Abstract
Purpose: The purpose of the Phase I component of this study was to find the maximally tolerated dose (MTD) of cisplatin administered within a regimen of fever-range whole body thermal therapy (FR-WB-TT), cisplatin, gemcitabine, and low-dose interferon-alpha (IFN-α). The Phase II component aimed to assess which cancer diagnoses responded to the regimen, the response rate, and response duration.
Materials and methods: The protocol design derived from a schedule-optimized preclinical regimen. Drugs were administered together, and also with thermal therapy in a schedule that optimized the therapeutic index. Eligible patients were those with therapy-resistant, metastatic or advanced solid malignancies. Beginning at 40 mg/m2, the cisplatin dose was escalated by 10 mg/m2 to the maximally tolerated dose (MTD) in successive cohorts of 3 patients. A treatment cycle consisted of cisplatin on day one, followed by thermal therapy and simultaneous gemcitabine 36 hours later; then a second dose of gemcitabine one week later; and daily IFN- α.
Results: Thirty-seven patients were treated on protocol. The MTD of cisplatin in the thermochemotherapy regimen was established to be 60 mg/m2. The dose limiting toxicities (DLT) were peripheral neuropathy and ototoxicity. Complete and partial responses combined were 43%. The therapy improved the quality of life of responding patients.
Conclusion: The protocol was well tolerated and was associated with antitumor activity in patients with a variety of advanced metastatic solid tumors. Tumor response occurred with the thermochemotherapy treatment despite treating malignancies that had progressed on the same chemotherapy drugs administered as standard treatment. Notably, good responses were observed in patients with high-grade neuroendocrine and pancreas cancers. This regimen will be tested in a phase II study.
Introduction
While systemic thermal therapy enhances chemotherapy-induced cancer response, it can also cause unacceptable toxicity Citation[1],Citation[2]. We hypothesized that the timing and scheduling of agents in a multimodality heat regimen could be optimized to enhance the therapeutic index (TI).
Based on a regimen developed in a pre-clinical tumor model Citation[3–6], a phase I-II clinical protocol was designed combining fever-range whole-body thermal therapy (FR-WB-TT; 6 hours at 40°C), cisplatin, gemcitabine, and interferon-alpha (IFN-α). The timing of administration of the chemotherapy drugs relative to thermal therapy was optimized to give a greater therapeutic index (i.e. the best anti-tumor response with the lowest toxicity) Citation[6]. Daily low-dose interferon-alpha was included in the regimen for its anti-angiogenic activity Citation[7–9] and to stimulate anti-tumor immunity Citation[10],Citation[11]. After enrollment of the number of patients needed to establish the maximally tolerated dose (MTD), inclusion of an additional 24–30 patients was planned for the phase II portion of the protocol to assess which cancer types respond to the therapy, the response rate and the duration of response. A maximum of eight treatment cycles was planned.
Cisplatin was chosen as the agent to be escalated because of the following considerations: In addition to nausea, cisplatin can cause both acute and chronic renal toxicity as well as a long-lasting neuropathy. Once manifest, the renal and neurological toxicities are not easily treated, and recovery takes months. In contrast, gemcitabine induces bone marrow suppression, mucositis, modest nausea, and less commonly, diarrhea. These toxicities are acute in nature and treatable. Low-dose, metronomic IFN-α usually causes minor toxicity Citation[7],Citation[12]. The therapeutic efficacy and, importantly, the minimal toxicity of FR-WB-TT in combination with chemotherapy, had already been established both in an animal model in the laboratory Citation[13] and in a previous phase I clinical protocol Citation[14],Citation[15].
Patients and methods
Approval and advisory bodies
The Committee for the Protection of Human Subjects, the Institutional Review Board (IRB) of the University of Texas Health Science Center at Houston, and the University Clinical Research Center Scientific Advisory Committee approved the protocol describing all treatments and procedures included in this study. All patients provided informed, written consent prior to study admission. A protocol Data Safety and Monitoring Board (DSMB) oversaw conduct of the protocol. Patients were enrolled in the phase I–II protocol between January 2000 and June 2004.
Patient inclusion criteria
General
Patients with chemotherapy-resistant metastatic cancers; i.e. tumors that progressed on chemotherapy were eligible for the protocol. Subjects had to have a Karnofsky performance status Citation[16] of 50% or greater, and cardiac, pulmonary, renal, hematological and hepatic functional requirements as described below. Other eligibility criteria included age greater than 18 years and a life expectancy greater than 2 months. There was no upper age limit. As many as 5 prior chemotherapy regimens were allowed, as well as prior therapy with cisplatin and/or gemcitabine. However, chemotherapy could not be given within four weeks of protocol treatment. Radiation therapy could not be given within six weeks of protocol treatment.
