L, Samulski V, Dewhirst MW, Alvarez-Secord A, Berchuck A, Clarke-Pearson D, Havrilesky LJ, Soper J, Prosnitz LR. A pilot phase II trial of concurrent radiotherapy, chemotherapy, and hyperthermia for locally advanced cervical carcinoma. Cancer 2003; 98: 277–282
- Van der Zee J, González D, van Rhoon G, van Dijk J, van Putten W, Hart A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomized, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L Malec MK, Lamb SA, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- International Collaborative Hyperthermia Group, Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WLJ, van Rhoon GC, van Dijk JDP, González González D, Princess Margaret Hospital/Ontario Cancer Institute, Liu F-F, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Overgaad J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Melloa O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European society for hyperthermic oncology. Lancet 1995; 345: 540–543
- Overgaad J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Melloa O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European society for hyperthermic oncology. Int J Hyperthermia 1996; 12: 3–20
- Xia H, Karasawa K, Hanyu N, Chang T-C, Okamoto M, Kiguchi Y, Kawakami M, Itazawa T. Hyperthermia combined with intra-thoracic chemotherapy and radiotherapy for malignant pleural mesothelioma. Int J Hyperthermia 2006; 22: 613–621
- Kamisawa T, Tu Y, Egawa N, Karasawa K, Matsuda T, Tsuruta K, Okamoto A. Thermo-chemo-radiotherapy for advanced bile duct carcinoma. World J Gastroenterol 2005; 11: 4206–4209
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Kregel KC. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002; 92: 2177–2186
- Gius D, Mattsony D, Bradbury CM, Smarty DK, Spitz DR. Thermal stress and the disruption of redox-sensitive signalling and transcription factor activation: Possible role in radiosensitization. Int J Hyperthermia 2004; 20: 213–223
- Lepock LR. How do cells respond to their thermal environment?. Int J Hyperthermia 2005; 21: 681–687
- Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (> or = 43°C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia 2004; 20: 131–139
- Hopfner K-P, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BAL, Karcher A, Henderson B, Bodmer J-L, McMurray CT, et al. The Rad50 zinc-hook is a structure joining MRE11 complexes in DNA recombination and repair. Nature 2002; 418: 562–566
- van den Bosch M, Bree RT, Lowndes NF. The MRN complex: Coordinating and mediating the response to broken chromosomes. EMBO Rep 2003; 4: 844–849
- Xu M, Myerson RJ, Straube WL, Moros EG, Lagroye I, Wang LL, Lee JT, Roti Roti JL. Radiosensitization of heat resistant human tumour cells by 1 hour at 41.1°C and its effect on DNA repair. Int J Hyperthermia 2002; 18: 385–403
- Xu M, Myerson RJ, Hunt C, Kumar S, Moros, EG, Straube WL, Roti Roti JL. Transfection of human tumour cells with MRE11 siRNA and the increase in radiation sensitivity and the reduction in heat-induced radiosensitization. Int J Hyperthermia 2004; 20: 157–162
- Dynlacht JR, Xu M, Pandita RK, Wetzel EA, Roti Roti JL. Effects of heat shock on the MRE11/Rad50/Nbs1 complex in irradiated or unirradiated cells. Int J Hyperthermia 2004; 20: 144–156
- Xu M, Wright WD, Higashikubo R, Roti Roti JL. 1996. Chronic thermotolerance with continued cell proliferation. Int J Hyperthermia 1996; 12: 645–660
- Xu M, Wright WD, Higashikubo R, Wang LL, Roti Roti JL. Thermal radiosensitization of human tumour cell lines with different sensitivities to 41.1°C. Int J Hyperthermia 1999; 15: 279–290
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Vujaskovic Z, Song CW. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia 2004; 20: 163–174
- Lepock JR. Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperthermia 2004; 20: 115–130
- Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis 2002; 23: 687–696
- Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: Spatiotemporal relationships among checkpoint and repair proteins. Cell 2004; 118: 699–713
- Huang J, Dynan WS. Reconstitution of the mammalian DNA double-strand break end-joining reaction reveals a requirement for an Mre11/Rad50/NBS1-containing fraction. Nucleic Acids Res 2002; 30: 667–674
- Yang Y-G, Saidi A, Frappart P-O, Min W, Barrucand C, Dumon-Jones V, Michelson J, Herceg Z, Wang Z-Q. Conditional deletion of Nbs1 in murine cells reveals its role in branching repair pathways of DNA double-strand breaks. EMBO J 2006; 25: 5527–5538
- Zhu W-G Seno JD, Beck BD, Dynlacht JR. Translocation of MRE11 from the nucleus to the cytoplasm as a mechanism of radiosensitization by heat. Radiat Res 2001; 156: 95–102
- Seno LD, Dynlacht JR. Intracellular redistribution and modification of proteins of the MRE11/Rad50/Nbs1 DNA repair complex following irradiation and heat-shock. J Cell Physiol 2004; 199: 157–170
- Iliakis G, Krieg T, Guan J, Wang Y, Leeper D. Evidence for an S-phase checkpoint regulating DNA replication after heat shock: A review. Int J Hyperthermia 2004; 20: 240–249
- Zolzer F, Streffer P. Quiescence in S-phase and G1 arrest induced by irradiation and/or hyperthermia in six human tumour cell lines of different p53 status. Int J Radiat Biol 2000; 76: 717–725
