Abstract
Antivascular and targeted therapy are now an integrated part of the treatment of myelogenous leukemias, GIST tumours, B-cell lymphomas and breast cancer. In various malignancies improved responses and prolongation of survival for several months is regularly reported. The progress in this field is relevant for hyperthermia. Heat has among other effects documented antivascular effects, and can be considered as one of the established methods in the field based on several randomised phase III studies. Hyperthermia should be considered for combination with other antiangiogenic agents.
Introduction
A vast number of reports on the preclinical and clinical effects of therapy directed against angiogenesis (i.e. formation of new vessels) and the vascular network in tumours have been published in the last five years. Currently there are more than 31 000 PubMed hits on the term ‘angiogenesis’, and more than 1 000 hits on ‘bevacizumab’. Awareness of this development is crucial for the judgement of the true value of progress in clinical hyperthermia. This review will highlight some aspects with special reference to randomised clinical trials. Judah Folkman has described the crucial role of the vasculature for tumour nutrition and growth. He initially postulated the ‘tumour angiogenic factor’, a concept which has been developed into a cascade of factors which induce or inhibit the formation of new vessels Citation[1–4]. Angiogenesis is now acknowledged as one of the ‘hallmarks of cancer’, and proper function of blood vessels is a prerequisite for delivery of drugs to solid tumours Citation[5], Citation[6].
Targeted therapy
The term ‘targeted therapy’ was originally introduced when monoclonal antibodies customised against specific cellular targets became available in the 1980s Citation[7]. Today, the term includes all molecules that target different signal transduction elements, or where the mechanism of action is thought to be at a particular molecule Citation[8]. Thus, it includes all antiangiogenic and antivascular therapies where the specific target molecules are known. It is interesting to note that the term by itself is a seducing one, as it implies that we actually know the mechanisms which are blocked or stimulated. Research in this field was boosted by the overwhelming success of imatinib which rapidly became accepted as standard therapy for chronic myelogenous leukemia worldwide Citation[9], Citation[10]. The discovery that the Philadelphia chromosome in chronic myelogenous leukemia consisted of a translocation between chromosome 9 and 22, leading to a constitutive activation of the Abelson tyrosine kinase (), which could be inhibited in culture and also in mice, paved the way for clinical testing Citation[11–17]. In addition, imatinib also inhibited the oncogen c-kit, and therefore proved to be especially effective in gastrointestinal stromal tumours (GIST) Citation[18]. The effect in these tumours was better than historical controls treated with doxorubicin Citation[19]. Imatinib also exhibited an inhibitory effect on the platelet derived growth factor receptor (PDGFR) in vitro, and this proved to be effective in dermatofibrosarcoma protuberans where a translocation of chromosome 17 and 22 leads to autocrine activation of platelet derived growth factor β-chain (PDGF β-chain) Citation[20], Citation[21]. In this neoplasm, imatinib induces complete or partial response in most patients treated for advanced disease. In eosinophilic leukemia and hypereosinophilic syndrome, different translocations lead to fusion products that involve PDGFR-α or PDGFR- β, both responding well to imatinib Citation[22–27]. An important lesson from the use of imatinib is that resistance can develop by mutations in the target molecules Citation[28]. Interestingly, the new drug dasatinib is able to induce response in imatinib-resistant leukemias Citation[29].
Figure 1. Mechanisms of action of selected antiangiogenic drugs that effect receptor tyrosine kinase pathways.
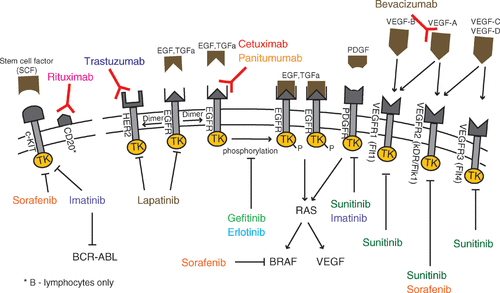
Another large success of targeted therapy has been the monoclonal antibody rituximab which interacts with the surface antigen CD20 in malignant non-Hodgkin B-cell lymphomas Citation[30], Citation[31]. Rituximab is effective administered alone, but especially when combined with conventional chemotherapy. Recent studies indicate that the mechanism of action is inhibition of the constitutively activated Akt pathways in B-cell lymphomas Citation[30].
These impressive results led to large press headings like the ‘smart bomb’ and ‘sea change’ Citation[32]. Some predicted that cancer soon could be changed to a chronic controllable disease like diabetes, provided the patients continued with an oral non-toxic compound. Such hopes have been the background for the unprecedented search for new targeted drugs and a large number of clinical trials. In the presentation below, the different antivascular agents will be addressed under the heading of the type of cancer in which they are mainly used.
Antiangiogenic therapy
VEGF inhibition
Colorectal cancer
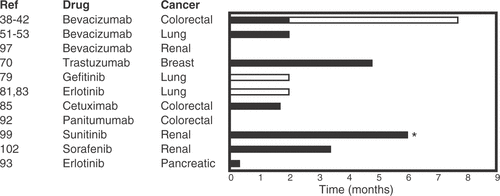
Vascular endothelial growth factor receptors (VEGFR-1, VEFGR-2, VEGFR-3) are specific tyrosine kinase growth factor receptors in endothelial cells. Based on the disclosure of the detailed mechanisms of angiogenesis Citation[4], Citation[33], Citation[34] and lymphangiogenesis Citation[35], Citation[36], a recombinant humanised monoclonal antibody, bevacizumab, was developed as an anticancer agent Citation[37]. Bevacizumab blocks the vascular endothelial growth factor A (VEGF-A). In metastatic colorectal cancer, bevacizumab added to a fluorouracil and folinate regimen (FU/LV) improved response rate (40% with 5 mg/week and 24% with 10 mg/week, vs. 17% for controls), produced longer time to progression (9.0, 7.2, vs. 5.6 months, respectively), and longer survival (21.5, 16.1 vs. 13.8 months) Citation[38]. These data were confirmed in subsequent studies Citation[39], Citation[40]. In a pivotal randomised phase III clinical trial, bevacizumab was added to bolus fluorouracil and folinate plus irinotecan (IFL) Citation[41]. This increased the median duration of survival from 15.6 to 20.3 months, while the secondary endpoint, median duration of progression-free survival increased from 6.2 to 10.6 months. Recently, bevacizumab added to a combination of fluorouracil, folinate and oxaliplatin (FOLFOX4) for patients with previously treated colorectal cancer metastases, improved survival by 2 months () Citation[42]. However, despite the success of this drug, the median survival remains about 20 months for metastatic colorectal cancer Citation[43–45]. Bevacizumab is currently widely used in advanced colorectal cancer, and several randomised phase III trials are ongoing to establish its role in adjuvant combination chemotherapy regimens. Recently, an oral small molecule, inhibiting the VEGFR-2 tyrosine kinase activity, showed clinical efficacy, making this treatment more convenient for the patients Citation[46]. New developments in imaging of angiogenesis clearly document the effect of anti-angiogenic directed therapy in colorectal cancer Citation[47–50].