A protocol candidate was required to demonstrate comprehension of the informed consent and also to understand other available therapeutic options.
The original histopathological slides were reviewed by a single pathologist in the Department of Pathology.
Cardiac function
During the week before the first treatment, cardiac function was assessed by an exercise MUGA or an ECHO assessment of ventricular ejection fraction. Protocol eligibility required a resting left ventricular ejection fraction of ≥40%. Subjects with a history of myocardial infarction were excluded.
Pulmonary function
Complete pulmonary function studies, including diffusion capacity were evaluated. The FEV-1 needed to be ≥70% of the predicted value, and the diffusion capacity ≥80%. The protocol required an arterial PO2 of 85 mm Hg or greater on room air with appropriate PCO2 and pH. Pleural effusion that significantly reduced pulmonary function excluded subjects from the protocol treatment.
Laboratory requirements
Eligibility included a WBC ≥1, 800 μL, a platelet count of ≥90, 000 μL, and hemoglobin of ≥9.5 g/dL. Patients were required to have a bone marrow cellularity of 50% or greater on biopsy. The serum creatinine had to be ≤1.5 mg/dL, BUN of ≤35 mg/dL, and a creatinine clearance ≥55 cc/min. Normal coagulation parameters with a prothrombin time (PT) less than 14 sec and a partial thromboplastin time (PTT) less than 35 sec were required. The bilirubin had to be ≤2.0 mg/dL and the AST/ALT <2X the upper limit of normal. A bone marrow aspiration and biopsy was performed within 30 days of the first treatment. To evaluate possible myelotoxicity and because the protocol subjects were heavily pretreated with chemotherapy, it was felt to necessary to evaluate bone marrow cellularity and/or tumor involvement prior to protocol therapy.
Radiological requirements
Baseline CT scans of the chest, abdomen, and pelvis, as well as a bone scan were obtained within 2 weeks of beginning the protocol. Additionally, potential subjects were evaluated by a contrast enhanced MRI of the brain within the week before the first treatment. Patients with untreated or uncontrolled brain metastases were ineligible for the study.
Tumor response
Tumor response was assessed according to RECIST criteria Citation[17], Citation[18] every two cycles. The duration of response was calculated from the first cisplatin infusion to the first date of documented disease progression.
Treatment plan
Timing and scheduling of the combination therapy
Chemotherapy drugs were administered with FR-WB-TT (40°C for 6 hours) using a defined schedule translated from preclinical laboratory studies Citation[5], Citation[6] (see Discussion). Based on preclinical data, cisplatin was administered on day 1, approximately 36 hours prior to heat induction Citation[3]. On day 3, as soon as the target core temperature of 40°C was reached, gemcitabine was infused Citation[4], Citation[5]. Low-dose IFN-α was begun on day one and given daily for one month Citation[19]. The month-long cycle was repeated up to seven times in the absence of unacceptable toxities or disease progression (see the treatment schema below).
The schema of the treatment regimen as shown above
Cycle 1, week 1, day 1: Patient evaluation, hydration, antiemetics, cisplatin (CIS) 4-hour intravenous (IV) infusion.
Cycle 1, week 1, day 1: Begin daily low-dose IFN-α 1 × 106 IU sc (continued daily for duration of protocol).
Cycle 1, week 1, day 3: FR-WB-TT + gemcitabine (GEM) 600 mg/m2 IV over 60 minutes (36 hours after cisplatin). Continue daily IFN-α.
Cycle 1, week 2, day 10: Gemcitabine (GEM) 600 mg/m2 IV over 60 minutes.
Cycle 1, week 3: No chemotherapy, continue daily IFN-α.
Cycle 2, week 5, day 29: Begin cycle 2, patient evaluation, hydration, antiemetics, cisplatin (CIS) 4 hours IV, continue daily IFN-α.
Cycle 2, week 5, day 31: FR-WB-TT + gemcitabine (GEM), continue daily IFN-α, etc.
Cycle 2, week 6, day 38: Gemcitabine (GEM) 600 mg/m2 IV over 60 minutes.
Chemotherapy and other pharmaceuticals
Drug dose calculations
Cisplatin, gemcitabine, and conscious-sedation drug doses were calculated based on body surface area (m2) using measured height and weight obtained immediately prior to each treatment cycle. The cisplatin dose was given according to the dose escalation schema (see below).
