Abstract
Purpose: To discuss the role of hyperthermia in optimizing the response to regional therapy for cancer.
Methods: A review of the current literature discussing mechanism of action, experimental models and prospective, randomized trials was performed.
Results: The mechanism of action of hyperthermia in combination with radiation and cytotoxic chemotherapy at the cellular and tissue levels is described. Data supporting the benefit of hyperthermia in conjunction with both regional, infusional chemotherapy, and locoregional radiation therapy is demonstrated. Several different histologic tumor types are covered, all with prospective evidence supporting the benefit of hyperthermia.
Conclusions: Although delivery methods for hyperthermia can be complex and difficult to implement, the data support its benefit and further endeavors to include hyperthermia as a component of regional therapy should be encouraged.
Introduction
Physicians and scientists in the field of oncology have been searching for a ‘magic bullet’ since the phrase was first coined by Paul Ehrlich at the turn of the twentieth century; however, one hundred years later we continue to treat cancer with multiple modalities across multiple specialities. Clinical trials today aim to add new therapies or tailor existing regimens to extract maximum clinical response and ameliorate toxicities. To this aim, hyperthermia has become a vital adjunct in regional tumor control due to its selective effect on cancer cells and its potentiation of chemotherapy and radiation therapy.
Cancer treatment can be separated into two major approaches: therapies aimed at locoregional control: generally surgical excision or radiation; and those aimed at systemic control: generally infusional therapy utilizing cytotoxic or, more recently, targeted chemotherapeutic agents. In a method first described by Oscar Creech in 1958, chemotherapy can also be delivered regionally by perfusing a limb or organ that has been isolated from the systemic circulation, thereby maximizing delivery of the agent and minimizing systemic toxicity Citation[1]. The addition of hyperthermia to regionally delivered agents has subsequently been demonstrated to enhance the efficacy of this approach.
The inherent acidity and low oxygen tension in tumors can provide resistance to both chemotherapy and radiation; however, these same factors render cells more susceptible to heat. Furthermore, the cytotoxicity of several antineoplastic drugs and the efficacy of radiation are enhanced at higher temperatures Citation[2–5]. Hyperthermia alone has shown complete response rates as high as 13% in several cancers, and clinical trials adding hyperthermia to current treatment regimens have reported as much as a 50% improvement in response rates, tumor control rates, and overall survival Citation[6].
History
Give me a chance to create fever and I will cure any disease.
Parmenides, 500 BC
Hyperthermia is one of the oldest recognized treatments for a multitude of ailments. Many papers point to the Egyptians and the Edwin Smith Surgical Papyrus (3000-2500 BC) as the first description of hyperthermia in medicine; however, the scrolls describe cautery and not hyperpyrexia in the cure of chest tumors. From Parmenides to Hippocrates, the Greeks believed heat was one of four body humors capable of both causing and curing disease. Hippocrates is credited with submersing patients in the desert sand or in hot baths to elevate core body temperature and effect cure. In the later nineteenth century, William Busch and Paul Bruns described complete remission of a facial sarcoma and recurrent melanoma, respectively, in patients suffering prolonged fever from erysipelas Citation[7], Citation[8]. At the turn of the century, a New York surgeon named William Coley took this a step further by actively infecting patients with streptococcus toxin to inflict fever. One inoculated patient developed a severe case of erysipelas followed by remission of tumors in his neck and tonsils Citation[9].
The first application of hyperthermia for regional cancer control dates to 1898 when the Swedish gynecologist F. Westermark treated cervical cancer by running hot water through an intracavitary spiral tube. He noted excellent clinical response in the seven patients treated Citation[10]. In 1959, Barnes Woodhall and colleagues perfused brain tumors with chemotherapeutics heated to 43°C. They later expanded the application to head and neck cancers; however, both experimental groups experienced little clinical response and severe morbidity and mortality Citation[11]. Renato Cavaliere followed but had limited success in 22 patients when he used hyperthermia alone to perfuse various limb malignancies Citation[12]. Severe complications occurred, including six deaths and three immediate amputations; however, he did note ten patients with complete response and seven with 3-year disease-free survival. In 1977 Jae Ho Kim used both wet heat, in the form of water bath immersion, and inductive heat, via electromagnetic fields, to treat cutaneous malignancies. When added to standard radiation, 27% of patients had improved disease-free survival, and when used alone, four of five patients had complete, though temporary, regression Citation[13], Citation[14]. It is due to these pioneers that the current use of hyperthermia in regional control has expanded to include cancers of soft tissue, gastrointestinal, neurologic, urologic, and gynecologic origin Citation[15–33].