Figure 2. Prolongation of median overall survival from randomized clinical trials using targeted antiangiogenic substances for different malignancies. Open bars represent variations between different studies. Note that the drugs are given in different settings. Use in 1. line or later will influence the results.
Other cancers
Bevacizumab also exhibited effect in lung cancer, where a median increase in progression-free survival and overall survival of 2 months has been reported by adding bevacizumab (15 mg/m2) to paclitaxel and carboplatin Citation[51–53]. The drug is now FDA approved for lung cancer patients who meet the original eligibility criteria for the studies, which excluded squamous cell histology, brain metastases, and being on anti-coagulants Citation[54]. Also in breast cancer and renal cancer, improved response was seen for bevacizumab added to chemotherapy, but generally the effect is less than that documented in colorectal cancer Citation[55]. Combinations of bevacizumab and new small molecular inhibitors are currently being investigated Citation[56]. VEGF inhibition also exerts pronounced effects on other angioproliferative disorders (neovascular macular degeneration, angioproliferating glaucoma) Citation[57–60]. Bevacizumab has been the leading drug for inhibition of angiogenesis, but a wide range of different drugs are now in early clinical testing Citation[37].
Multiple myeloma
Thalidomide was originally introduced as an anticonvulsant. Later thalidomide became popular as a sedative and antiemetic drug during pregnancy, until its teratogenous effect was discovered in the 1960s Citation[61]. This drug has important immunomodulating properties, and decreases VEGF and beta fibroblast growth factor (bFGF), both known proangiogenic factors. Thalidomide is today standard of care, alone or in combination with chemotherapy, for multiple myeloma, which is the second most common haematological malignancy Citation[61–63]. In controlled studies it delays time to progression with 5 months (from 4 to 9 months). Newer more potent derivatives are currently under investigation. There is, however, substantial toxicity associated with this drug (see below).
EGFR inhibition
Epidermal growth factor receptor (EGFR) belongs to the ErbB family of growth factor receptors expressed on the surface of many cells. The ErbB receptors regulate specific pathways that are crucial for cell growth (proliferation, apoptosis, angiogenesis) Citation[64]. Treatment with EGFR tyrosine kinase inhibitors inhibits angiogenesis by decreasing VEGF Citation[65]. Specific activating molecules (ligands) exist for the different types of receptors. The receptors generally consist of an extracellular growth factor receptor part, a transmembrane domain and an intracellular tyrosine kinase regulatory site (). The tyrosine kinase activity can be blocked by specific small molecules, while the growth factors or the external receptor domain can be blocked by specific monoclonal antibodies. The receptors exist as monomers that form dimers when activated. ErbB receptors are up-regulated in many tumour cells. However, EGFR inhibitors have effects in tumour cells expressing less number of receptors per cell than their corresponding normal cells.
Breast cancer
The human epidermal growth factor 2 (HER-2/ErbB2) is a receptor tyrosine kinase overexpressed in 20-30% of human breast cancers, and is associated with poor prognosis Citation[66]. Currently, no specific ligand is associated with HER-2, which tends to form heterodimers with other members of the ErbB family. A humanised monoclonal antibody, trastuzumab, was developed for inactivation of the extracelluar part of HER-2 receptor after intravenous injection Citation[66]. In a phase II study in HER-2 positive, heavily pretreated breast cancer patients with advanced metastases, trastuzumab yielded objective response in 15%. This included eight complete responses and 26 partial responses among 222 patients, and the median duration of response was 9.1 months Citation[67]. In a subsequent study on metastatic disease, trastuzumab alone as first line therapy exhibited an objective response rate of 26% (seven complete responses) in 114 patients Citation[68]. The response was 35% in a subgroup having 3+ or 2+ HER-2 overexpression. Half of the patients had no disease progression at 12 months follow up. These promising results were confirmed in a randomised study where trastuzumab was added to standard chemotherapy (an anthracycline and cyclophosphamide or paclitaxel) vs. chemotherapy alone in 469 patients Citation[69]. There were significantly better results in the group treated with trastuzumab: objective response in 50% vs. 32%; longer time to disease progression, 7.4 vs. 4.6 months, and longer survival, 25.1 vs. 20.3 months. However, the trial also revealed an important difference in side effects as cardiac dysfunction occurred in 27% of the patients treated with trastuzumab in contrast to 8% in the chemotherapy alone group. Based on the effect observed in advanced metastatic disease, trastuzumab was added to paclitaxel after doxorubicin and cyclophosphamide in a randomised adjuvant study, including 133 patients in the trastuzumab arm, and 261 in the control arm Citation[70]. After three years the trastuzumab group had 12% better survival than the control group. Another trial reported the effect of one year trastuzumab administered as an injection every third week after locoregional therapy with surgery and/or radiation therapy, and at least four cycles of neoadjuvant or adjuvant chemotherapy Citation[71]. The initial analysis revealed an absolute difference in disease-free survival of 8.4% after two years. In summary, five studies have used different adjuvant regimens with trastuzumab, and clearly demonstrated a benefit. More impressive, a review of more than 13,000 patients, included in four adjuvant studies, reported that trastuzumab almost halved the risk of recurrence in this population Citation[72–74]. Therefore, adjuvant trastuzumab is current standard of care for HER-2 positive primary tumours after local curative treatment. The new small molecule, lapatinib, is an oral receptor tyrosine kinase inhibitor that blocks both HER-2 and EGFR Citation[75]. Lapatinib plus carboplatin vs. carboplatin alone were used as salvage therapy in a randomised trial in locally advanced or metastatic breast cancer after progression on regimens including an anthracycline, a taxane and trastuzumab Citation[76]. The overall response rate was 22% after combination therapy vs. 14% in the carboplatin group (p = 0.09). The median time to progression was significantly prolonged in the combined group, 8.4 vs. 4.4 months, respectively (P < 0.001), while the survival was similar in both groups.