Cisplatin
After appropriate hydration and administration of granisetron, a serotonin 3-HT3 receptor antagonist anti-emetic, cisplatin (cisplatin, Bristol Myers-Squibb Company, Princeton, NJ) was administered intravenously on day 1 of each therapy cycle.
Cisplatin dose escalation schema
The cisplatin dose escalation was based on a standard phase I design, with 3 patients per dose level cohort Citation[20–22]. The cisplatin dose was escalated by 10 mg/m2 for each patient cohort, starting with an initial dose of 40 mg/m2. There was no dose escalation within a cohort. Grade III renal, neurotoxicity, or ototoxicity was considered a dose limiting toxicity (DLT). If no patient within a dose cohort experienced a DLT, the cisplatin dose was escalated by 10 mg/m2 for the next cohort. If one patient in a cohort experienced a DLT, 3 more patients were enrolled at the same dose level. If no additional patients experienced a DLT, the dose was escalated one level for the next cohort of 3 patients. However, if a second patient developed a DLT, the dose was considered non-tolerable and the next lower cisplatin dose was considered the MTD. Only toxicities that occurred during the first 2 courses of therapy were considered in the dose escalation scheme.
Patients were taken off protocol for grade III-IV renal or neurotoxicity.
Gemcitabine
Gemcitabine hydrochloride (Gemzar, Eli Lilly and Co., Indianapolis, IA) was administered approximately 36 hours after cisplatin, as soon as core temperature first reached target 40°C. Gemcitabine was administered at a fixed dose of 600 mg/m2 IV over 60 min. The dose and schedule were chosen because 600 mg/m2 of gemcitabine by 60 minute infusion yields a larger dose area under the curve (AUC) compared to the more commonly used 1000 mg/m2 over 30 min. A greater AUC has been correlated with a better survival Citation[23–25].
Gemcitabine dose reductions
The second dose of gemcitabine was omitted for a week-two grade I-II neutropenia or thrombocytopenia. Subsequent cycles could be delayed for two weeks for a week-four grade I-II neutropenia, or thrombocytopenia. Gemcitabine was decreased by 50% during subsequent cycles for grade III neutropenia, thrombocytopenia, mucositis or diarrhea. Patients were taken off protocol for grade IV hematological or gastrointestinal toxicities.
Metronomic low-dose interferon-α
IFN-α (Roferon, Schering-Plough, Kenilworth, NJ) was administered as a daily subcutaneous injection (SC) at a fixed dose of 1 × 106 international units (IU) beginning day one, and continued for the duration of the protocol. Celecoxib (Celebrex) or acetaminophen (Tylenol) was taken with IFN-α to reduce side effects.
Sargramostim
Sargramostim (Leukine, GM-CSF, Berlex; Wayne, NJ) was administered for therapy-induced leucopenia grade ≥I (a leucocyte count ≤1500 cells/dl).
Thermal therapy
Fever-range whole-body thermal therapy
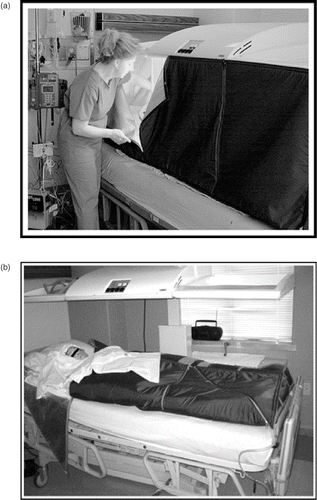
Thermal therapy was induced and maintained using the Heckel-HT2000 infrared radiant heat device (Heckel Medizintechnik Gmbh; Esslingen, Germany)Citation[26]. Systemic temperature was elevated to target 40°C using four 300 W infrared halogen radiant heat lamps located in the overhead pane of the device. During heat induction the soft sides of the infrared radiant heat bed were up, and the unit resembled a soft-sided box (as shown in ). During the induction phase, clear plastic windows in the unit are positioned above, behind, and on each side of the patient's head, preventing claustrophobia. When target temperature was achieved, the flexible soft sides of the box were folded over the patient to insulate and retain heat, as shown below in . Additional use of the radiant heat lamps was typically not necessary. After 6 hours at 40°C, patients were cooled by air convection to a rectal temperature below 38°C. The thermal dose (6 hours at 40°C) was the same for each patient.
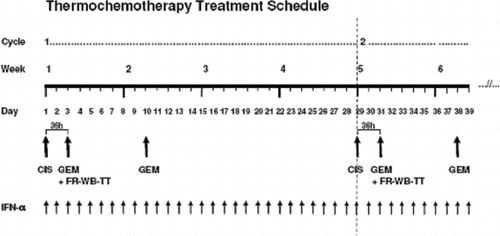
Figure 1. (a) The HT2000 infrared radiant heat device being closed. (b) HT2000 infrared radiant heat device with heating lights off, and soft tent sides folded over patient.