Mechanism of effect
Macroscopic
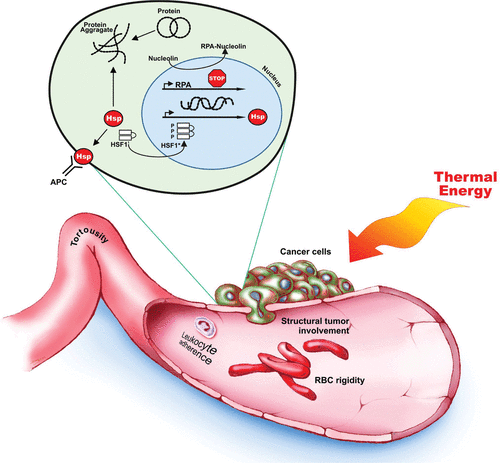
The rise in temperature in a tissue is dependent on the amount and duration of heat exposure, which is physiologically controlled by afferent heat delivery and efferent flow dissipation. In general, tissues with low blood flow will reach higher temperatures as heat accumulates. The appropriate physiologic response to heat is vasodilation via the noradrenergic sympathetic system followed by the recruitment of capillaries via bradykinin and histamine stimulation Citation[34]. The response of tumor vasculature is distinct from healthy vessels and provides for preferential heating. Upon initial heating, blood flow and oxygenation in tumors increases, thereby aiding in drug delivery and radiation sensitivity Citation[35]. As temperatures increase above 42°C, vascular collapse and necrosis ensue Citation[36]. This is due in part to the haphazard organization and construction of cancer neo-vascularization. Tumor vessels are bent, coiled, maximally dilated at rest, and often clogged with cancer cells Citation[37]. As temperatures increase in the tumor, vascular collapse ensues due to a preferential (i) increase in red blood cell rigidity, (ii) endothelial cell swelling, (iii) hemorrhage into capillary lumens, and (iv) leukocyte adherence to vessel walls () Citation[37]. This process is first seen in tissues at approximately 42°C and overwhelms all human tissue at 45°C Citation[36].
The architecture of a growing tumor also renders neoplastic cells more sensitive to heat. As cancer cells divide they distance themselves from a vascular source, decreasing their nutrient supply and oxygenation. In addition, the decline in available oxygen that accompanies heat-induced vascular collapse contributes to an overall drop in pH. Glycolitic metabolism and lactic acidosis, inherent to tumor cells, significantly increase upon heating and remain elevated for several hours Citation[37]. The acidic, hypoxic, and nutrient poor condition within a cancer, subsequently exacerbated by hyperthermia, makes an otherwise survivable condition incompatible with cell life Citation[38], Citation[39].
Microscopic
Cells resist heating; however, thermal damage will occur when the amount of heat delivered provides energy in the range of 100–150 kcal/mole Citation[40], Citation[41]. Heat imparts energy to cells in the form of molecular motion causing increased metabolism and transitions in cellular structures. Metabolism and enzymatic reactions require little activation energy (3−20 kcal/mol). A transition in DNA, macromolecules, or proteins refers to a change from an ordered, native state to a denatured state and requires a large input of energy (100–200 kcal/mole). Thus, the rate limiting step in thermal damage is likely to be the denaturation of proteins and DNA resulting in disruption of cellular structure and function Citation[41]. When exposed to sufficient energy, proteins unfold, are rendered insoluble, and aggregate into non-functioning clusters Citation[42]. Moreover the aggregates attract healthy proteins from the nucleus and cytosol due to exposed hydrophobic binding sites. As a result, heated cells show inactivation of membrane receptors, altered enzyme activity, altered cellular structures, and chromosomal damage and mis-repair.
Heat exposure preferentially affects cells in the synthesis phase (S phase) of the cell cycle Citation[5]. This has been shown to be the result of heat-induced damage to chromatin structure and nucleolin inactivation of Replication Protein A, a replication initiation and elongation regulatory protein Citation[5]. Inhibition of replication may actually be a protective mechanism to avoid heat induced errors in DNA synthesis and/or DNA replication. Thermal energy is known to cause poor initiation of replicons, disrupted elongation of DNA fibers at the replication fork, and inhibition of chromatin formation Citation[5], Citation[43–45] Elevated temperatures or prolonged exposures that overcome the heat shock response or disrupt DNA replication result in thermal injury and cell death.
Healthy cells exposed briefly to elevated temperatures will show resistance to future heating, so called thermotolerance. Heat shock proteins (HSPs) provide this tolerance by refolding insoluble proteins and preventing the formation of protein aggregates Citation[41]. These proteins are active under normal conditions; however, under stress, a heat shock transcription factor, HSF1, dramatically increases their production. HSF1 is normally bound to HSP and rendered inactive. An abundance of denatured proteins engages HSPs and frees HSF1 to transcribe more protein Citation[42], Citation[46]. Cells have been shown to increase production of HSPs when exposed to temperatures greater that 39°C or when laden with denatured protein aggregates. These proteins also translocate to the cell membrane after thermal exposure and act as membrane receptors for natural killer cells, macrophages, and antigen presenting cells. Of interest to tumor biology, HSP cell surface signaling is only seen in malignant cells and may provide prolonged immunologic tumor control Citation[47].