Lung cancer
Great expectations were raised when a clinical trial of a small molecule EGFR tyrosine kinase inhibitor, gefitinib, 250 mg orally daily, caused objective tumour response in 13.6% for a duration of 8.9 months in non-small cell lung cancer (NSCLC) patients failing two previous chemotherapy combinations, or failing chemotherapy due to toxicity Citation[77]. Then two randomised phase II trials (Ideal 1 and Ideal 2) in NSCLC reported an objective response approaching 20% in second-line patients, and around 10% in those patients having two or more previous chemotherapy regimens Citation[78]. The median survival in the two studies approached 6–8 months. However, two large randomised studies (Intact 1 and Intact 2), including more than 1,000 patients, failed to show an improvement of tumour response or survival when gefitinib was administered as first line therapy in combination with two different chemotherapy regimens Citation[78]. Later, a retrospective molecular analysis of EGFR mutations and amplifications from the above mentioned studies, revealed that the response to gefitinib was related to EGFR mutations, which correlated with previously identified clinical factors like adenocarcinoma histology, absence of smoking history, female sex, and Asian ethnicity Citation[79]. Then a randomised phase III clinical trial reported a significant survival benefit for patients with advanced NSCLC treated by another small molecule inactivating the tyrosine kinase ATP binding site, erlotinib Citation[80]. In a study including 731 patients, the response rate in the erlotinib group was 8.9% vs. <1% in the control group, while the median duration of response was 7.9 vs. 3.7 months, respectively. Overall survival was prolonged by two months. The difference in response in the gefitinib and erlotinib trials may be related to the fact that gefitinib was used at a dose of 42% of the maximum tolerated dose while erlotinib was used at the maximum tolerated dose Citation[81]. A recent trial failed to show any benefit from erlotinib combined with cisplatin and gemcitabine in advanced NSCLC Citation[82]. EGFR inhibition is still considered a promising therapy in aerodigestive tumours, including squamous tumours from the head and neck area Citation[83].
Colorectal cance
EGFR is up-regulated in 60–80% of colorectal cancer tumours Citation[84]. In 57 patients failing an irinotecan containing regimen for metastatic colorectal cancer, 10.5% obtained a partial response on cetuximab, a humanised monoclonal antibody binding to EGFR Citation[85]. The time to tumour progression was only 1.4 months, with a median duration of response of 4.2 months. In another phase II study on 346 patients with metastatic colorectal cancer failing irinotecan, oxaliplatin and fluoropyrimidines, cetuximab achieved similar results with median progression-free survival of 1.4 months and survival of 6.6 months Citation[86]. In a phase III trial patients failing an irinotecan-based regimen, received either cetuximab alone (111 patients) or cetuximab plus the same chemotherapy dose and schedule they previously failed on (218 patients) Citation[84]. The objective response was 10.8% in the cetuximab group, but 22.9% in the combined group. The median time to progression (1.5 vs. 4.1 months) and median survival (6.9 vs. 8.6 months) also favoured the combined group. However, side effects like asthenia, acne-like skin rash, nausea and vomiting were also more frequent in the combined group.
While initially only given to EGFR expressing tumours, the drug seems also to be effective in tumours that do not express EGFR Citation[87]. Patients with tumours expressing high levels of the EGFR ligands epiregulin and amphiregulin seemed more likely to have disease control by cetuximab Citation[88]. Based on the effects in second and third line chemotherapy, cetuximab combined with chemotherapy is now in phase III clinical trials as first line therapy for metastases from colorectal cancer Citation[89], Citation[90]. Another monoclonal antibody directed against EGFR, panitumumab, was administered to 463 patients in a randomised trial including chemotherapy-refractory metastatic colorectal cancer as best supportive care alone vs. best supportive care and panitumumab Citation[91]. Despite a significant difference in median progression-free survival, the difference was only 7.3 vs. 8 weeks (5 days). Recently the effect of monoclonal antibodies against EGFR seems to depend on wild type of KRAS as mutations in this gene predict resistance Citation[92–94].
In pancreatic cancer, 569 patients were randomised between standard gemcitabine and erlotinib and gemcitabine Citation[95]. The overall survival was statistically significant longer in the combined group (P = 0.038), but the difference in median overall survival was hardly of clinical significance: 5.91 months in the gemcitabine group and 6.24 months in the combined group.
Renal cell carcinoma
Renal cell carcinomas are refractory to most standard chemotherapy regimens. It is well known that primary tumours and metastases from renal cell carcinomas generally are well vascularised, often causing bleedings during surgical resections. The hypoxia-inducible factor 1α (HIF-1α) is an important regulator of angiogenesis Citation[96]. New insights into the molecular mechanisms in renal cell carcinomas reveal that for clear cell carcinomas there is accumulation of HIF-1α due to a defect in the von Hippel-Lindau protein which normally addresses HIF-1α for degradation (ubiquitinaton) Citation[97], Citation[98]. HIF-1α then causes overexpression of VEGF, transforming growth factor α (TGF-α, a ligand for EGFR), and platelet derived growth factor chain β (PDGF-β) which also stimulates angiogenesis Citation[99]. On this background, bevacizumab was tested in metastatic renal cell cancer in a randomised phase II trial Citation[100]. A dose of 10 mg/kg improved progression-free survival by 2–3 months compared to placebo, but there was no survival benefit. Then an oral, multi-targeted tyrosine kinase inhibitor, sunitinib, targeting VEGFR type 1-3 and PDGFR (α and β), proved to be effective in metastatic clear cell renal cancer in a pooled analysis of two phase II studies Citation[101]. The drug induced partial response in 45% of the patients (36% when assessed by an independent review committee), and the median progression-free survival was 8.1 months. In a randomised phase III study including 750 patients, the same regimen of sunitinib (50 mg orally each day) was compared with interferon α which can be considered standard therapy Citation[102]. In this study the sunitinib arm yielded about 20% objective responses and a median progression free survival of 11 months, in contrast to interferon α where the corresponding endpoint was 5 months. It is also important that the patients on sunitinib reported significantly better quality of life. Others used a combination of bevacizumab (10 mg/kg daily) and erlotinib (150 mg orally daily) for metastatic renal cell cancer to attack different targets (VEGF, EGFR, respectively) in 59 evaluable patients Citation[103]. The combination was well tolerated and resulted in 25% partial responses and a median progression free survival of 11 months. Sorafenib, another tyrosine kinase inhibitor, targets both tumour cells and tumour vasculature as it inhibits VEGFR type 2, B-Raf, PDGFR, Fms-like tyrosine kinase (Flt-3) and stem cell factor receptor (c-Kit) Citation[104]. When sorafenib was given orally twice daily for 12 weeks, 73 out of 202 patients with metastatic renal cell cancer obtained tumour shrinkage of > 25% Citation[104]. The responding patients were then randomised to continued therapy or placebo, which resulted in a median progression-free survival of 24 weeks in the treated group vs. 6 weeks for the placebo group. Then a follow-up randomised phase III study including 903 patients showed a median progression-free survival of 5.5 months in the sorafenib group against 2.8 months in the placebo group Citation[105]. Thus, the multi-targeted tyrosine kinase inhibitors yield currently the best available therapy in clear cell renal cancer.