After the first treatment, the patient was observed for 12 hours after cooling, and then discharged. For subsequent treatments, patients who were awake and stable were discharged 1.5 to 2.5 hours after cooling.
Fluid maintenance
Lactated Ringers solution was used to maintain stable urinary function. Urine output and urine osmolarity were evaluated every 60 minutes (as described below).
Physiological monitoring
Standard critical care cardiovascular monitoring instruments were used to monitor and record vital signs. The cardiac waveform, cardiac rate, CVP, systolic/diastolic blood pressure, respiratory rate and depth, and O2 saturation were continuously monitored and recorded. Cardiac rate as well as systolic and diastolic blood pressures were maintained within 50% of baseline. An oxygen (O2) saturation monitor was placed on the ear lobe or a finger for continuous monitoring of O2 saturation. Oxygen saturation was maintained at ≥90%. Urine output and urine osmolarity were measured every hour. Urine osmolarity was assessed using a hand-held refractometer. The rapidly available urine osmolarity values allowed for accurate replacement of insensible and urinary fluid losses. The volume of intravenous fluid was adjusted to maintain urine osmolarity at 1.10.
Temperature monitors
Thermistor probes were used to continuously monitor bladder, rectal, axillary, and 3 skin temperatures. The probes were recorded by a computer and also the probe temperatures were manually recorded on paper every 15 min. At plateau, the core temperature was maintained at 40°C ± 0.2°C.
Conscious sedation
The induction heating phase from beginning core temperature (range 35.6°–37.4°C) to 40°C (104°F) took a median of 120 minutes (range: 50–180 minutes). The patient remained supine for the 6 hours of treatment at target temperature. The entire temperature elevation lasted 7 to 9 hours, including both the induction time to reach the target temperature of 40°C and the 6 hours at 40°C. The induction heat phase is associated with restlessness and irritability, as also experienced with infectious fevers caused by viral or bacterial organisms. Also, lying supine in one position for the entire treatment duration is generally found stressful. Light conscious sedation was therefore used to provide a comfortable, safe therapy. A nurse trained and experienced in conscious sedation techniques administered the sedation, supervised by the thermal therapy critical care physician. The conscious sedation drugs used were midazolam (Versed) 2 mg IV/hour or lorazepam (Ativan) 1 mg IV/hour, promethazine (Phenergan) 12.5 to 25 mg IV/hour, and fentanyl (Sublimaze) 25 to 50 µg IV/15 min (limit: 300 µg/hour).
Interim physical, and radiological evaluation
Weekly laboratory analysis
A complete blood count (CBC) was performed weekly. Sargramostim was administered for a leucocyte count below 1500 cells/dl.
Medical history, physical examination, laboratory and radiology
Immediately before each treatment cycle the principal investigator (PI) obtained an interim medical history, including assessment of pain, appetite, and daily activity and a physical examination. Additionally, the thermal nurses assessed pain, appetite, and daily activity. A chest radiograph, an EKG, a CBC with differential and platelet counts, a PT/PTT, serum creatinine, BUN, electrolytes, liver function studies and appropriate tumor marker levels were obtained prior to each treatment. Also, a chest radiograph, CBC, and an electrolyte panel were repeated after treatment. A CBC and an electrolyte panel were performed weekly after each treatment.
Toxicity exclusion criteria
Toxicities were assessed for the first two treatment cycles using the NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 Citation[27].
Treatment delay
Treatment could be delayed for up to two weeks for a grade I-III hematological toxicity, or for a grade II non-hematological toxicity. Sargramostim was administered for leucopenia.
Treatment termination
Treatment was discontinued for disease progression, withdrawal of consent, or a cycle delay beyond two weeks. Treatment was also terminated for grade IV hematological toxicity, or for ≥grade III non-hematological toxicity.
Response assessment
Tumor response was assessed every two cycles using contrast CT scans of the chest, abdomen, and pelvis, MRI scans of the brain, and bone scans. Tumor response was defined by RECIST criteria Citation[17], Citation[18].
Study end points
The study end points were response as defined by RECIST criteria, and duration of response. Time to disease progression was measured in months from treatment day 1 until documentation of progression. Complete response (CR) was defined as disappearance of all target lesions; partial response (PR) was a 30% decrease in the sum of the longest diameter of target lesions. Stable disease (SD) was defined as changes that did not meet the above criteria for PR; and progressive disease (PD) was defined as a 20% increase in the sum of the longest diameter of target lesions.