Experimental models
Animal models are of great utility to study the results of supra-normal temperatures on cancer cells. Giovanella and colleagues were some of the earliest to utilize the nude mouse as a vehicle to study the in vivo effects of heat on human tumors Citation[48]. A radio frequency generator was applied to heat tumors to between 40° and 43°C. Compared to controls, the authors noted a 75% growth inhibition in 11 of 16 superficial tumors and a 37% to 63% inhibition in 5 of 7 deep tumors. The benefits were from a single application of hyperthermia for 30 minutes and were seen in all tumor types. Recently, Pelz et al. used a rat model to differentiate between the benefits of adding hyperthermia to intraperitoneal chemoperfusion of colorectal carcinomatosis Citation[49]. Five groups of animals were treated: negative controls, sham operated controls, hyperthermic infusion without chemotherapeutics, chemotherapeutics without heat, and hyperthermia with intraperitoneal chemotherapeutics (HIPEC). Tumor load was significantly reduced in the heat alone and drug only groups while the combination HIPEC treatment group experienced the largest benefit (p < 0.036). These are but a few examples of animal models proving the efficacy of hyperthermia. Many others exist which demonstrate the utility of hyperthermia delivery methods, drug and radiation potentiation, and treatment related toxicities Citation[48], Citation[50–58].
Clinical trials/results
As previously described, initial accounts of hyperthermic therapy were subjective, anecdotal, and retrospective in nature. Fortunately, many groups from around the world have undertaken trials to evaluate the clinical efficacy and toxicity of heat therapy. For the purpose of this review, we will include only prospective, randomized, and well-controlled trials evaluating the role of hyperthermia in optimizing tumor response to regional cancer therapy ().
Table I. Randomized trials assessing the efficacy of hyperthermia.
Melanoma
Approximately 5–10% of patients who develop recurrent melanoma present as in-transit disease Citation[59]. Local therapy often requires multiple surgical resections, systemic treatment or, historically, individual injections into each lesion. For extremity disease, the evolution of regional chemotherapy has provided an excellent treatment option. In 1984, Ghussen et al. conducted the first prospective, randomized trial assessing the addition of hyperthermia to regional extremity perfusion of in-transit melanoma Citation[16], Citation[17]. One hundred and seven patients were randomized to receive either surgical excision or surgical excision followed by hyperthermic isolated limb perfusion (HILP) with melphalan, a nitrogen mustard compound. The primary end-point was disease-free survival, and the study was prematurely stopped (median follow-up 550 days) due to a highly significant advantage in the HILP arm (disease-free survival: 89% vs. 52%). In addition, there was an improvement in overall survival (98% vs. 86%) while avoiding local and systemic complications that had plagued previous perfusion reports. The efficacy of HILP was evident; however, the study was not designed to analyse the influence of hyperthermia alone.
In 1998, the European Organization for Research and Treatment of Cancer (EORTC), the World Health Organization, and the Southwest Oncology Group collaborated in Trial 18832 to assess the value of adjuvant HILP in high-risk, MD Anderson Stage I primary melanoma Citation[19]. At the time the study was initiated, the effect of HILP on recurrent melanoma and in-transit melanoma was clear; however, many retrospective studies had questioned whether HILP could improve tumor control at operation. A total of 832 patients with primary cutaneous melanomas greater than 1.5 mm in thickness were randomized to excision alone or excision plus HILP using melphalan. At a median follow-up of 6.4 years, the results showed that HILP decreased the development of in-transit metastases and regional lymph node metastases without impacting time to recurrence or overall survival. Due to high costs and limited benefits, the group concluded that HILP is not appropriate as an adjuvant therapy for primary melanoma.
The Danish Cancer Society evaluated the influence of hyperthermia on radiation by randomizing 70 patients with recurrent or metastatic melanoma to radiotherapy with or without hyperthermia Citation[18]. Heat was applied by microwave or radiofrequency equipment. The study yielded an overall high response rate and significant palliative effect, and the group receiving the combined treatment modalities had a significantly higher complete response initially and better local control at two years (46% vs. 28%). These results were observed in spite of the fact that only 14% of the hyperthermia treatments reached the pre-determined minimum temperature of 43°C. Nevertheless, they concluded that hyperthermia with radiation increases local tumor control of recurrent melanoma.
Soft tissue sarcoma (STS)
Patients with intermediate and high-grade STS are at considerable risk for local recurrence, and therefore limb-sparing surgery is generally combined with radiation therapy to improve local control. Despite the efficacy of combined surgery and radiation, as many as 25% of patients will have recurrences; additionally, many patients present with primary lesions abutting or involving vital structures. Neoadjuvant treatments may facilitate limb-sparing surgical approaches in these cases.
In 1996, several participants from the aforementioned EORTC 18832 trial for melanoma published data evaluating the benefit of HILP with melphalan and TNFα for limb-threatening sarcoma Citation[20]. The objectives were to achieve improved tumor response and increased limb salvage. One hundred and eighty-six patients received a 90-minute limb perfusion at 39° to 40°C followed by tumor resection several months later. HILP resulted in an 82% objective response rate and 82% limb salvage rate in a patient population with grade II and III sarcoma. Unfortunately, the study lacked a control group so results were compared to historical reports. Roughly 20% of patients experienced transient Grade 3 and 4 systemic toxicities, and a single patient underwent amputation due to a severe perfusion reaction.