Matrix metalloproteinase inhibitors
Matrix metalloproteinases (MMP) are a family of proteolytic enzymes with effects on various components of the extracellular matrix, where the effect of MMPs are controlled by endogenous tissue inhibitors of MMPs (TIMPs) Citation[106]. This is important for vascular remodelling and angiogenesis, and MMPs are partly regulated by VEGF Citation[107]. The role of MMP inhibitors is currently unclear as randomised phase III trials have demonstrated negative effect on survival Citation[108].
Vascular disrupting agents
The vascular targeting drugs selectively destruct the vasculature of malignant tumours, thereby causing rapid and selective shutdown of blood supply and thus indirect killing of the tumour cells Citation[109–111]. The concept was introduced in the 1980's by Denekamp who underlined that the tumour vascular endothelial cells are proliferating, and that the undifferentiated vessels differ from corresponding normal ones, making them particularly vulnerable for vascular targeting drugs Citation[112], Citation[113]. In animal studies, the Vinka alkaloids vincristine and vinblastine, the microtubule disrupting agent combretastatine A4, arsenic trioxide, flavone acetic acid, tumour necrosis factor and 5,6-dimethylxanthenone-4-acetic acid are the most studied drugs Citation[114–123]. These drugs are now in clinical phase II studies after showing some objective effect in phase I studies Citation[110], Citation[124]. It is too early to judge their true clinical role as side effects like myocardial infarction, tumour bleeding, tumour pain, as well as fatigue and nausea are associated with their use. However, these drugs are of particular interest as their anti-tumour effects are enhanced by hyperthermia Citation[75], Citation[116], Citation[119], Citation[120].
Low dose or metronomic chemotherapy
While cytotoxic chemotherapy generally is administered as courses at highest tolerable doses for some days, with rest intervals of 2 to 3 weeks, metronomic chemotherapy is administered as regularly repeated (i.e. metronomic) low, nontoxic doses, aiming at destruction of endothelial cells in the irregular, newly formed tumour vessels Citation[125–129]. The aim of the therapy is therefore indirect tumour cell killing by cutting off the supply of nutrition and oxygen, necessary for maintenance of the tumour cell population. This dosage strategy not only works well in experimental animal tumours, but also show objective tumour regressions in advanced human malignancies, and even in some tumours generally considered resistant to standard chemotherapy Citation[130–132]. One of the known mechanisms of metronomic therapy is induction of gene and protein expression of thrombospondin 1, a potent inhibitor of angiogenesis Citation[126], Citation[127]. A new aspect of metronomic dosing is the reduction of cells with stem cell characteristics in experimental glioma xenografts Citation[133]. Even more intriguing is the reduction of circulating endothelial stem cells after metronomic dosed chemotherapy in animal models Citation[107], Citation[134–136]. However, the contribution of bone marrow derived endothelial cells to the tumour vasculature seems smaller in man than in rodents Citation[137]. As there are reduced side effects with metronomic dosed therapy, it seems particularly well suited for palliative treatment.
Hyperthermia as an antivascular agent
The biological rationale for hyperthermia as a method for increasing the effect of radiation and chemotherapy is well documented Citation[138–141]. From experimental and clinical studies it is well known that hyperthermia has effects on blood flow, causing vascular shut down at high temperatures, but also a positive effect on tumour tissue oxygenation at mild temperatures Citation[122], Citation[142–146]. It has been clearly demonstrated that hyperthermia exhibits antiangiogenic effects, and proliferating endothelial cells in vessels from malignant tumours seem more sensitive to heat than the microvessels of normal tissues Citation[116] Citation[147–149]. Possible mechanisms may be changes in HIF-1α, VEGF or enhancement of the tumour necrosis factor Citation[142], Citation[150–152], but the exact mechanisms of action remains unclear. The known positive interaction with vascular disrupting drugs indicates that hyperthermia also influences some crucial mechanisms for endothelial cell proliferation and maintenance.
The important progress of hyperthermia in the clinic is well documented in randomised controlled studies. When hyperthermia was given with adequate equipment it enhanced the effect of radiation in localised head and neck lymph node metastases Citation[153], recurrent cutaneous malignant melanomas Citation[154], Citation[155], recurrent breast cancer Citation[156], Citation[157], soft tissue sarcomas Citation[158–160] and localised brain tumours Citation[161]. Of pelvic malignancies, particularly the effect in cervical cancer is remarkable Citation[162–164]. The effect of radiation and heat seems even further improved by adding cisplatin, and two controlled studies are now ongoing testing this trimodality treatment in cervical cancer Citation[165]. Combined with chemotherapy, hyperthermia has significantly enhanced the effect observed in superficial bladder cancer Citation[166] and recently for locally advanced sarcomas Citation[167]. It is outside the scope of this paper to go into detail on the clinical results. However, it can be concluded that hyperthermia can increase the control rate of radiation alone by about 50% (i.e. from 40% to 60%), and the effects observed in primary situations tend to be durable, i.e. the effect is not only a transient phenomenon.
Hyperthermia may also be used as a method to direct drugs towards the local tumour, both for nanoparticles and liposomes Citation[168–173]. Recently, several groups have used the local effects to selectively deliver gene therapy to tumours by activation of a heat responsive promoter construct Citation[174–177]. Hyperthermia has many mechanisms of action, but hyperthermia deserves also to be considered as one of the most clinically established antivascular treatments.