Quality of life
Although quality of life was not formally assessed, questions about well-being, including queries about pain, weight gain, and daily activity, were asked during each pre-therapy clinic visit by the principal investigator and thermal therapy nurses.
Statistical analysis
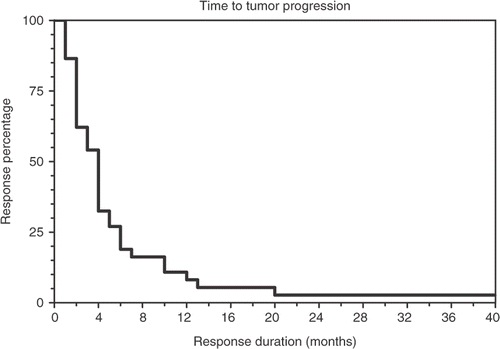
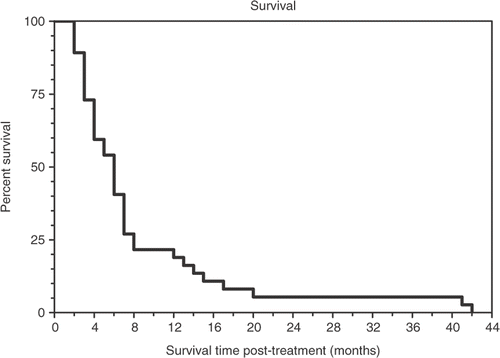
Descriptive statistics were calculated for patient characteristics, toxicities, and responses. Curves showing time to tumor progression and survival were calculated using the method of Kaplan-Meier Citation[28].
Results
Patient Population
As shown in , 37 patients with advanced, metastatic solid malignancies were treated on the protocol. The median age of the subjects was 60 years (range: 25–78 years). Fourteen patients were female (38%), and 23 patients were male (62%). Twenty-eight patients were Caucasian (76%), seven were African-American (19%), and two were Hispanic (5%). The subjects had undergone a median of three prior chemotherapy regimens (range: 0–5).
Table I. Tumor diagnosis of individual patients, age, sex, race, stage, number of treatment cycles, response, time to tumor progression (duration in months), and prior therapy.
The tumors included a variety of malignancies. Thirty-six tumors were of epithelial origin; one was a sarcoma. The tumors of 12 patients had progressed with cisplatin chemotherapy. Eighteen malignancies had progressed on gemcitabine, and six had progressed on a prior regimen of cisplatin and gemcitabine. An additional eight malignancies had progressed with carboplatin chemotherapy. Five patients had been previously treated with radiation therapy.
Number of treatment cycles
The 37 protocol patients completed at least one and as many as eight thermochemotherapy cycles. As detailed below, protocol treatment was discontinued for 29 patients because of progressive disease (PD). The protocol therapy was stopped for two patients because of grade III non-hematological toxicity. Therapy was discontinued for six patients because of temporary closure of the University Clinical Research Center (UCRC)Footnote1. The number of doses of daily IFN-α was dependent on the number of cycles the patient received. Those patients receiving only one therapy cycle had 28–30 daily, low-dose IFN-α injections. Those patients treated with 8 cycles received 224 to 240 daily IFN-α injections.
Eleven patients received only one treatment cycle. Treatment was discontinued after one cycle for ten patients because of disease progression documented by history, physical examination, laboratory studies or chest radiograph. The therapy was discontinued for one of the 11 patients because of temporary facility closureFootnote1.
Eleven patients completed two therapy cycles. Treatment was discontinued for eight patients because of disease progression. One therapy was discontinued because of toxicity. Two patients’ treatments ended because of temporary facility closureFootnote1.
One patient completed three cycles, and was taken off protocol because of prolonged hematological toxicity.
Ten patients completed four therapy cycles. Nine patients were taken off protocol for progressive disease and one patient's treatment was stopped for facility closureFootnote1.
Two patients completed five treatment cycles. For one patient therapy was stopped because of disease progression, documented by physical examination. The second patient's therapy was discontinued because of temporary facility closureFootnote1.
Two patients completed eight therapy cycles.
To summarize, of the 37 patients treated on protocol, two patients completed eight therapy cycles; 28 patients (76%) were taken off protocol because of disease progression, either early, or after tumor response, the disease later progressed; two patients (5%) were taken off protocol because of toxicity, and five patients (14%) because of temporary facility closure.
Time to temperature
The median time to target 40°C temperature was 120 min (range 55 to 210 min).