Preliminary results of the EORTC 62961 trial, a study comparing neo-adjuvant chemotherapy with or without regional hyperthermia (RHT) in the treatment of high-risk soft tissue sarcomas, were recently presented at the 2007 meeting of the American Society of Clinical Oncology Citation[21]. Three hundred and forty-one patients were stratified according to site (extremity vs. non-extremity) and treated with radiation, a chemotherapy cocktail of etoposide, ifosfamide, and adriamycin with and without hyperthermia, and surgery. At a median follow-up of 24.9 months, the addition of hyperthermia resulted in a superior disease-free survival (31.7 months vs. 16.2 months) and local progression-free survival (45.3 months vs. 23.7 months). Results were noted in both extremity and non-extremity sarcomas.
These trials demonstrate the utility of hyperthermia in conjunction with a perfusate of cytotoxic chemotherapy, while the latter trial demonstrates the benefit of hyperthermia compared to non-hyperthermic conditions.
Breast cancer
The treatment of recurrent breast cancer poses a significant clinical dilemma. A previously irradiated field hinders surgical resection, decreases the delivery of drug therapies, and limits the radiation dose that can safely be administered often to a suboptimal level Citation[25], Citation[60]. Between 1988 and 1993, The International Collaborative Hyperthermia Group supervised five prospective trials with the purpose of determining if the addition of heat to radiation could enhance response rates in recurrent or inoperable breast cancer Citation[25]. In all, 306 patients were randomized to receive either radiotherapy alone or radiotherapy with electromagnetically induced hyperthermia. Radiation was given in ‘radical’ doses up to 50 Gy or in ‘palliative’ doses (less than 35 Gy) for those patients previously irradiated. Of note, the treatment goal of a minimum of 43°C for at least one hour was reached in a minority of patients. Analysis of the five trials showed that survival was similar in all groups, but the addition of hyperthermia increased local response rate by 50% (p < 0.001) and decreased local relapses by 40% (p = 0.007). Unfortunately, the response was only seen in those receiving the palliative dose of radiotherapy and there was discordance in response rates between centers.
A recent prospective, randomized trial was conducted to assess whether improved heat delivery, as measured by the duration of time that 90% of the tumor exceeds 43°C, can improve response and local control in superficial, recurrent cancers of the chest, head and neck, and skin Citation[61]. Patients were treated with either palliative or radical doses of radiation depending on their radiation history. Thereafter, either a single dose of radiofrequency induced hyperthermia was given or patients underwent multiple hyperthermic treatments (MHT) in order to achieve a duration of treatment above 43°C of 10 to 100 minutes. Results showed that the MHT group subjected to a longer duration of higher temperatures experienced improved complete response (42% vs. 66%) and duration of local control (p = 0.02). The study did not analyze the results by tumor type; however, in this study comprised of 65% breast and chest wall recurrences improved heating techniques resulted in improved clinical outcome.
Glioblastoma
Aggressive multimodal interventions for glioblastoma are considered palliative but in some instances can extend survival to 40% at 2 years Citation[62], Citation[63]. In one trial of brachytherapy, The Brain Tumor Cooperative Group demonstrated a significant survival benefit to this modality, however regional control was not enhanced Citation[64]. A year later, the Radiation Therapy Oncology Group attempted to boost local control with adjuvant interstitial hyperthermia; however, inadequate heating techniques inhibited the study Citation[65]. In a follow-up study, Sneed and colleagues at UCSF were able to maintain tumor temperatures above 42.5°C using implanted catheters and helical-coil microwave antennas. They performed a prospective, randomized trial of 68 patients with primary glioblastoma comparing external beam radiation and brachytherapy boost with or without interstitial hyperthermia Citation[30]. Patients in the hyperthermia arm required more re-operations and experienced more Grade 3 toxicities; however, adjuvant hyperthermia increased time to progression by 50% (49 weeks vs. 33 weeks) and doubled 2-year survival probabilities (31% vs. 15%). Current research efforts aim to improve heat-delivery techniques via radiated magnetic nanoparticles and to integrate hyperthermia with intra-arterial chemotherapeutic injections Citation[33], Citation[66].
Squamous cell cancer (SCC): Head, neck and esophageal
Squamous cell carcinoma of the head and neck that is diagnosed early can be treated effectively, but lymphatic spread to a single node reduces a patient's 3-year survival to 25% Citation[67]. In 1988, Valdagni et al. reported the results of 44 patients with metastatic head and neck squamous cell cancers randomized to receive either conventional fractionated radical radiation alone or radiation combined with hyperthermia Citation[23]. The end-points of the study were local control at 3 months and treatment toxicity. At interim analysis, the study was closed due to a significant advantage in local control for the hyperthermia arm (82.3% vs. 36.8%). Toxicity was similar between the groups except that one patient in the combined arm died from carotid rupture. The study concluded that addition of hyperthermia to radiation enhances local control without increasing toxicity.
Squamous cell cancer of the esophagus has a poor prognosis, with less than a quarter of patients surviving to five years. In an attempt to improve treatment response and survival, Sugimachi and colleagues added heat via intraluminal radiofrequency current (RFC) to a standard pre-operative chemoradiotherapy regimen Citation[24]. Thirty-two patients were treated with hyperthermo-chemoradiotherapy (HCR) and 34 patients were treated with chemoradiotherapy alone. The HCR patients had an improved complete response (25% vs. 5.9%), and twice as many survived to 3-year follow-up (50.4% vs. 24.2%). The role of hyperthermia as an adjunct to the treatment of SCC is evident.
