Abstract
The results from experimental studies indicate that hyperthermia is both an effective complementary treatment to, and a strong sensitiser of, radiotherapy and many cytotoxic drugs. Since the first international hyperthermia conference in 1975, Washington DC, techniques to increase tumour temperature have been developed and tested clinically. Hyperthermia can be applied by several methods: local hyperthermia by external or internal energy sources, perfusion hyperthermia of organs, limbs, or body cavities, and whole body hyperthermia. The clinical value of hyperthermia in combination with other treatment modalities has been shown by randomised trials. Significant improvement in clinical outcome has been demonstrated for tumours of the head and neck, breast, brain, bladder, cervix, rectum, lung, oesophagus, for melanoma and sarcoma. The addition of hyperthermia resulted in remarkably higher (complete) response rates, accompanied by improved local tumour control rates, better palliative effects, and/or better overall survival rates. Toxicity from hyperthermia cannot always be avoided, but is usually of limited clinical relevance. In spite of these good clinical results, hyperthermia has received little attention. Problems with acceptance concern the limited availability of equipment, the lack of awareness concerning clinical results, and the lack of financial resources. In this paper the most relevant literature describing the clinical effects of hyperthermia is reviewed and discussed, and means to overcome the lack of awareness and use of this modality is described.
General introduction
Hyperthermia is the elevation of temperature above the physiological level with the objective to achieve therapeutic gain. Hyperthermia is generally defined as a modest elevation of temperature to a range of 39° to 45°C. Higher temperatures are used for thermal ablation. Only clinicians using classical hyperthermia participated in the Kadota meeting; therefore, this report is limited to the use of temperatures in the range of 39° to 45°C.
The use of elevated temperatures for the treatment of cancer has been well documented for centuries Citation[1]. The first international congress on hyperthermic oncology held in 1975 in Washington DC ignited worldwide interest in hyperthermia. As with most new treatment modalities, hyperthermia was initially met with ever increasing enthusiasm reflected by an exponential increase in the number of papers and participants at meetings. Interest in hyperthermia waned thereafter due to disappointing clinical results of the first randomised studies in the USA, accompanied by reluctant sponsoring authorities and hospital boards concerned over support of future research.
However, more recent results of several randomised studies have shown great improvement in treatment outcome by the addition of hyperthermia to radiotherapy or chemotherapy, provided adequate heating procedures are used. Nevertheless, this treatment modality has failed to garner broad acceptance. In this report, the consensus of the participants at the Kadota forum on clinical aspects of hyperthermia are summarised and the problems involved in gaining acceptance, along with ways to overcome these problems, are discussed.
Methods to increase tumour temperatures
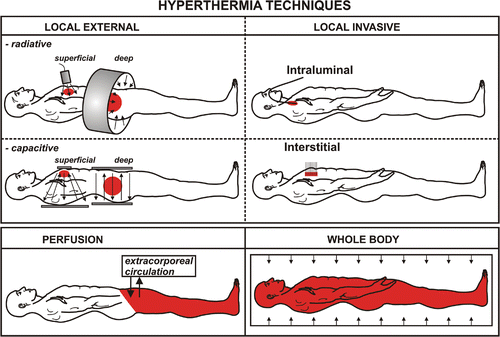
Clinical hyperthermia is achieved by exposing tissues to conductive heat sources, or non-ionising radiation (e.g. electromagnetic or ultrasonic fields). Although these modalities deposit energy in tissue by different physical mechanisms, they have general similarities. They are sensitive to the heterogeneity of tissue properties, geometry of blood flow, and the practical problems of coupling the energy source into tissue. Hyperthermia can be administered either invasively, or noninvasively using externally applied power. It can be delivered locally, by perfusion or as a whole body hyperthermia ().
Local hyperthermia
The aim of local hyperthermia is to achieve the optimal thermal dose in the tumour tissue without exceeding the tolerance limits of the surrounding normal tissues. Local hyperthermia can be applied by external, intraluminal or interstitial methods. Electromagnetic or ultrasound energy is directed at the treatment volume. The volume that can be heated depends on the physical characteristics of the energy source and on the type of applicator (array) Citation[2]. Methods to apply hyperthermia externally can be divided into superficial and deep techniques. For deep heating, the energy is directed from around the part of the body in which the target volume is located. The energy distribution within the tissues is strongly dependent on tissue characteristics and thereby is inhomogeneous. The temperature variance is not simply a result of the energy distribution, but also depends on thermal tissue characteristics and blood flow. During local hyperthermia, the systemic temperature may increase as well, depending on both the heated volume and the measures taken to help the patient lose energy.
Perfusion hyperthermia
Perfusion of a limb, organ, or body cavity with heated fluids produces regional heating Citation[3], Citation[4]. When this approach is applied to limbs, and without a cytotoxic agent, the temperature can be safely increased to about 43°C for a duration of two hours. When used in combination with cytotoxic drugs, the perfusion fluid temperature should be adjusted to avoid unacceptable toxicity.
Whole-body hyperthermia
For whole-body hyperthermia, several methods have been used. A common characteristic is the introduction of energy into the body, while at the same time energy losses are minimised. The temperature increase is usually limited to 41.8°–42°C. Experience with radiant heat methods, for which the patients need deep sedation during the treatment, has shown that this procedure is well tolerated Citation[5], Citation[6]. A newer approach is to increase the temperature to about 40°C for a long duration, which, in combination with cytokines and/or cytotoxic drugs is expected to lead to a greater therapeutic index than whole-body hyperthermia at the maximum tolerated level for a short duration Citation[7].