Toxicity
Hematological toxicities
As described in , in the cohort of three patients receiving 40 mg/m2 cisplatin, one grade I leucopenia (33%) was observed. One patient developed a grade I leucopenia (33%) at cisplatin 50 mg/m2.
Table II. Toxicities per cisplatin dose: Hematological and non-hematological.
Twenty-nine patients were treated with the MTD dose of 60 mg/m2 of cisplatin. Nine (31%) of the 29 patients experienced grade I leucopenia, and 16 patients (55%) had a grade II leucopenia at cisplatin 60 mg/m2.
Two patients were treated at cisplatin 70 mg/m2. Two episodes of grade II leucopenia (100%) occurred at cisplatin 70 mg/m2. The leucopenia occurred on days 8–10, and recovered by day 28. Because of leucopenia during week 2, the second dose of gemcitabine planned for week 2 was administered to only eight of the 37.
protocol patients (22%). Neutropenia was not associated with fever and resolved within 3 to 7 days after initiation of sargramostim.
No thrombocytopenia was observed at cisplatin 40 or 50 mg/m2. At cisplatin 60 mg/m2 18 of the 29 patients treated at that dose, (62%), experienced a grade I thrombocytopenia. There were six patients (21%) with grade II thrombocytopenia at day 28 of the cycle, which delayed the next treatment cycle for 1–2 weeks. One patient was taken off protocol because of persistence of thrombocytopenia beyond two weeks. At 70 mg/m2 grade II thrombocytopenia occurred in the two patients (100%).
One (33%) grade I anemia was seen with 40 mg/m2 cisplatin and one grade I anemia (33%) was seen at 50 mg/m2 cisplatin. At 60 mg/m2 cisplatin (MTD) there were 15 patients (52%) with grade I anemia and six patients (21%) with grade II anemia. Two patients treated with cisplatin 70 mg/m2 experienced grade II anemia (100%).
In summary, at 40–50 mg/m2 cisplatin there was essentially no hematological toxicity. At 60 mg/m2, as currently used for this protocol, hematological toxicity was tolerable. Cisplatin at 70 mg/m2 induced a grade II thrombocytopenia in both patients, and a prolonged thrombocytopenia in one of the two. No grade III-IV hematological toxicity was observed at 40–70 mg/m2 cisplatin.
Non-hematological toxicities
As shown in , no renal toxicity was seen at cisplatin dose levels of 40–70 mg/m2.
No peripheral sensory or motor neuropathy or ototoxicity (cranial nerve VIII) was observed for cisplatin dose levels at 40–50 mg/m2. At 60 mg/m2 cisplatin, two patients (7%) experienced grade I peripheral sensory neuropathy, and one patient (3%) experienced grade II peripheral sensory neuropathy. Both patients (100%) treated with 70 mg/m2 cisplatin experienced grade III peripheral sensory neuropathy.
No ototoxicity was observed for cisplatin doses 40–60 mg/m2. However, one of the two patients (50%) treated with 70 mg/m2 cisplatin experienced grade III ototoxicity.
Granisetron, a serotonin 5-HT3 receptor antagonist, was given prior to cisplatin infusion to prevent nausea. No nausea occurred at 40–50 mg/m2 cisplatin. Five patients (17%) experienced grade I nausea and one patient (3%) had grade II nausea with 60 mg/m2 cisplatin. One of two patients (50%) had grade II nausea at 70 mg/m2 cisplatin. The nausea responded to promethazine and granisetron every 12 hours. There was no diarrhea at cisplatin doses of 40–70 mg/m2.
During the first 6-month time period when the protocol was first initiated, two patients experienced pressure injuries to a heel, one at 40 mg/m2 cisplatin, and one at 50 mg/m2. The lesions appeared as red tender areas where the heel rested on the bed mattress. The pressure injury was caused by the heel resting on the bed in one position for the entire ≥8-hour duration of the heat procedure. Subsequently, the leg position was modified by placing rolled towels under ankles during the treatment. Thereafter, pressure injuries were not observed.
One heavy-set patient treated with 60 mg/m2 cisplatin experienced a grade II thermal burn to the chest wall during the first thermal treatment. She recovered uneventfully with local treatment of the burn. Padding was subsequently added over her chest and she was treated on three further occasions without injury.
MTD of cisplatin
As discussed above, at 70 mg/m2 cisplatin a grade III peripheral sensory neuropathy occurred in two patients (100%) and grade III ototoxicity occurred in one patient (50%). On the basis of neurological toxicity, 60 mg/m2 of cisplatin was established as the MTD in the regimen using one dose of gemcitabine per treatment cycle.