Gastric cancer
Loco-regional failure rates for gastric cancer approach 65% in some studies, and five-year survival is well below 50% once the lesion has spread to lymphatics Citation[68–70]. Adjuvant chemoradiotherapy has become the standard treatment paradigm in the United States due to improved disease-free survival and overall survival Citation[70].
Two large prospective trials have tested the addition of hyperthermia to adjuvant treatment regimens for advanced gastric cancer. In the first study, 293 patients at a single center were randomized to receive surgery alone (OR), surgical resection with pre-operative radiation (OR + RT), or resection with pre-operative radiation and local microwave hyperthermia (OR + RT + HT) Citation[26]. Compared to surgery alone, the addition of radiation did not show a benefit. In contrast, the combination of radiation with hyperthermia enhanced survival (3-year survival: 57.6% vs. 35.5%, 5-year survival: 51.4% vs. 30.1%). The authors determined that the best response was seen in tumors heated to 41°–43°C, and the least toxicity was experienced when heat was applied two hours after radiation. In the second study, Fujimoto et al. combined intraperitoneal hyperthermia with chemotherapy to treat advanced gastric cancer Citation[27]. One hundred forty-one patients received surgery alone or surgery combined with hyperthermic chemoperfusion with mitomycin C. Thereafter, both groups received standard adjuvant chemotherapy including the anti-tumor polysaccharide Sizofiran. Peritoneal recurrence and all-site recurrence were significantly decreased in the hyperthermic perfusion group, and overall survival was greatly improved in follow-up to 8 years (62% vs. 49%). These studies indicate that hyperthermic perfusion improves outcomes when combined with standard surgical, chemotherapy, and radiation protocols.
Bladder, cervical and rectal cancers
Local control of deep-seated pelvic tumors is critical to avoid future spread of disease, and local treatment failures generally suggest an aggressive and fatal course. Furthermore, pelvic recurrences are notoriously difficult to manage surgically, and thus aggressive measures to avoid such occurrences are routine. In 2000, the Dutch Deep Hyperthermia Group published results evaluating the role of hyperthermia in enhancing control of locally-advanced bladder, cervical, and rectal cancer Citation[31]. A total of 358 patients with T2-T4 bladder cancer (n = 101), stage IIB, IIIB, or IV cervical cancer (n = 114), and M0-M1 rectal cancer (n = 143) were randomized to receive radiation therapy alone (median dose = 65 Gy) or radiotherapy with heat provided by various microwave applicators. There were a number of limitations to this trial, particularly the heterogeneity of tumor types and stages that were studied. For example, rectal cancer patients showed a trend toward improved response; however, the overall survival was actually worse in the hyperthermia group. The authors speculated that the poor results for rectal cancer may have been due to the dose of radiotherapy used and the high number of recurrent lesions. In addition, local hyperthermia is unlikely to benefit the systemic M1 lesions included in the rectal cancer group. As a result, Subgroup analysis of the Dutch Deep Hyperthermia Group trial showed that the overall improvements in response rate and local control were seen entirely in the cervical cancer patients. Only cervical cancer treated with hyperthermia and radiotherapy experienced an improved 3-year survival (51% vs. 27%).
Complicating these results were five randomized, clinical trials published soon after the Dutch trial showing an improved response in cervical cancers treated with combined cisplatin and radiation therapy, thereby proving the control arm of the study inadequate Citation[71–75]. Recently, a contradicting report was published finding no survival benefit and increased toxicity from thermochemotherapy for cervical cancer Citation[32]. Therefore, an ongoing international Phase III trial testing the benefit of adding hyperthermia to cisplatin based chemoradiotherapy is underway to shed some light on the optimal therapeutic regimen for advanced cervical cancer Citation[76].
Despite the results of the Dutch Group Study, intravesicular mitomycin-C combined with microwave hyperthermia has been successfully used for over a decade as an alternative to transurethral bladder resection (TURB) for superficial bladder cancer Citation[29]. Recently the same group that advocated this technique examined the ability of intravesicular thermochemotherapy (IVTC) to prevent recurrence in superficial transitional cell carcinoma (TCC) Citation[28]. Eighty-three patients with intermediate to high-risk TCC were resected and given either intravesicular mitomycin-C alone or in combination with local microwave-induced hyperthermia. Pelvic pain and bladder wall injury were greater in the IVTC treated patients but not significant enough to prevent completion of the course of therapy. At two years post-TURB, the IVTC group had a 3-fold decrease in recurrences (17.1% vs. 57.5%) and no patients had progression of their disease. One of the criticisms of this trial however was that the dose of mitomycin-C was sub-optimal and as such there was an unusually high rate of recurrence in the control arm. Nevertheless, these studies attest to the benefit of adding heat to chemotherapy as an adjuvant to the treatment of superficial bladder cancers.