Clinical studies
In order to gain wide acceptance as a new treatment modality, hyperthermia has to fulfil the following criteria:
It has to be necessary–i.e. there must exist a need for improvement in treatment outcome over standard treatment;
It has to be effective–the effectiveness of the treatment needs to be shown in preclinical and clinical studies;
It has to be efficient: results should show a favourable balance between cost and effect.
The treatment must result in improvements which are relevant to the patient. In this respect, endpoints are distinguished into ‘surrogate’ and ‘true’ endpoints. True endpoints are relevant to the patient, such as palliation, an improved quality of life, or longer survival. Surrogate endpoints are defined as response rate and improvement in laboratory results, so long as these indications of treatment effect do not coincide with improvement of true endpoints.
The quality of evidence from clinical studies, which may lead to acceptance of a new treatment modality, has been grouped to the following three levels.
Level 1. Evidence obtained from at least one properly randomised controlled clinical trial;
Level 2-1. Evidence obtained from well-designed controlled trials without randomisation;
Level 2-2. Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one centre or research group;
Level 2-3. Evidence obtained from comparisons between times or places with or without the intervention;
Level 3. Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees.
It is clear that, in cancer treatment, there are many situations where improvement of clinical outcome is necessary.
Both preclinical and clinical studies have shown hyperthermia to be effective.
Clinical results
Strong proof of effectiveness of hyperthermia comes from clinical studies in which hyperthermia was used alone. A review of 14 such studies including a total of 343 patients, reported complete response rates varying from 0 to 40% (overall 13%) and partial response rates from 0 to 56%, with an overall objective response rate of 51% Citation[8]. Three additional studies reported complete response rates of 11, 16 and 18% Citation[9–11]. Since adequate heating the whole tumour volume is difficult except for superficially located small tumours, and in general the reported response duration is short, the use of hyperthermia alone is not recommended.
Relatively strong evidence comes from several studies on ‘matched lesions’: multiple lesions of the same patients were treated with radiotherapy, with or without hyperthermia. In some studies, the treatment was selected at random, in others the larger lesions received combination treatment. In all these studies, a higher complete response rate for combined treated lesions was demonstrated. A summation of the data from these studies (total 713 lesions) shows an increase in complete response rate from 31% to 67% Citation[12].
Level I evidence
Level I evidence from randomised trials showing significantly better results following the addition of hyperthermia to other treatment modalities is summarised in Citation[13–32]. The majority of these studies were included in a previous review Citation[33]. Publications concerning randomised trials were retrieved from PubMed, and information on additional studies came from meeting proceedings. Since the review was written, two more randomised studies have been published Citation[30], Citation[34]. Of the total 28 randomised trials, 19 showed significantly better results in the treatment arm with hyperthermia. The majority of this level I evidence concerns the addition of local hyperthermia to radiotherapy. The tumour types in which significant improvements were observed include head and neck tumours, melanoma, sarcoma, breast cancer, glioblastoma multiforme, rectum, bladder and cervix cancer, oesophageal cancer and various superficial tumours. Two studies have shown that local hyperthermia in addition to radiotherapy plus chemotherapy improved the results in oesophageal carcinoma. Perfusion hyperthermia in addition to chemotherapy, applied either pre- or postoperatively, improved results in bladder cancer. Whole body hyperthermia added to chemotherapy gave better results than chemotherapy alone for lung cancer.
Table I. LEVEL I evidence from randomised trials showing significantly better results following combination of radiotherapy (RT), chemotherapy (CT), or RT plus CT with hyperthermia (HT), compared to the same treatment without HT. LHT, local HT; RHT, regional HT; WBHT, whole body HT; CR, complete response.
Nine randomised studies failed to show a significant benefit from addition of hyperthermia. The following four studies showed a more than 10% higher (complete) response or local control rate with the addition of hyperthermia, but the numbers of patients were too small to make the difference statistically significant. In head and neck tumours, complete response rate was 58% following radiotherapy and 74% following radiotherapy plus hyperthermia Citation[35]. In patients with multiple lesions, with inpatient randomisation, the response rate following radiotherapy was 7% and following combined treatment 47% Citation[36]. In oesophageal cancer, pathological complete response rate after preoperative treatment with chemotherapy increased from 19% to 40% thanks to the addition of hyperthermia Citation[37]. In cervical cancer, 18-months local control rate was 50% after radiotherapy and 70% after combined treatment Citation[38]. Five studies showed smaller or no differences in clinical outcome between treatment with or without hyperthermia. Addition of hyperthermia to preoperative radiotherapy in breast cancer was reported to result in a significant improvement in local control rate, but only a tendency to improved survival was found, from 67% to 73% Citation[39]. In gastric cancer, 5-year overall survival following preoperative radiotherapy was 45%, while it was 51% after combined treatment Citation[40].
In three studies, which are further discussed in the paragraph ‘Importance of adequate local heating techniques’, inadequate heat delivery has been indicated as the cause of failure to achieving a beneficial effect from the addition of hyperthermia Citation[34], Citation[41–43].
Level II evidence
A few non-randomised studies comparing combined treatment to radiotherapy or chemotherapy alone in well-matched control groups have led to the acceptance of hyperthermia as part of standard care in The Netherlands and Germany.
In patients with thoracic wall invasion of malignant mesothelioma, addition of hyperthermia to radiotherapy resulted in considerably higher response and palliation rates, and much lower tumour regrowth and pain recurrence rates Citation[44]. In children with a first relapse of germ cell tumours, addition of hyperthermia to chemotherapy resulted in much better disease free and overall survival Citation[45–46].