Toxicity issues
Terms like smart bombs and targeted therapy imply that these drugs find their targets selectively, thus avoiding the side effects tightly associated with classic anticancer agents, often termed cytotoxic agents. Due to the presumed selective effect of ‘designer’ drugs, the established maximum tolerated dose was frequently claimed to be irrelevant. However, as large number of patients have been exposed to the drugs, it has become clear that apart from some drug-specific side effects, many of the general side effects (nausea and vomiting, diarrhoea, allergic reactions and bone marrow suppression) are also a problem with targeted drugs () Citation[107], Citation[178–180]. This may simply reflect the disturbance on growth factor signalling pathways involved in many normal processes Citation[181]. It has especially been focused on dermatological side effects for EGFR inhibiting agents Citation[182] and cardiotoxic effects of trastuzumab and imatinib Citation[183–185]. For anti-angiogenic agents, effects on wound healing are relevant when surgical treatment is planned or must be performed during therapy Citation[186], and endothelial effects may also initiate thromboembolic events Citation[56], Citation[181], Citation[187]. For bevacizumab, hypertension, proteinuria and bleeding should be carefully addressed Citation[188]. Thalidomide is associated with substantial toxicity: thromboembolism which can be controlled by anticoagulants, somnolence, neurotoxicity, obstipation, and skin rash Citation[61–63]. The main acute side effects from hyperthermia are general discomfort and some localised pain during the treatment sessions. Serious late complications after local and regional heating, apart from some local blistering, have generally not reached statistical significance above that of radiation and chemotherapy alone. In a study of advanced rectal cancer, addition of hyperthermia to radiation therapy had no detrimental effect on the patient's quality of life Citation[189]. However, severe neurological side effects and tissue necrosis have been described in some adult patients after regional heating Citation[190], Citation[191], and avascular femoral osteonecrosis appeared in 14% of children less than 5 years after multiple hyperthermia sessions Citation[192]. The neurological damage is probably determined by the sensitivity of the nerve vasculature which should be kept under a dose of 30 min at 44°C or equivalent. Hopefully, the introduction of specific absorption rate (SAR) based treatment planning and continuous non-invasive monitoring, will help in delivering homogenous heating and reduce thermal hot spots Citation[193–197].
Table 1. Some of the side effects recently associated with use of antiangiogenic drugs.
Discussion and conclusions
The major curative treatment modalities for cancer remain surgery and/or radiation therapy. For some tumour types (malignant lymphomas, testicular germ cell tumours and leukemias) chemotherapy may be a curative treatment. For the majority of patients treated with chemotherapy, the goal of the therapy is palliation and extension of lifetime, except in adjuvant settings where the addition of chemotherapy to standard locoregional therapy increases cure rate (often in the range of 10–15%). Hyperthermia remains the most reliable sensitiser to increase the efficacy of radiation and chemotherapy Citation[141], Citation[198]. The tumour response tends to be durable as evidenced by parallel survival curves in the hyperthermia group and the control group. For most targeted therapy, with important exceptions for imatinib in chronic myelogenous leukemias and GIST, rituximab in malignant lymphomas, and trastuzumab in breast cancer, the effects are most often palliative for a few months duration. Bevacizumab improves the survival of metastatic colorectal cancer by several months, but its role combined with chemotherapy as adjuvant therapy after surgery is under investigation. Antiangiogenic drugs are expensive, but both trastuzumab and cetuximab are considered cost effective Citation[199], Citation[200]. However, the investment in hyperthermic equipment and staff to run the facilities are also cost effective Citation[163]. These two treatment options should both be further evaluated in the clinic. Combinations of antiangiogenic drugs and hyperthermia may prove effective both experimentally and in the clinic. Far more money is presently invested in potential new drugs, instead of letting patients with suitable tumours gain access to hyperthermia for conditions where phase III studies have documented effects. Certainly, many new drugs will give us better treatment options in the years to come. Nevertheless, hyperthermia is already a valuable option for selected malignancies, and the machines for administering heating are today much more reliable than those used 30 years ago. Hyperthermia must therefore get the necessary funding to become available in major cancer centres.
References
- Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med 1971; 285: 1182–1186
- Folkman J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg 1972; 175: 409–416
- Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974; 34: 2109–2113
- Folkman J. Angiogenesis: An organizing principle for drug discovery?. Nat Rev Drug Discov 2007; 6: 273–286
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6: 583–592
- Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol 2004; 5: 292–302
- Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 2006; 6: 714–727
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1031–1037
- Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med 2007; 357: 258–265
- Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Gilliland DG, Druker BJ. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood 1997; 90: 4947–4952
- Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest 2000; 105: 3–7
- Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1084–1086
- le Coutre P, Mologni L, Cleris L, Marchesi E, Buchdunger E, Giardini R, Formelli F, Gambacorti-Passerini C. In vivo eradication of human BCR/ABLpositive leukemia cells with an ABL kinase inhibitor. J Natl Cancer Inst 1999; 91: 163–168
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 2000; 289: 1938–1942
- Thiesing JT, Ohno-Jones S, Kolibaba KS, Druker BJ. Efficacy of STI571, an abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against bcrabl-positive cells. Blood 2000; 96: 3195–3199
- Vigneri P, Wang JY. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat Med 2001; 7: 228–2234
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001; 344: 1052–1056
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JTY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 2004; 364: 1127–1134
- Shimizu A, O'Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, Heldin CH, Dumanski JP, Ostman A. The dermatofibrosarcoma protuberans associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res 1999; 59: 3719–3723
- McArthur G. Dermatofibrosarcoma protuberans: Recent clinical progress. Ann Surg Oncol 2007; 14: 2876–2886
- Curtis CE, Grand FH, Musto P, Clark A, Murphy J, Perla G. Minervini MM, Stewart J, Reiter A, Cross NC. Two novel imatinib-responsive PDGFRA fusion genes in chronic eosinophilic leukaemia. Br J Haematol 2007; 138: 77–81