Ovarian cancer
Cytoreduction and post-operative, platinum-based chemotherapy is the standard treatment for ovarian cancer Citation[77]. The inability to obtain a complete pathological response is the chief obstacle in advanced disease, and on second-look operation only half of patients show a complete response Citation[78]. Although no Phase III clinical trials exist, a few groups have recently evaluated the effects of adding intraperitoneal hyperthermic chemotherapy (HIPEC) during a second-look operation. In 2004, Ryu et al. published a review of 117 patients with Stages IC to III ovarian cancer treated with surgery and chemotherapy alone or surgery and chemotherapy followed by second-look HIPEC Citation[78]. The HIPEC group (n = 57) had a significantly improved disease-free period (48.7 months vs. 19.8 months) and 5-year survival rate (63.4% vs. 52.8%). On multivariate analysis, the survival benefit was found to be solely in Stage III tumors. Four cases of intestinal perforation occurred in Stage III tumors in the HIPEC group likely due to the temperature of the perfusate and the extensive cytoreduction undertaken for this advanced stage of disease. A recent follow-up study using both carboplatin and paclitaxil has corroborated these results Citation[79]. Gori et al. added second-look laparotomy and HIPEC to standard surgery and post-op chemotherapy of Stage IIIB and IIIC ovarian cancer Citation[80]. Twenty-nine patients in the experimental group experienced better, but not significant, improvements in recurrence rates and 5-year survival (p = 0.227 and p = 0.264 respectively). Unfortunately, these studies were not randomized nor can they differentiate the benefit of a second surgical excision from hyperthermia-enhanced intraperitoneal chemotherapy. The results are measurable and demonstrate a role for a Phase III trial assessing HIPEC in advanced ovarian tumors.
Colorectal carcinomatosis (CC) and pseudomyxoma peritonei (PP)
Cytoreduction and HIPEC as a component of the treatment of peritoneal carcinomatosis of colorectal origin has received considerable attention in recent literature Citation[81]. Unfortunately, much of the published data is poorly supported, with little in the way of randomized, prospective data offering conclusive evidence of the utility of this approach. In 2003, the Netherlands Cancer Institute published the results of a Phase III trial assessing the efficacy of aggressive cytoreduction and mitomycin-C based HIPEC for 105 patients suffering from CC Citation[82]. The control group received only systemic chemotherapy consisting of fluorouracil and leucovorin. The experimental group survived almost twice as long (22.3 months vs. 12.6 months); however, similar to trials for ovarian cancer, the study is limited in that it cannot differentiate the effect from the two treatment regimens: aggressive surgery and HIPEC.
There are no prospective, randomized trials comparing the efficacy of hyperthermia in pseudomyxoma peritonei, although a number of centers have reported their experiences. This is partly due to the extremely low incidence of this disease and is compounded by the fact that different centers use different approaches, making a multi-institutional trial nearly impossible. Despite these limitations, various studies performed in the last seven years have shown that combined modality treatment employing HIPEC seems favorable compared to serial debulking surgery with non-standardized intraperitoneal chemotherapy [83–85]. Although there are associated morbidities and mortalities, the addition of hyperthermia appears to aid in microscopic cytoreduction and to decrease recurrence rates Citation[83].
Conclusions
Since Creech first reported the delivery of chemotherapy for regional tumor treatment, there have been efforts to enhance the efficacy of this approach. The effects of hyperthermia at the cellular and tissue level have been extensively studied in a multitude of tumor models. The consequences on the pharmacokinetics of different chemotherapeutic agents, its synergism with radiation, and the added survival observed in clinical trials have all been reported. Most importantly, at the clinical level hyperthermia is proven to enhance response rates of regional treatments, whether radiation or chemotherapy, in a litany of tumor histologies. Due to advances in application technology, thermal therapy is well tolerated with minimal treatment-related side effects. Its benefits therefore come with minimal cost.
Some of the strongest evidence to date has supported the use of hyperthermia as an adjunct to radiation. Radiation therapy is a powerful tool for local control of both primary and recurrent lesions, and in settings where dosage is limited, hyperthermia has been shown to be a sensitizer enhancing the effects of low doses of radiation. In breast and central nervous system tumors, the benefits of this combined approach are very impressive, although it has yet to receive widespread acceptance.
In regional limb perfusion for the treatment of melanoma, hyperthermia is an essential component. Recent studies have shown that mild temperature hyperthermia selectively increases oxygenation in tumors thereby enhancing drug uptake and radiation susceptibility Citation[35]. At present, there is active interest in isolated limb infusion, a minimally invasive approach using temperatures slightly lower than those traditionally utilized for limb perfusions. Advances like this which aim to improve response rates and toxicity profiles are possible due to the research and trials presented in this review. Although not completely explicit, there is certainly enough data to advocate the continued use of hyperthermia as an adjuvant to regional cancer therapy.