Level III evidence
Two studies have led to the acceptance in The Netherlands of combined hyperthermia and cisplatin in patients with locoregional recurrences of cervix following radiotherapy. Simultaneous combination of cisplatin and hyperthermia resulted in 50% response Citation[47–48], compared to 15% (maximum expected) with cisplatin alone. In six of 49 patients, the combination resulted in long-term disease free survival.
Further experience
Hyperthermia is used in some countries such as Japan to treat recurrent tumours, which are untreatable with any conventional treatment modalities and thus the patients would otherwise be denied further treatment. Because of the nature of the tumours, the results of such treatment are difficult to document by conventional means. However, it has been observed that hyperthermia of such tumours often causes noticeable retardation of tumour growth and confers significant palliative benefit, thereby improving quality of life of the patients Citation[49]. The potential usefulness of hyperthermia for palliation and improvement of quality of life of patients with tumours untreatable with conventional treatment merits further investigation, especially in recurrent lung and liver cancers.
Interesting results were seen in patients with anal cancer. A randomised phase II trial, studying the effect of adding hyperthermia to radiotherapy and chemotherapy showed that with hyperthermia, patients had significantly fewer local recurrences and higher anorectal function preservation Citation[50].
Importance of adequate local heating techniques
The results of the first two randomised studies performed in the USA were disappointing, as these studies failed to show a beneficial effect of adding hyperthermia to radiotherapy. Retrospectively, these negative results have been explained by the use of hyperthermia treatment techniques that were inadequate for the patients included in these studies Citation[41–43]. In the study by Perez et al. Citation[41–42] the more easily heated lesions (smaller than 3 cm in diameter) showed a difference in complete response rate (52% versus 39%), while the larger lesions did not (25% versus 27%). Recently, a randomised study on addition of hyperthermia to radiotherapy in patients with uterine cervical cancer also failed to show an advantage of hyperthermia Citation[34]. This also appears a matter of inadequate heating techniques Citation[51–52].
Over the years, the importance of adequate heating has become clear. A study on recurrent breast cancer showed that the complete response rate in tumours larger than 3 cm increased from 31% to 65% with the use of a better heating technique (433 MHz compared to 2450 MHz) in addition to re-irradiation Citation[53]. It is not easy to translate measured temperatures directly into adequacy of heating technique. The measured temperature distribution depends strongly on number of thermometry sites and location of thermometry probes. Thermometry procedures differ between institutes and may, within the same institute, change over time. For example, within the afore mentioned study Citation[53], the specific absorption rate (SAR) distribution was more homogeneous over the treatment volume when 433 MHz was used. Nevertheless, the measured temperatures were not better with the 433 MHz technique compared to the 2450 MHz technique, due to a difference in number of thermometry sites. In the five combined randomised trials on breast cancer Citation[16], the improvement of tumour response due to addition of hyperthermia to radiotherapy and the measured temperatures were not related. This probably was also due to differences between the thermometry procedures.
A better measure for adequacy appears to be the use of SAR coverage of the treatment volume: the energy level that encompasses the volume at risk. Lee et al. Citation[54] found that when the tumour volume was covered by 25% SAR, a higher complete response rate resulted than when SAR fell below 25%. Besides, SAR is a more attractive measure for adequacy since it can be prescribed. Nevertheless, many experimental and clinical studies have shown that higher temperature levels, converted into a large variety of hyperthermia dose descriptors, result in better outcomes. A recent study comparing the effect of a prospectively prescribed thermal dose of more than 10 CEM 43°C T90 with that of less than 1, demonstrated a considerable local control benefit from the higher dose Citation[55]. Therefore, it is important to apply treatments with optimum available treatment techniques and with temperature measurements in or near the volume at risk to the maximum achievable temperature levels.
Cost of treatment
Toxicity
Normal tissue toxicity will result directly from hyperthermia when the tolerance limits are exceeded. Experimental studies have shown that most normal tissues are not damaged when the temperature over 1 hour treatment does not exceed 43°C Citation[56]. Only nervous and gastro-intestinal tissues appear more sensitive. For the central nervous tissue, irreversible damage was found after treatment at 42°–42.5°C for longer than 40–60 minutes Citation[57]. Treatment of peripheral nervous tissue for >30 minutes at 44°C, or an equivalent dose, results in temporary functional loss, which recovers within 4 weeks Citation[58]. Gastro-intestinal mucosa may be damaged by a one-hour treatment when the temperature exceeds 42°C Citation[59]. During local hyperthermia, it is not always possible to avoid high temperatures in normal tissues adjacent to tumours, due to the heterogeneity of the temperature distribution, and the limited thermometry. The patient is not always able to feel temperature hot spots, e.g. when the target area has been subject to surgery in the past and sensitivity is disturbed. The undesirable side effect from superficial hyperthermia is usually a skin burn (in about 25% of the patients with recurrent breast cancer Citation[16], Citation[53–54], which can be healed with conservative treatment. During hyperthermia for deep-seated tumours, the skin is extensively cooled, through which the hot spots will develop in deeper tissues. A too high temperature in subcutaneous fat or muscle tissue results in a feeling of pressure, which is not always recognised by the patient. Thereby, patients may be reluctant to mention unpleasant sensations. Subcutaneous fat or muscle tissue burns usually do not cause much discomfort: the patient feels a subcutaneous lump, tender for a few days to maximum a few weeks, which disappears spontaneously. Subcutaneous fat burns were seen in 3-12% of the patients treated with deep hyperthermia. The risk of developing skin burns may be higher following treatment with a radiofrequency capacitive heating technique (5–16%) than with a radiative heating technique (0–3%) [18, 60-62. However, pre-cooling of skin was found to significantly reduce the skin damage in capacitive heating Citation[63]. The randomised studies did not show an increase in acute or late toxicity of radiotherapy. Whether the toxicity of chemotherapy is enhanced will depend on the temperature in the drug-sensitive tissues.