Metronomic interferon-α toxicity
Approximately 60% of patients developed a low-grade fever (<39°C) and mild malaise during the first 1 to 5 daily doses of low-dose interferon-α; however, the symptoms disappeared after 2 to 5 daily doses. No neurological or psychological dysfunction or other interferon-α associated toxicity was observed.
Conscious sedation
There were no adverse effects using conscious sedation. Patients spontaneously awoke within 5 to 45 min after therapy.
In summary, 70 mg/m2 cisplatin caused intolerable oto- and neurological toxicities, thus establishing the maximally tolerated dose as 60 mg/m2.
Tumor response
shows responses by cisplatin dose. At 60 mg/m2 cisplatin there was one (3%) complete response (CR).
Table III. Tumor response by cisplatin dose (40–70 mg/m2) evaluated using response evaluation criteria in solid tumors (RECIST).
There was a total of 15 (41%) out of the 37 protocol patients with documented partial response (PR). Breaking the responses down by dose level of cisplatin, there were two patients (66%) with PR at cisplatin 40 mg/m2 and at cisplatin 50 mg/m2 there was one patient (33%) with PR. Of the 29 patients receiving cisplatin 60 mg/m2 there were 12 (21%) with PR.
Seven (19%) of the 37 protocol patients experienced stable disease (SD) of greater than two months’ duration. At 50 mg/m2 cisplatin one patient had SD. At 60 mg/m2 cisplatin, six (21%) of the 29 patients had SD.
Fourteen patients (38%) experienced progressive disease (PD). At 40 mg/m2 cisplatin, one patient (33%) had PD. One patient (33%) also had PD at a dose of 50 mg/m2 cisplatin. At 60 mg/m2 cisplatin, ten patients (34%) had PD, and two patients treated with 70 mg/m2 cisplatin had PD (100%).
Time to progression and survival
Patient survival with therapy was estimated using the Kaplan-Meier method. shows the Kaplan-Meier curve of time to tumor progression. The mean time to disease progression was 5.5 months with a SEM of 1.8. A Kaplan-Meier survival curve is shown in . The mean overall survival was 8.1 months with a SEM of 1.4; however, in a small fraction of patients survival was more than double this, and two patients survived for more than 40 months.
Quality of life
A formal quality of life survey was not included as part of this Phase I-II clinical trial, however, clear changes in patient's well being were evident and were documented in the medical records of those patients whose disease responded to the therapy.
Pain
Twenty-eight of the 37 patients (76%) had pain requiring narcotic drug control prior to treatment. Of the 28 patients with pain prior to treatment, there were 13 patients (46%) with objective tumor response. All 13 responding patients reported a decrease of pain and were able to decrease their pain medication. Eight of the 13 responding patients (62%) were able to stop narcotic pain medication altogether.
Weight
Twenty-nine of the 37 protocol patients (78%) reported a weight loss of 5 to 35 pounds prior to treatment. Fourteen of the 16 patients with objective tumor response (CR + PR) with weight loss regained weight (range 45–100%, median 76%).
Fatigue
Prior to treatment, 34 of the 37 protocol patients (92%) reported grade I -II fatigue, with one patient reporting grade III fatigue. All 16 patients with objective tumor responses reported increased energy, an improved sense of well-being, and 15 of the 16 patients (79%) resumed normal activities, including seven patients who resumed their former employment.
Discussion
The 37 patients treated with the protocol therapy all had advanced metastatic disease. Thirty-six of the 37 advanced tumors had progressed through one chemotherapy regimen or as many as five regimens. A 43% response rate of meaningful duration, accompanied with improvement in pain, body weight, and increased daily activities suggests that the therapy has notable activity in advanced cancer.
A combination of fever-range whole-body thermal therapy with chemotherapy differentiated this protocol from standard chemotherapy regimens. Thermal therapy has been shown to enhance the antitumor activity of chemotherapy drugs in part by increasing drug penetration to the tumor. Heat increases the drug concentration to the tumor by selectively increasing blood flow to the tumor Citation[29], Citation[30], and also by decreasing tumor oncotic pressure Citation[31–33].
Because this was a phase I-II protocol, a comparison of thermochemotherapy to standard therapy was not possible. However, using pancreas cancer as an example, the literature shows that gemcitabine chemotherapy gives a survival benefit compared to comfort care. In a meta-analysis there was a significant survival benefit for gemcitabine chemotherapy over best supportive care in patients with advanced pancreatic cancer Citation[34]. However, with standard gemcitabine chemotherapy, patients with metastatic pancreas cancer survived eight months or less. In a multi-modality phase II trial of 47 patients with locally advanced pancreas cancer treated with cisplatin/gemcitabine and radiation therapy, the survival at 1 year was 40% Citation[36].