References
- Creech O, Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958; 148(4)616–32
- Crile G. Heat as an adjunct to the teatment of cancer: Experimental studies. Cleveland Clin Quart 1961; 28: 75–89
- Takemoto M, Kuroda M, Urano M. The effects of various chemotherapeutic agents with mild hyperthermia on different types of tumours. Int J Hyperthermia 2003; 19: 193–203
- Mhalaey MSJ, Woodhall B, Knisely WH. Selection of anti-cancer agents for regional brain cancer perfusion. Surg Forum 1960; 10: 774–777
- Iliakis G, et al. Evidence for an S-phase checkpoint regulating DNA replication after heat shock: A review. Int J Hyperthermia 2004; 20(2)240–249
- van der Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13(8)173–184
- Bruns P. Die Heilwirkung des Erysipels auf Geschwulste. Bietr Klin Chir 1887; 3: 443–466
- Busch W. Uber den Einfluss welchen Heftigere Erispeln Zuweilen auf Organisierte Neubildungen Ausuben. Verhandl Naurth Preuss Rhein Westphal 1866; 23: 28–30
- Coley WB. A Preliminary Note on the treatment of inoperable sarcoma by the toxic product of erysipelas. Post-Graduate 1893; 8: 278–286
- Westermark F. Uber die Behandlung des Ulcerirended Cervixcarcinoms. Mittel Konstanter Warme. Zbl Gynak 1898; 00: 1335–1339
- Woodhall B, Pickrell KL, Georgiade NG, Mahaley MS, Dukes HT. Effect of hyperthermia on cancer chemotherapy–application to external cancer of head and face structures. Annals Surg 1960; 151: 750–759
- Cavaliere R, Ciocatto EC, Giovanella BC, Heidelberger C, Johnson RO, Margottini M, Mondovi B, Moricca G, Rossi-Fanelli A. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967; 20(9)1351–1381
- Kim JH, Hahn EW, Tokita N. Combination hyperthermia and radiation therapy for cutaneous malignant melanoma. Cancer 1978; 41(6)2143–2148
- Kim JH, Hahn EW, Tokita N, Nisce LZ. Local tumor hyperthermia in combination with radiation therapy. 1. Malignant cutaneous lesions. Cancer 1977; 40(1)161–169
- Ghussen F, Kruger I. Technical aspects of isolation extremity perfusion: Experimental studies and clinical experience. J Invest Surg 1989; 2(4)487–496
- Ghussen F, et al. The role of regional hyperthermic cytostatic perfusion in the treatment of extremity melanoma. Cancer 1988; 61(4)654–659
- Ghussen F, Nagel K, Groth W, Muller JM, Stutzer H. A prospective randomized study of regional extremity perfusion in patients with malignant melanoma. Ann Surg 1984; 200(6)764–768
- Overgaard J, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345(8949)540–543
- Koops HS, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: Results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol 1998; 6(9)2906–2912
- Eggermont AM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 224(6)756–764, discussion 764–765
- Issels RD, et al. Regional hyperthermia (RHT) improves response and survival when combined with systemic chemotherapy in the management of locally advanced, high grade soft tissue sarcomas (STS) of the extremities, the body wall and the abdomen: A Phase III randomised prospective trial. Proc Am Soc Clin Oncol Annual Meeting Part I 2007; 25(18s)
- Issels RD. High-risk soft tissue sarcoma: Clinical trial and hyperthermia combined chemotherapy. Int J Hyperthermia 2006; 22(3)235–239
- Valdagni R, Amichetti M, Pani G. Radical radiation alone versus radical radiation plus microwave hyperthermia for N3 (TNM-UICC) neck nodes: A prospective randomized clinical trial. Int J Radiat Oncol Biol Phys 1988; 5(1)13–24
- Kitamura K, et al. Prospective randomized study of hyperthermia combined with chemoradiotherapy for esophageal carcinoma. J Surg Oncol 1995; 60(1)55–58
- Vernon CC, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35(4)731–744
- Shchepotin IB, et al. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol 1994; 3(1)37–44
- Fujimoto S, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999; 85(3)529–534
- Colombo R, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003; 21(23)4270–4276
- Colombo R, et al. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol 1996; 155(4)1227–1232
- Sneed PK, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40(2)287–295
- van der Zee J, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355(9210)1119–1125
- Vasanthan A, et al. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys 2005; 61(1)145–153
- Maier-Hauff K, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol 2007; 81(1)53–60
- Shea SM, Caulfield JB, Burke JF. Microvascular ultrastructure in thermal injury: A reconsideration of the role of mediators. Microvascular Research 1973; 5: 87–96
- Song C, et al. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21(8)761–768
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22(3)197–203
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res 1984; 44(10 Suppl)4721s–4730s
- Overgaard J, Bichel P. The influence of hypoxia and and acidity on the hyperthermic response of malignant cells in vitro. Radiology 1977; 23(2)511–514
- Overgaard J, Nielson O. The role of tissue environmental factors on the kinetics and morphology of tumor cells exposed to hyperthermia. Ann New York Acad Sci 1980; 335: 254–280
- Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of chinese hamster cells in vitro. Int J Radiat Biol Realt Stud Phys Chem Med 1971; 9(5)467–477
- Lepock JR. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int J Hyperthermia 2003; 9(3)252–266
- Lepock J. Protein denaturation during heat shock. Adv Molec Cell Biol 1997; 19: 223–259
- Wong R, Kapp L, Dewey W. DNA fork displacement rate meaasurements in heated Chinese hamster ovary cells. Biochim Biophys Acta 1989; 1007: 224–228