Toxicity from whole body hyperthermia depends on, besides temperature, the patient's general condition, condition of organ systems, and the physiological conditions during the treatment Citation[7]. Serious toxicity from hyperthermic perfusion with modern technology and proper choice of perfusate composition, flow rate and pressure, blood gas values, drug doses, temperature dose and scheduling, is limited Citation[64]. During any application of hyperthermia it is important to avoid pressure on normal tissues. Pressure may hinder blood circulation, thereby increasing tissue temperature, and/or cause hypoxia. Either one of, or both these conditions will increase the risk of toxicity.
Economic aspects
The application of hyperthermia is relatively labour intensive. With local hyperthermia, the energy distribution and the resulting temperature distribution can only be partially monitored with interstitial placed temperature sensors. During treatment, the information given by the patient, especially on (painful) hot spots, is mandatory to prevent the development of thermal burns. The clinical staff have to be continuously alerted in interpreting both the measured temperature distribution and the symptoms mentioned by the patient, in order to appropriately adjust the applied energy distribution. Both whole body and perfusion hyperthermia are time-consuming procedures requiring appropriate equipment and skilled personal. Nevertheless, thanks to the large therapeutic gain achieved, the cost-effectiveness of hyperthermia appears acceptable. Within e.g. the Dutch randomised trial on intrapelvic tumours, the cost-per-life-year-gained for cervical cancer was less than €4000 Citation[65].
Acceptance of hyperthermia
In several countries, hyperthermia has been accepted as a part of standard care for cancer for a number of years. The acceptance is often restricted to certain techniques (i.e. superficial treatments only), or to specified indications. However, hyperthermia has not yet been accepted as a standard cancer treatment modality in many countries. There appear to be several reasons for this lack of acceptance.
Quality of published studies
Problem. Many of the studies listed in are relatively small studies, including less than 100 patients or lesions. Even with these small numbers, the results were sufficient to show a significant improvement by the addition of hyperthermia, because of the large gain. However, small studies are not easily accepted as the basis for new treatment guidelines by the medical community.
Solution
The institutes using hyperthermia have the obligation to perform more and larger high quality studies providing level I and level II evidence, and to publish these results. The quantity and quality of clinical studies of hyperthermia may be expected to improve when the treatment becomes available in more medical centres.
Lack of knowledge
Problem
Both the medical community and the general public are not well informed of hyperthermia and of what can be achieved with it. Scientific journals often reject manuscripts on the subject, stating that there is no interest for it.
Solution
Results of clinical studies should be reported more often and in a scientifically accepted format in radiation and medical oncology meetings, on both the national and international levels. Some basic knowledge of hyperthermia should be included in the teaching programme of medical students. The public should be informed more widely by the use of brochures and Internet. The web sites of national and international hyperthermia societies should be linked to each other.
Heating technique and thermometry
Problem
The application of hyperthermia is labour intensive and requires specifically trained staff. It is hard to know precise temperature distribution in tumour and normal tissues during heating. The tumour sites where hyperthermia can be adequately applied are limited. The number of institutions where hyperthermia treatment is available is limited. These last two problems are related: since the number of indications for the use of hyperthermia is limited, institutes are reluctant to invest in equipment and staff.
Solution
The efforts to develop better heating and thermometry techniques should be expanded. Further research should aim at improving existing techniques Citation[66–68] and also developing new techniques or devices to be able to adequately heat more tumour sites. Much attention should be given to the development and verification of hyperthermia treatment planning systems Citation[69–71] and to the development of non-invasive thermometry Citation[72–73]. It appears to be possible to develop non-invasive thermometry systems utilising MRI, and to use this during hyperthermia treatment of patients Citation[74]. The appropriate combination of such a thermometry system with heating equipment may vastly improve our capability to develop treatment planning programmes. With these tools the application of hyperthermia can, eventually, lead to real time treatment planning and a computer controlled feed-back system. This will make the treatment easier to apply, less labour intensive, and at the same time result in a better tolerance by patients.
Lack of quality control and staff training
Problem
The available quality assurance guidelines on how to apply hyperthermia cannot be translated directly into clinical practice. Demands concerning education of clinicians, physicists and technicians involved in the application of treatment are not formulated.
Solution
Quality assurance guidelines for each heating system and for each tumour site must be developed. Hyperthermia device companies should take an active part in this development. Educational programmes must be developed to train newcomers in the field. Users’ meetings must be organised often to exchange new experience and to maintain guidelines for quality assurance and education up-to-date.
Perception of unfavourable economic cost-benefit ratio
Problem
Hyperthermia requires investment in equipment and trained personnel. For the Dutch Deep Hyperthermia Trial, for example, the cost of a series of 5 treatments was calculated to be €6800. About half of this amount was for personnel, and one third for equipment. On the other hand, the maximum discounted cost-per-life-year-gained in cervical cancer patients, with the conservative assumption that a difference in eventual overall survival would be 11%, was only €3790, which is within the range calculated for accepted types of treatment.