- Fletcher S, Bain B. Eosinophilic leukaemia. Br Med Bull 2007; 81-82: 115–127
- Gotlib J, Cools J, Malone JM, III, Schrier SL, Gilliland DG, Coutre SE. The FIP1L1- PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: Implications for diagnosis, classification, and management. Blood 2004; 103: 2879–2891
- Kalac M, Quintas-Cardama A, Vrhovac R, Kantarjian H, Verstovsek S. A critical appraisal of conventional and investigational drug therapy in patients with hypereosinophilic syndrome and clonal eosinophilia. Cancer 2007; 110: 955–964
- Pardanani A, Reeder T, Porrata LF, Li CY, Tazelaar HD, Baxter EJ, Witzig TE, Cross NC, Tefferi A. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood 2003; 101: 3391–3397
- Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, Erben P, Popp H, Walz C, Hochhaus A, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood 2007; 109: 4635–4640
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med 2005; 353: 172–187
- Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood 2007; 109: 4143–4150
- Cheson BD. CHOP plus rituximab - Balancing facts and opinion. N Engl J Med 2002; 346: 280–282
- Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene 2007; 26: 3603–3613
- Stephenson J. Researchers optimistic about sea change in cancer treatment. JAMA 2001; 285: 2841–2842
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–4380
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8: 464–478
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005; 438: 946–953
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21: 60–65
- Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–3712
- Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol 2005; 23: 3697–3705
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007; 25: 1539–1544
- Goldberg RM. Therapy for metastatic colorectal cancer. Oncologist 2006; 11: 981–987
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004; 22: 209–1214
- Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005; 352: 476–487
- Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007; 25: 3045–3054
- McDonald DM, Choyke PL. Imaging of angiogenesis: From microscope to clinic. Nat Med 2003; 9: 713–725
- Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: Applications and potential for drug development. J Natl Cancer Inst 2005; 97: 172–187
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–147
- Willett CG, Duda DG, di Tomaso E, Boucher Y, Czito BG, Vujaskovic Z, Vlahovic G, Bendell J, Cohen KS, Hurwitz HI, et al. Complete pathological response to bevacizumab and chemoradiation in advanced rectal cancer. Nat Clin Pract Oncol 2007; 4: 316–321
- Sandler A. Bevacizumab in non small cell lung cancer. Clin Cancer Res 2007; 13: 4613s–4616s
- Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: Bevacizumab (avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non small cell lung cancer. Oncologist 2007; 12: 713–718
- Stinchcombe TE, Socinski MA. Bevacizumab in the treatment of non-small-cell lung cancer. Oncogene 2007; 26: 3691–3698
- Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: Focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol 2007; 8: 15–27
- de Castro Junior G, Puglisi F, de Azambuja E, El Saghir NS, Awada A. Angiogenesis and cancer: A cross-talk between basic science and clinical trials (the ‘do ut des’ paradigm). Crit Rev Oncol Hematol 2006; 59: 40–50
- Los M, Roodhart JM, Voest EE. Target practice: Lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 2007; 12: 443–450
- Arroyo JG. A 76-year-old man with macular degeneration. JAMA 2006; 295: 2394–2406
- Steinbrook R. The price of sight–Ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med 2006; 355: 1409–1412
- Stone EM. A very effective treatment for neovascular macular degeneration. N Engl J Med 2006; 355: 1493–1495
- Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM.2 Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol 2006; 142: 1054–1056
- Melchert M, List A. The thalidomide saga. Int J Biochem. Cell Biol 2007; 39: 1489–1499
- Morgan GJ, Krishnan B, Jenner M, Davies FE. Advances in oral therapy for multiple myeloma. Lancet Oncol 2006; 7: 316–325
- Zervas K, Mihou D, Katodritou E, Pouli A, Mitsouli Ch, Anagnostopoulcs A, Delibasi S, Kyrtsonis M, Anagnostopoulos N, Terpos E, et al. VAD-doxil vs. VAD-doxil plus thalidomide as initial treatment for multiple myeloma: Results of a multicenter randomized trial of the Greek myeloma study group. Ann Oncol 2007; 18: 1369–1375
- Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: A review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol 2003; 4: 397–406
- Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res 2006; 66: 3197–3204
- Nahta R, Esteva FJ. Trastuzumab: Triumphs and tribulations. Oncogene 2007; 26: 3637–3643
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17: 2639–2648
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–726
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–1684
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–1672
- Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: A milestone in the treatment of HER-2-positive early breast cancer. Oncologist 2006; 11 Suppl. 1: 4–12
- Pritchard Kl, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 2006; 354(20)2103–2111
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, et al. Two-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 2007; 369: 29–36
- Engel RH, Kaklamani VG. HER2-positive breast cancer: Current and future treatment strategies. Drugs 2007; 67: 1329–1341
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733–2743
- Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belanj CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with nonsmall cell lung cancer: A randomized trial. JAMA 2003; 290: 2149–2158
- Giaccone G. The role of gefitinib in lung cancer treatment. Clin Cancer Res 2004; 10: 4233s–4237s
- Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, Sgroi DC, Muir B, Riemenschneider MJ, Lacona RB, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: Molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 2005; 23: 8081–8092
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123–132
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–181
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007; 25: 1545–1552
- Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA 2007; 298: 70–82
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–345
- Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004; 22: 1201–1208
- Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol 2006; 24: 4914–4921
- Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 2005; 23: 1803–1810
- Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007; 25: 3230–3237
- Galizia G, Lieto E, De Vita F. Orditura M, Castellano P, Troiani T, Imperatore V, Ciardiello F. Cetuximab, a chimeric human mouse antiepidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene 2007; 26: 3654–3660
- Hildebrandt B, le Coutre P, Nicolaou A, Koble K, Riess H, Dorken B. Cetuximab: Appraisal of a novel drug against colorectal cancer. Recent Results Cancer Res 2007; 176: 135–143
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25: 1658–1664