- Wong R, Thompson L, Dewey W. Recovery from effects of heat on DNA synthesis in Chinese hamster ovary cells. Radiat Res 1988; 114: 125–137
- Warters R, Lyons B. Inhibition of replicon cluster ligation into chromosomal DNA at elevated temperatures. J Cell Physiol 1990; 142: 365–372
- Zuo J, Rungger D, Voellmy R. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol 1995; 15: 4309–4331
- Multhoff G, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 1995; 61(2)272–279
- Giovanella B, et al. Hyperthermic treatment of human tumors heterotransplanted in nude mice. Cancer Res 1979; 39: 2236–2241
- Pelz J, et al. Histologic response of peritoneal carcinomatosis after hyperthermic intraperitoneal chemoperfusion. BMC Cancer 2006; 6(162)
- Abdel-Wahab OI, et al. The role of hyperthermia in regional alkylating agent chemotherapy. Clin Cancer Res 2004; 10(17)5919–5929
- Ko SH, et al. Optimizing a novel regional chemotherapeutic agent against melanoma: Hyperthermia-induced enhancement of temozolomide cytotoxicity. Clin Cancer Res 2006; 12(1)289–297
- Brucker C, Sieg P. [Effect of combined carboplatin and ifosfamide with local hyperthermia on human mouth carcinoma in the animal model]. Mund Kiefer Gesichtschir 1999; 3((Suppl 1)S144–146
- Ferron G, et al. Feasibility of laparoscopic peritonectomy followed by intra-peritoneal chemohyperthermia: An experimental study. Gynecol Oncol 2005; 99(2)358–361
- Lang H, et al. A porcine model for investigation of hyperthermic isolated liver perfusion. J Invest Surg 1998; 11(6)401–408
- Johannsen M, et al. Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate 2006; 66(1)97–104
- Myerson RJ, et al. Simultaneous superficial hyperthermia and external radiotherapy: report of thermal dosimetry and tolerance to treatment. Int J Hyperthermia 1999; 5(4)251–266
- Oleson JR, et al. Regional hyperthermia by magnetic induction in a beagle dog model: Analysis of thermal dosimetry. Radiat Res 1984; 98(3)445–455
- Zakris EL, et al. Pharmacokinetics and toxicity of intraperitoneal cisplatin combined with regional hyperthermia. J Clin Oncol 1987; 5(10)1613–1620
- Clary BM, et al. Sentinel lymph node biopsy in the management of patients with primary cutaneous melanoma: Review of a large single-institutional experience with an emphasis on recurrence. Ann Surg 2001; 233(2)250–258
- Jones E, Marks L, Prosnitz L. Point: Hyperthermia with radiation for chest wall recurrences. J Nat Comp Cancer Network 2007; 5(3)339–344
- Jones EL, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23(13)3079–3085
- Mirimanoff R-O, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: Recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 Phase III randomized trial. J Clin Oncol 2006; 24(16)2563–2569
- Wagner W, et al. Survival rates of patients with glioblastoma multiforme treated with combined radiochemotherapy at first line. J Clin Oncol (Meeting Abstracts) 2005; 23(16Suppl.)1576
- Selker RG, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: A randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurg 2002; 51(2)343–355, discussion 355–357
- Emami B, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors: A prospectively controlled randomized study by the radiation therapy oncology group. Int J Radiat Oncol Biol Physics 1996; 34(5)1097–1104
- Uzuka T, Takahashi H, Tanaka R. Interstitial hyperthermia with intra-arterial injection of adriamycin for malignant glioma. Neurol Med Chir (Tokyo) 2006; 46(1)19–23, discussion 23
- Cummings B, Kim J, O'Sullivan B. Radiation therapy and managment of the cervical lymph nodes. Cummings B, Otolaryngology: Head and neck surgery4th. Mosby, Inc. 2005; 2589–2613
- Yoo C, et al. Recurrence following curative resection for gastric carcinoma. Brit J Surg 2000; 87(2)236–242
- Roviello F, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Brit J Surg 2003; 90(9)1113–1119
- Macdonald JS, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345(10)725–730
- Keys HM, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999; 340(15)1154–1161
- Morris M, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999; 340(15)1137–1143
- Abstracts presented for the thirtieth annual meeting of the Society of Gynecologic Oncologists. Gynecol Oncol 1999; 72(3)443
- Rose PG, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999; 340(15)1144–1153
- Whitney CW, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999; 17(5)1339–1348
- Dahl O, Mella O. Referee: Hyperthermia alone or combined with cisplatin in addition to radiotherapy for advanced uterine cervical cancer. Int J Hyperthermia 2002; 8(1)25
- Cannistra SA. Cancer of the Ovary. N Engl J Med 1993; 329(21)1550–1559
- Ryu KS, et al. Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol 2004; 4(2)325–332
- Bae J, et al. Treatment of ovarian cancer with paclitaxil- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 2007; 106: 193–200
- Gori J, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer 2005; 5(2)233–239
- Sugarbaker P. Colorectal carcinomatosis: A new oncologic frontier. Current Opin Oncol 2005; 17(4)397–399
- Verwaal V, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21(20)3737–3743
- Smeenk RM, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei. Cancer Treat Rev 2007; 33(2)138–145
- Loungnarath R, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: A prospective study. Dis Colon Rectum 2005; 48(7)1372–1379
- Guner Z, et al. Cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei. Int J Colorectal Dis 2005; 20(2)155–160