Solution
Information concerning the real cost of hyperthermia should be made public. Hyperthermia investigators should provide more information on the costs of treatment. Since, in general, hospitalisation nor extensive laboratory tests nor treatment of side effects are necessary, the overall cost of hyperthermia probably is less than the cost of many other types of cancer treatment. As long as the indications for hyperthermia are limited, one centre could provide the therapy for near-by centres without the facility, which allows economically attractive investments.
Reimbursement for hyperthermia treatments is lacking, or insufficient
Problem
Reimbursement for hyperthermia treatment often is much lower than the real cost, or even completely lacking.
Solution
The insurance companies should be informed about the real costs of the treatment, and of the fact that the cost-benefit ratio is relatively favourable in comparison to other types of cancer treatment.
Lack of sufficient funding in general
Problem
Unlike the pharmaceutical companies, hyperthermia companies are small and lack financial resources for promotional activities and supporting clinical studies.
Solution
Several more recent results from hyperthermia may be of interest to pharmaceutical and/or biotech companies. Examples are the development of temperature sensitive liposomes for therapeutic or diagnostic applications Citation[75–77], the exploration of heat shock proteins for triggering tumour immunogenecity Citation[78–81], enhancement of gene expression with a heat shock promoter Citation[82–85], the use of hyperthermia as an additional bone marrow purging modality Citation[86–90], and improvement of the effect of tumour vasculature targeting agents Citation[91–94].
Another way to raise funds to promote hyperthermia is to interest charitable foundations and/or companies which have programmes to support health improvements, to form a new international non-profit foundation to promote the benefits of hyperthermia, support cooperation in clinical trials, and support in establishing guidelines for quality assurance and training.
Conclusions and recommendations
Hyperthermia is the elevation of temperatures above the physiological level with the objective of achieving therapeutic gain.
Hyperthermia is a feasible, quantifiable and reproducible treatment modality. When added to other treatment modalities, hyperthermia results in relevant benefit to the patient.
When applying local hyperthermia, the aim must be to achieve temperatures as high as possible within the tumour volume, while normal tissue temperatures remain within tolerable levels.
Level I evidence for the benefit of hyperthermia when added to radiotherapy has been established for the following tumours: head and neck, melanoma, sarcoma, breast, glioblastoma multiforme, bladder, cervix, rectum, oesophagus and various superficial tumours.
Level I evidence has been established for the addition of hyperthermia to chemotherapy in tumours of the bladder, lung, and oesophagus (with radiotherapy as well).
Level II evidence has been established for the addition of hyperthermia to radiotherapy in malignant mesothelioma, and for addition to chemotherapy in pediatric germ cell tumours.
Level III evidence has been established in recurrent cervix carcinoma after previous radiation treatment when hyperthermia is added to chemotherapy.
Problems:
Scientific problems concern patient selection, quality assurance, statistical design, assessment of the thermal dose, and the need for better understanding of thermal biology.
Socioeconomic problems concern perception of the cost-benefit, reimbursement, lack of industry support and labour intensiveness, lack of medical community awareness and acceptance, and lack of public information.
Solutions:
Achievement of more level I/II evidence, i.e. more well-designed randomised clinical trials, and high quality prospective trials with a well matched control group. Incorporation of translational research in clinical trials. Development of quality assurance guidelines per system and site. Improvement of thermal dosimetry, when feasible, use of non-invasive methods, and development of treatment techniques more friendly to users and patients. Extension of treatment techniques to other tumour sites. Expansion of relationships with pharmaceutical and biotech companies. More agressive promotion of the benefits of hyperthermia in the medical community and to the general public.
Recommendation
To initiate the establishment of an international non-profit foundation to promote a wider use of hyperthermia and support improvement in quality control.
References
- Seegenschmiedt MH, Vernon CC. A historical perspective on hyperthermia in oncology. Thermoradiotheraphy and thermochemotheraphy, Volume 1, MH Seegenschmiedt, P Fessenden, CC Vernon. Springer Verlag, Berlin 1995; 3–44
- Myerson RJ, Moros E, Roti Roti JL. Hyperthermia. Principles and practice of radiation oncology, Third, CA Perez, LW Brady. Lippincott-Raben Publishers, Philadelphia 1997; 637–683
- Coit DG. Hyperthermic isolation limb perfusion for malignant melanoma: A review. Cancer Investigation 1992; 10: 277–284
- Ceelen WP, Hesse U, De Hemptinne B, Pattyn P. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surgery 2000; 87: 1006–1015
- Robins HI. on behalf of SHOWG members. Meeting report. Systemic Hyperthermia Oncological Working Group. Oncology 1995; 52: 260–263