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF Côte JF, Tomasic G, Penna C, Ducreux M, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006; 66: 3992–3995
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S, Bardelli A. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007; 67: 2643–2648
- Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 2007; 96: 1166–1169
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med 2003; 9: 677–684
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005; 353(23)2477–2490
- Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol 2005; 23: 1028–1043
- Brugarolas J. Renal-cell carcinoma–molecular pathways and therapies. N Engl J Med 2007; 356: 185–187
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–434
- Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, et al. Sunitinib in patients with metastatic renal cell carcinoma. 2. Jama 2006; 295: 2516–2524
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib vs. interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124
- Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol 2005; 23: 7889–7896
- Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24: 2505–2512
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356: 125–134
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2007; 7, Online Jul
- Gasparini G, Longo R, Toi M, Ferrara N. Angiogenic inhibitors: A new therapeutic strategy in oncology. Nat Clin Pract Oncol 2005; 2: 562–577
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 2001; 93: 178–193
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin. Cancer Res 2004; 10: 415–427
- Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer 2004; 100: 2491–2499
- Patterson DM, Rustin GJ. Vascular damaging agents. Clin Oncol (R Coll Radiol) 2007; 19: 443–456
- Denekamp J, Hill SA, Hobson B. Vascular occlusion and tumour cell death. Eur J Cancer Clin Oncol 1983; 19: 271–275
- Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol Oncol 1984; 23: 217–225
- Hill SA, Lonergan SJ, Denekamp J, Chaplin DJ. Vinca alkaloids: Anti-vascular effects in a murine tumour. Eur J Cancer 1993; 29A(9)1320–1324
- Eikesdal HP, Bjerkvig R, Dahl O. Vinblastine and hyperthermia target the neovasculature in BT(4)AN rat gliomas: Therapeutic implications of the vascular phenotype. Int J Radiat Oncol Biol Phys 2001; 51: 535–44
- Eikesdal HP, Bjerkvig R, Mella O, Dahl O. Combretastatin A-4 and hyperthermia: A potent combination for the treatment of solid tumors. Radiother Oncol 2001; 60: 147–154
- Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest 2005; 115: 2992–3006
- Salmon BA, Siemann DW. Characterizing the tumor response to treatment with combretastatin A4 phosphate. Int J Radiat Oncol Biol Phys 2007; 68: 211–217
- Griffin RJ, Monzen H, Williams BW, Park H, Lee SH, Song CW. Arsenic trioxide induces selective tumour vascular damage via oxidative stress and increases thermosensitivity of tumours. Int J Hyperthermia 2003; 19: 575–589
- Murata R, Horsman MR. Tumour-specific enhancement of thermoradiotherapy at mild temperatures by the vascular targeting agent 5,6-dimethylxanthenone-4-acetic acid. Int J Hyperthermia 2004; 20: 393–404
- ten Hagen TL, Eggermont AM. Changing the pathophysiology of solid tumours: The potential of TNF and other vasoactive agents. Int J Hyperthermia 2006; 22: 241–246
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22: 197–203
- Brunstein F, Rens J, van Tiel ST, Eggermont AM, ten Hagen TL. Histamine, a vasoactive agent with vascular disrupting potential, improves tumour response by enhancing local drug delivery. Br J Cancer 2006; 95: 1663–1669
- Thorpe PE, Chaplin DJ, Blakey DC. The first international conference on vascular targeting: Meeting overview. Cancer Res 2003; 63: 1144–1147
- Albertsson P, Lennernas B, Norrby K. On metronomic chemotherapy: Modulation of angiogenesis mediated by VEGE-A. Acta Oncol 2006; 45: 144–155
- Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, Del Tacca M. Cyclophosphamide-methotrexate 'metronomic' chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol 2005; 16: 1243–1252
- Damber JE, Vallbo C, Albertsson P, Lennernas B, Norrby K. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother Pharmacol 2006; 58: 354–360
- Gasparini G. Metronomic scheduling: The future of chemotherapy?. Lancet Oncol 2001; 2: 733–740
- Kerbel RS. Antiangiogenic therapy: A universal chemosensitization strategy for cancer?. Science 2006; 312: 1171–1175
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000; 60: 1878–886
- Broxterman HJ, Lankelma J, Hoekman K. Resistance to cytotoxic and anti-angiogenic anticancer agents: Similarities and differences. Drug Resist Updat 2003; 6: 111–127
- Kamat AA, Kim TJ, Landen CN, Jr, Lu C, Han LY, Lin YG, Merritt WM, Thaker PH, Gershenson DM, Bischoff F, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res 2007; 67: 281–288
- Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem–like cell fraction in glioma xenograft tumors. Cancer Res 2007; 67: 3560–3564
- Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003; 63: 4342–4346
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer 2006; 6: 835
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy?. Nat Rev Cancer 2002; 2: 826–835
- Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer 2006; 6: 626–635
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Dahl O, Overgaard J. Hyperthermia. Oxford Textbook of oncology. 2, RL Souhami , IF Tannock, P Hohenberger, J-C Horiot . Oxford University Press, Oxford 2002; 1: 511–525
- Jackson IL, Batinic-Haberle I, Sonveaux P, Dewhirst MW, Vujaskovic Z. ROS production and angiogenic regulation by macrophages in response to heat therapy. Int J Hyperthermia 2006; 22: 263–273
- Lyng H, Monge OR, Bohler PJ, Rofstad EK. Relationships between thermal dose and heat-induced tissue and vascular damage after thermoradiotherapy of locally advanced breast carcinoma. Int J Hyperthermia 1991; 7: 403–415
- Rofstad EK, Brustad T. Response of human malignant melanoma xenografts to hyperthermia: Effect of vascular occlusion. Int J Radiat Oncol Biol Phys 1981; 7: 1685–1687
- Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia 2006; 22: 365–373
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–767
- Fajardo LF, Prionas SD. Endothelial cells and hyperthermia. Int J Hyperthermia 1994; 10: 347–353
- Fajardo LF, Prionas SD, Kowalski J, Kwan HH. Hyperthermia inhibits angiogenesis. Radiat Res 1988; 114: 297–306
- Eikesdal HP, Bjorkhaug ST, Dahl O. Hyperthermia exhibits anti-vascular activity in the s.c. BT4An rat glioma: Lack of interaction with the angiogenesis inhibitor batimastat. Int J Hyperthermia 2002; 18: 141
- Kanamori S, Nishimura Y, Okuno Y, Horii N, Saga T, Hiraoka M. Induction of vascular endothelial growth factor (VEGF) by hyperthermia and/or an angiogenesis inhibitor. Int J Hyperthermia 1999; 15: 267–278
- Sawaji Y, Sato T, Takeuchi A, Hirata M, Ito A. Anti-angiogenic action of hyperthermia by suppressing gene expression and production of tumour-derived vascular endothelial growth factor in vivo and in vitro. Br J Cancer 2002; 86: 1597
- Srinivasan JM, Fajardo LF, Hahn GM. Mechanism of antitumor activity of tumor necrosis factor alpha with hyperthermia in a tumor necrosis factor alpha-resistant tumor. J Natl Cancer Inst 1990; 82: 1904–1910
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994; 28: 163–169