- Robins HI, Dennis WH, Neville AJ, Shecterle LM, Martin PA, Grossman J, Davis TE, Neville SR, Gillis WK, Rusy BF. A nontoxic system for 41.8°C whole-body hyperthermia: Results of a phase I study using a radiant heat device. Cancer Research 1985; 45: 3937–3944
- Bull JCM. Clinical practice of whole-body hyperthermia: New directions. Thermoradiotheraphy and thermochemotheraphy, MH Seegenschmiedt, P Fessenden, CC Vernon. Springer Verlag, Berlin 1995; 2: 303–322
- Hetzel FW, Mattiello J. Interactions of hyperthermia with other modalities. In: Paliwal BR, Hetzel FW, & Dewhirst MW, editors. Medical Physics Monograph. Biological, physical and clinical aspects of hyperthermia. Am Inst Phys 1987; 16: 30–56
- Manning MR, Cetas TC, Miller RC, Oleson JR, Connor WG, Gerner EW. Clinical hyperthermia: Results of a phase I trial employing hyperthermia alone or in combination with external beam or interstitial radiotherapy. Cancer 1982; 49: 05–216
- Dunlop PRC, Hand JW, Dickinson RJ, Field SB. An assessment of local hyperthermia in clinical practice. Int J Hyperthermia 1986; 2: 39–50
- Gabriele P, Orecchia R, Ragona R, Tseroni V, Sannazzari GL. Hyperthermia alone in the treatment of recurrences of malignant tumors. Cancer 1990; 66: 191–2195
- Van der Zee J, Treurniet-Donker AD, The SK, Helle PA, Wijnmaalen AJ, Van den Berg AP, Van Rhoon GC, Broekmeyer-Reurink MP, Reinhold HS. Low dose reirradiation in combination with hyperthermia: a palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int J Radiat Oncol Biol Phys 1988; 15: 1407–1413
- Valdagni R, Amichetti M, Pani G. Radical radiation alone versus radical radiation plus microwave hyperthermia for N3 (TNM-UICC) neck nodes: A prospective randomized clinical trial. Int J Radiat Oncol Biol Phys 1988; 15: 13–24
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymphnodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1993; 28: 163–169
- Overgaard J, González González D, Hulshof MCCM, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995; 345: 540–543
- International Collaborative Hyperthermia Group: Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, Van der Zee J, Van Putten WLJ, Van Rhoon GC, Van Dijk JDP, González González D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Sneed PK, Stauffer PR, McDermott W, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- Van der Zee J, González González D, Van Rhoon GC, Van Dijk JDP, Van Putten WLJ, Hart AAM,. for the Dutch Deep Hyperthermia Group. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000; 355: 119–1125
- Berdow BA. Menteshashvili., Thermoradiotherapy of patient with locally advanced carcinoma of the rectum. Int J Hyperthermia 1990; 6: 881–890
- Datta NR, Bose AK, Kapoor HK. Thermoradiotherapy in the management of carcinoma cervix (stage IIIb): A controlled clinical study. Indian Med Gazette 1987; 121: 68–71
- Egawa S, Tsukiyama I, Watanabe S, Ohno Y, Morita K, Tominaga S, Onoyama Y, Hashimoto S, Yanagawa S, Uehara S, et al. A randomized clinical trial of hyperthermia and radiation versus radiation alone for superficially located cancers. J Jpn Soc Ther Radiol Oncol 1989; 1: 135–140
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIb cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, Nakatsugawa S. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia 1990; 6: 719–740
- Strotsky AV, Fradkin SZ, Zhavrid EA, Karpovich UA. Combined therapy of bladder cancer with the use of hyperthermia. Strahlenther Onkol 1991; 167: 346
- Wang J, Li D, Chen N. Intracavitary microwave hyperthermia combined with external irradiation in the treatment of esophageal cancer. Zhonghua Zhong Liu Za Zhi 1996; 18: 51–54
- You Q-S, Wang R-Z, Suen G-Q, Yan F-C, Gao Y-J, Cui S-R, Zhao J-H, Zhao T-Z, Ding L. Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: Long-term results of 44 patients. Int J Hyperthermia 1993; 9: 19–24
- Colombo R, Da Pozzo LF, Gev A, Freschi M, Gallus G, Rigatii P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urology 1996; 155: 1227–1232
- Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, Caldarera E, Pavone-Macalusa M. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003; 21: 4270–4276
- Engelhardt R, Neumann H, Müller U, Löhr GW. Clinical studies in whole body hyperthermia. Hyperthermic oncology, T Sugahara, M Saito. Taylor and Francis, London 1989; 2: 509–510
- Issels RD, Lindner LH, Wust P, Hohenberger P, Jauch K, Daugaard S, Mansmann U, Hiddemann W, Blay J, Verweij J. Regional hyperthermia (RHT)improves response and survival when combined with systemic chemotherapy in the management of locally advanced, high grade soft tissue sarcomas (STS) of the extremities, the body wall and the abdomen: A phase III randomized pros. ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007; 25(18S)10009
- Kitamura K, Kuwano H, Watanabe M, Nozoe T, Yasuda M, Dumiyoshi K, Saku M, Sugimacahi K. Prospective randomized study of hyperthermia combined with chemoradiotherapy for esophageal carcinoma. J Surg Oncol 1995; 60: 55–58
- Sugimachi K, Kitamura K, Baba K, Ikebe M, Morita M, Matsuda H, Kuwano H. Hyperthermia combined with chemotherapy and irradiation for patients with carcinoma of the oesophagus: A prospective randomized trial. Int J Hyperthermia 1992; 8: 289–295
- Van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002; 13: 1173–1184
- Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y, Hiraoka M. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2005; 61: 145–153
- Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: Results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia 1990; 6: 479–486
- Marmor JB, Hahn GM. Combined radiation and hyperthermia in superficial human tumors. Cancer 1980; 46: 1986–1991
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 4: 485–493
- Sharma S, Patel FD, Sandhu APS, Gupta BD, Yadav NS. A prospective randomized trial of local hyperthermia as a supplement and radiosensitizer in the treatment of carcinoma of the cervix with radiotherapy. Endocuriether Hyperthermia Oncol 1989; 5: 151–159
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys 1989; 16: 535–549
- Shchepotin IB, Evans SRT, Chorny V, Osinsky S, Buras RR, Maligonov P, Shabahang M, Nauta RJ. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol 1994; 3: 37–44
- Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1989; 16: 551–558
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol (CCT) 1991; 14(2)133–141
- Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, Montesano A, Rubin P, Curran W, Delrowe J, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors: a prospectively controlled randomized study by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1996; 34: 1097–1104
- De Graaf-Strukowska L, van der Zee J, van Putten WLJ, Senan S. Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura–A single institution experience with 189 patients. Int J Radiat Oncol Biol Phys 1999; 43: 511–516
- Wessalowski R, Kruck H, Pape H, Kahn T, Willers R, Göbel U. Hyperthermia for the treatment of patients with malignant germ cell tumors. A phase I/II study in ten children and adolescents with recurrent or refractory tumors. Cancer 1998; 82: 793–800
- Wessalowski R, Schneider D, Mils O, Calaminus G, Engelbrecht V, Pape H, Engert J, Harms D, Göbel U. An approach for cure: Regional deep hyperthermia and PEI-chemotherapy in children and adolescents with unresectable malignant tumors. Presented at the 21th Annual Meeting of the European Society for Hyperthermic Oncology 2003
- Rietbroek RC, Schilthuis MS, Bakker PJM, Van Dijk JDP, Postma AJ, González González D, Bakker AJ, Van der Velden J, Helmerhorst TJM, Veenhof CHN. Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with a previously irradiated recurrent carcinoma of the uterine cervix. Cancer 1997; 79: 935–943
- De Wit R, Van der Zee J, Van der Burg MEL, Kruit WH, Logmans A, Van Rhoon GC, Verweij J. A phase I/II study of combined weekly systemic cisplatin and locoregional hyperthermia in patients with previously irradiated recurrent carcinoma of the uterine cervix. Br J Cancer 1999; 80: 1387–1391
- Jo S, Sugahara T, Yamamoto T. Clinical response of hyperthermia using heating equipment Thermotron-RF8 in Japan. Biomed Eng Appl Basis Comm 1994; 6: 340–362
- Kouloulias V, Plataniotis G, Kouvaris J, Dardoufas C, Gennatas C, Uzunoglu N, Papavasiliou C, Vlahos L. Chemoradiotherapy combined with intracavitary hyperthermia for anal cancer. Feasibility and long-term results from a phase II randomized trial. Am J Clin Oncol 2005; 28: 91–99
- Van der Zee J, Van Rhoon GC, Wust P. In regard to Dr Vasanthan et al. (Int J Radiat Oncol Biol Phys 2005;61:145-153). Int J Radiat Oncol Biol Phys 2005; 62: 940–941
- Jones EL, Prosnitz LR, Dewhirst MW, Vujaskovic Z, Samulski TV, Oleson JR, Yu D, Myerson RJ, Moros EG, Hurwitz MD, et al. In regard to Vasanthan et al. (Int J Radiat Oncol Biol Phys 2005;61:145-153). Int J Radiat Oncol Biol Phys 2005; 63: 644
- Van der Zee J, Van der Holt B, Rietveld PJM, Helle PA, Wijnmaalen AJ, Van Putten WLJ, Van Rhoon GC. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer 1999; 79: 483–490
- Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, Emami B, Molmenti EP, Buckner A, Lockett MA. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int J Radiat Oncol Biol Phys 1998; 40: 365–375
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Fajardo LF. Pathological effects of hyperthermia in normal tissues. Cancer Research 1984; 44: 4826s–4835s
- Haveman J, Sminia P, Wondergem J, Van der Zee J, Hulshof MCCM. Effects of hyperthermia on the central nervous system: What was learnt from animal studies? Int J Hyperthermia 2005; 21: 185–192
- Haveman J, Van der Zee J, Wondergem J, Hoogeveen JF, Hulshof MCCM. Effects of hyperthermia on the peripheral nervous system: A review. Int J Hyperthermia 2004; 20: 371–391
- Hume SP, Marigold JC, Michalowski A. The effect of local hyperthermia on non-proliferative, compared with proliferative, epithelial cells of the mouse intestinal mucosa. Radiation Research 1983; 94: 252–262
- Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors II. Effect of thermoradiotherapy. Cancer 1987; 60: 128–135
- Lee CK, Song CW, Rhee JG, Foy JA, Levitt SH. Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: Results of a phase I/II study. Int J Radiat Oncol Biol Phys 1995; 32: 733–745
- Petrovich Z, Langholz B, Gibbs FA, Sapozink MD, Kapp DS, Stewart RJ, Emami B, Oleson J, Senzer N, Slater J, et al. Regional hyperthermia for advanced tumors: A clinical study of 353 patients. Int J Radiat Oncol Biol Phys 1989; 16: 601–607
- Ohguri T, Imada H, Kato F, Yahara K, Morioka T, Nakano K, Korogi Y. Radiotherapy with 8 MHz radiofrequency-capacitive regional hyperthermia for pain relief of unresectable and recurrent colorectal cancer. Int J Hyperthermia 2006; 22: 1–14
- Cavaliere R, Di Filippo F, Cavaliere F, Carlini S, Schiratti M, Anzà M, Garinei R, Callopoli A, Capua A, Impiombato FA, . Clinical practice of hyperthermic extremity perfusion in combination with radiotherapy and chemotherapy. Thermoradiotherapy and thermochemotherapy, MH Seegenschmiedt, P Fessenden, CC Vernon, et al. Springer Verlag, Berlin 1996; 2: 323–345
- Van der Zee J, González González D. The Dutch Deep Hyperthermia Trial: Results in cervical cancer. Int J Hyperthermia 2002; 18: 1–12
- Wust P, Fahling H, Helzel T, Kniephoff M, Wlodarczyk W, Monich G, Felix R. Design and test of a new multi-amplifier system with phase and amplitude control. Int J Hyperthermia 1998; 14: 459–477
- Van Rhoon GC, Rietveld PCM, Van der Zee J. A 433 MHz Lucite Cone waveguide applicator for superficial hyperthermia. Int J Hyperthermia 1998; 14: 13–27
- Rietveld PJM, Van Putten WLJ, Van der Zee J, Van Rhoon GC. Comparison of the clinical effectiveness of the 433 MHz Lucite Cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys 1999; 43: 681–687
- Wust P, Seebass M, Nadobny J, Deuflhard P, Monich G, Felix R. Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int J Hyperthermia 1996; 12: 477–494
- Lagendijk JJW. Hyperthermia treatment planning. Phys Med Biol 2000; 45: R61–R76
- Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, Hege H-C, Deuflhard P, Felix R. Clinical evaluation and verification of the hyperthermia treatment planning system Hyperplan. Int J Radiat Oncol Biol Phys 2000; 47: 1145–1156
- Carter DL, MacFall JR, Clegg ST, Wan X, Prescott DM, Charles HC, Samulski TV. Magnetic resonance thermometry during hyperthermia for human high-grade sarcoma. Int J Radiat Oncol Biol Phys 1998; 40: 815–822
- Hentschel M, Dreher W, Wust P, Roll S, Leibfritz D, Felix R. Fast spectroscopic imaging for non-invasive thermometry using the Pr[MOE-DO3A] complex. Phys Med Biol 1999; 44: 2397–2408
- Gellermann J, Wlodarczyk W, Hildebrandt B, Ganter H, Nicolau A, Rau B, Tilly W, Fähling H, Nadobny J, Felix R, et al. Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res 2005; 65: 5872–5880
- Yatvin MB, Mühlensiepen H, Porschen W, Weinstein JN, Feinendegen LE. Selective delivery of liposome-associated cis-Dichlorodiammineplatinum(II) by heat and its influence on tumor drug uptake and growth. Cancer Res 1981; 41: 1602–1607
- Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PA, Dewhirst MW, Needham D, Thrall DE. Hyperthermia increases accumulation of Technetium-99M-labeled liposomes in feline sarcomas. Clin Cancer Res 2000; 6: 3748–3755
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 1995; 61: 272–279
- Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD-3 large granular lymhocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood 1995; 86: 1374–1384
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 2000; 12: 1539–1546
- Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother 2001; 50: 515–522
- Gerner EW, Hersh EM, Pennington M, Tsang TC, Harris D, Vasanwala F, Brailey J. Heat-inducible vectors for use in gene therapy. Int J Hyperthermia 2000; 16: 171–181
- Huang Q, Hu JK, Lohr F, Zhang L, Braun R, Lanzen J, Little JB, Dewhirst MW, Li C-Y. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res 2000; 60: 3435–3439
- Okamota K, Shinoura N, Egawa N, Asai A, Kirino T, Shibasaki F, Shitara N. Adnovirus-mediated transfer of p53 augments hyperthermia-induced apoptosis in U251 glioma cells. Int J Radiat Oncol Biol Phys 2001; 50: 525–531
- Lohr F, Hu K, Huang Q, Zhang L, Samulski TV, Dewhirst MW, Li C-Y. Enhancement of radiotherapy by hyperthermia-regulated gene therapy. Int J Radiat Oncol Biol Phys 2000; 48: 1513–1518
- Benowitz S. Bone marrow experts are still debating the value of purging. J Nat Canc Inst 2000; 92: 190–192
- Moriyama Y, Narita M, Sato K, Urushiyama M, Koyama S, Hirosawa H, Kishi K, Takahashi M, Takai K, Shibata A. Application of hyperthermia to the treatment of human acute leukemia: Purging human leukemic progenitor cells by heat. Blood 1986; 67: 802–804
- Iwasawa T, Hirabayashi Y, Kubota N, Inoue T, Kakehi M, Matsui K. Hyperthermic purging in vitro of murine leukemia cells (MK-8057): Surviving fractions of normal and leukemic stem cells and the long-term survival of mice injected with the post-hyperthermic leukemia cells. Exp Hematol 1991; 19: 332–337
- Osman Y, Moriyama Y, Shibata A. Enhanced elimination of Ph+ chromosome cells in vitro by combined hyperthermia and other drugs (AZT, IFN-alpha, TNF, and quercetin): Its application to autologous bone marrow transplantation for CML. Exp Hematol 1995; 23: 444–452
- Wierenga PK, Setroikromo R, Vellenga E, Kampinga HH. Purging of acute myeloid leukaemia cells from stem cell grafts by hyperthermia: Enhancement of the therapeutic index by the tetrapeptide AcSDKP and the alkyl-lysophospholipid ET-18-OCH(3). Br J Haematol 2000; 111: 1145–1152
- Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, Park H, Song CW. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia 2000; 2: 555–560
- Horsman MR, Murata R, Overgaard J. Improving local tumor control by combining vascular targeting drugs, mild hyperthermia and radiation. Acta Oncol 2001; 40: 497–503
- Eikesdal HP, Bjerkvig R, Dahl O. Vinblastine and hyperthermia target the neovasculature in BT4AN rat gliomas: Therapeutic implications of the vascular phenotype. Int J Radiat Oncol Biol Phys 2001; 51: 535–544
- Eikesdal HP, Bjerkvig R, Mella O, Dahl O. Combrestatin A-4 and hyperthermia: A potent combination for the treatment of solid tumors. Radiother Oncol 2001; 60: 147–154