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996; 12: 3–20
- Hand JW, Machin D, Vernon CC, Whaley JB. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia 1997; 13: 343–364
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Prosnitz LR, Maguire P, Anderson JM, Scully SP, Harrelson JM, Jones EL, Dewhirst M, Samulski TV, Powers BE, Rosner GL, et al. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys 1999; 45: 941–949
- Maguire PD, Samulski TV, Prosnitz LR, Jones EL, Rosner GL, Powers B, Layfield LW, Brizel DM, Scully SP, Harrelson JM, et al. A phase II trial testing the thermal dose parameter CEM43 degrees T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia 2001; 17: 283–290
- Issels RD. High-risk soft tissue sarcoma: Clinical trial and hyperthermia combined chemotherapy. Int J Hyperthermia 2006; 22: 235–239
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- van der Zee J, van Rhoon GC. Cervical cancer: Radiotherapy and hyperthermia. Int J Hyperthermia 2006; 22: 229–234
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy vs. thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, Uitterhoeve AL, de Wit R, van der Velden J, Burger C, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 2005; 104: 763–770
- Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, Caldarera E, Pavone-Macaluso M. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003; 21: 4270–4276
- Issels RD, Lindner LH, Wust P, Hohenberger P, Jauch K, Daugaard S, Mannsmann U, Hiddemann W, Blay J, Verweij J. Regional hyperthermia (RHT) improves response and survival when combined with systemic chemotherapy in the management of locally advanced, high grade soft tissue sarcomas (STS) of the extremities, the body wall and the abdomen: A phase III randomised pros. J Clin Oncol 2007; 10009: 18s
- Alvarez Secord A, Jones EL, Hahn CA, Petros WP, Yu D, Havrilesky LJ, Soper JT, Berchuck A, Spasojevic I, Clarke-Pearson DL, et al. Phase I/II trial of intravenous Doxil and whole abdomen hyperthermia in patients with refractory ovarian cancer. Int J Hyperthermia 2005; 21: 333–347
- Johannsen M, Gnevckow U, Taymoorian K, Thiesen B, Waldöfner N, Scholz R, Jung K, Jordan A, Wust P, Loening SA. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int J Hyperthermia 2007; 23: 315–323
- Liu P, Zhang A, Xu Y, Xu LX. Study of non-uniform nanoparticle liposome extravasation in tumour. Int J Hyperthermia 2005; 21: 259–270
- Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic resonance imaging of temperaturesensitive liposome release: Drug dose painting and antitumor effects. J Natl Cancer Inst 2007; 99: 53–63
- Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia 2006; 22: 205–213
- Wust P, Gneveckow U, Johannsen M, Böhmer D, Henkel T, Kohmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy–feasibility, tolerance and achieved temperatures. Int J Hyperthermia 2006; 22: 673–685
- Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang X, Lame SM, Avery PR, Dewhirst MW, Ullrich RL. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int J Hyperthermia 2006; 22: 587–606
- Siddiqui F, Li CY, Zhang X, Larue SM, Dewhirst MW, Ullrich RL, Avery PR. Characterization of a recombinant adenovirus vector encoding heat-inducible feline interleukin-12 for use in hyperthermia-induced genetherapy. Int J Hyperthermia 2006; 22: 117–134
- Zhang S, Xu G, Liu C, Xiao S, Sun Y, Su X, Cai Y, Li D, Xu B. Clinical study of recombinant adenovirus-p53 (Adp53) combined with hyperthermia in advanced cancer (a report of 15 cases). Int J Hyperthermia 2005; 21: 631–636
- Li GC, He F, Ling CC. Hyperthermia and gene therapy: Potential use of microPET imaging. Int J Hyperthermia 2006; 22: 215–221
- Branford S, Hughes T, Milner A, Koelmeyer R, Schwarer A, Arthur C, Filshie R, Moreton S, Lynch K, Taylor K. Efficacy and safety of imatinib in patients with chronic myeloid leukemia and complete or near-complete cytogenetic response to interferon-alpha. Cancer 2007; 110: 801–808
- Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: Amidst the hype, the neglected potential for serious side effects. Circulation 2001; 104: 115–119
- Van Glabbeke M, Verweij J, Casali PG, Simes J, Le Cesne A, Reichardt P, Issels R, Judson IR, van Oosterom AT, Blay JY. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: A study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG). Eur J Cancer 2006; 42: 2277–2285
- Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 2007; 7: 475–485
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006; 6: 803–812
- Chien KR. Herceptin and the heart - a molecular modifier of cardiac failure. N Engl J Med 2006; 354: 789–790
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007; 7: 332–344
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, et al. Trastuzumab-Associated Cardiac Adverse Effects in the Herceptin Adjuvant Trial. J Clin Oncol 2007; 25: 3859–3865
- Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar S, Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005; 91: 173–180
- Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007; 99: 1232–1239
- van Heeckeren WJ, Ortiz J, Cooney MM, Remick SC. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: Clinical toxicity, therapeutic target, or novel biomarker?. J Clin Oncol 2007; 25: 2993–2995
- Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R, Rau B. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia 2006; 22: 301–318
- Haveman J, Van Der Zee J, Wondergem J, Hoogeveen JF, Hulshof MC. Effects of hyperthermia on the peripheral nervous system: A review. Int J Hyperthermia 2004; 20: 371–391
- Wiggenraad R, Koning C, Westermann C, Jansen C, van der Zee J. Two cases of fatal necrosis of the lesser pelvis in patients treated with combined radiotherapy and hyperthermia for cervical carcinoma. Int J Hyperthermia 2005; 21: 185–192
- Balzer S, Schneider DT, Bernbeck MB, Jager M, Mils O, Schaper J, Willers R, Krauspe R, Gobel U, Wessalowski R. Avascular osteonecrosis after hyperthermia in children and adolescents with pelvic malignancies: A retrospective analysis of potential risk factors. Int J Hyperthermia 2006; 22: 451–461
- Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, Felix R, Tunn PU, Reichardt P, Wust P. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006; 107: 1373–1382
- Gellermann J, Wlodarczyk W, Feussner A, Fahling H, Nadobny J, Hildebrandt B, Felix R, Wust P. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia 2005; 21: 497–513
- Hornsleth SN, Frydendal L, Mella O, Dahl O, Raskmark P. Quality assurance for radiofrequency regional hyperthermia. Int J Hyperthermia 1997; 13: 169–185
- van Haaren PM, Kok HP, Zum Vorde Sive Vording PJ, van Dijk JD, Hulshof MC, Fockens P, van Lanschot JJ, Crezee J. Reliability of temperature and SAR measurements at oesophageal tumour locations. Int J Hyperthermia 2006; 22: 545–561
- van Rhoon GC, Wust P. Introduction: Non-invasive thermometry for thermotherapy. Int J Hyperthermia 2005; 21: 489–495
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007; 19: 418–426
- Norum J, Olsen JA, Wist EA, Lonning PE. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol 2007; 46: 153–164
- Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/irinotecan vs. active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 2007; 96: 206–212