Seven patients with metastatic pancreas cancer were treated in this thermochemotherapy trial. Six of the seven tumors had previously demonstrated resistance to gemcitabine or to a combination of gemcitabine and cisplatin (i.e. tumor had progressed while being treated with gemcitabine or gemcitabine/cisplatin). Despite the resistance at ambient temperature to the same chemotherapy drugs used in the protocol, there were five patients with partial responses to the thermochemotherapy of 6, 7, 7, 9, and 13 months duration, and one patient with stable disease of 4 months duration. The one patient with progressive disease had not received prior chemotherapy, however, very unusually, this patient with pancreas cancer was found to already have bone marrow involvement prior to treatment. These observations suggest the thermochemotherapy may benefit a patient with metastatic pancreas cancer at least comparably to aggressive multi-modality chemo-radiation therapy.
A schedule/timing optimized administration of the therapeutic agents was tested in the preclinical laboratory to determine a schedule with both the greatest antitumor effect, and the least toxicity, i.e. the best therapeutic index Citation[6]. The timing of cisplatin with gemcitabine, cisplatin with FR-WB-TT, and gemcitabine with FR-WB-TT were all studied preclinically, and the optimal pre-clinical schedule formed the basis of the Phase I-II protocol design.
The thermo-chemo-biotherapy regimen induced one complete response and eight partial responses in 37 protocol patients with high tumor burden cancers that had progressed while on standard chemotherapy. The responses were of reasonable duration, and, while not formally documented, the responding patients appeared to experience improved quality of life.
Notably, one patient with small-cell neuroendocrine lung cancer, and five patients with progressive high-grade neuroendocrine cancer experienced >90% partial responses. A 66-year-old patient with chemotherapy-resistant small-cell lung cancer with lung, liver, and bone metastases experienced the complete response. She remained disease free after four treatment cycles, and died of unrelated causes 3.6 years (42 months) after her last thermochemotherapy treatment.
Certainly the most important difference between this treatment and standard chemotherapy is the use of whole-body thermal therapy. Thermal therapy, including fever-range thermal therapy has been previously demonstrated to increase chemotherapy drug delivery to tumor Citation[30–33].
Another distinction between this thermochemotherapy regimen and standard therapy is the optimized scheduling of the agents. That is, the schedule of administration of the chemotherapy drugs together, as well as each drug with thermal therapy, was optimized to give the best antitumor effect with the least toxicity (i.e. the best therapeutic index). The optimized scheduling to give the best therapeutic index was carried out in a pre-clinical model Citation[5], Citation[6]. Attention to the administration schedule of chemotherapy drugs together is not standard practice. This protocol used both an optimized timing/schedule of administration of the drugs together as well as an optimized administration timing/schedule of the cytotoxic drugs with systemic thermal therapy Citation[5], Citation[6]. We suggest that an optimized scheduling of the chemotherapy drugs together and with the thermal therapy likely enhanced the regimen's efficacy.
The doses of the chemotherapy agents used in the protocol, cisplatin, gemcitabine, and IFN-α, are lower than standard doses. It may be noteworthy that three of the eight partial responses occurred at less than the MTD dose of cisplatin. Whether this occurred because small numbers skewed the data, or because a lower drug dose is an important factor cannot be answered by this study. However, pre-clinical studies suggest that lower drug doses combined with fever-range systemic thermal therapy are beneficial. This issue is being further investigated in the laboratory.
In addition to its effects on drug delivery directly to the tumor, fever-range systemic thermal therapy may enhance host immune defense. This has been shown pre-clinically Citation[36]. Burd et al. demonstrated that fever-range whole-body thermal therapy increased the influx of NK lymphocytes into tumors of SCID mice, which was associated with increased tumor cell apoptosis Citation[37]. Fever-range whole-body thermal therapy increases the trafficking of lymphocytes into lymphoid tissue, enhancing host immunity Citation[30], Citation[38]. Ostberg et al. have shown that mild heat increases the proliferation and maturation of dendritic cells and also increases the antigen-presenting ability of dendritic cells to boost the host immune response to cancer Citation[39].
Additionally, the therapy included a metronomically administered low dose of interferon-α, a drug known to increase host immunity. IFN-α increases host immune response in part by increasing dendritic cell stimulation of cytotoxic T lymphocytes and by acting as a ‘third signal’ to transform ineffective naïve CD8+ T lymphocytes into effective cytotoxic memory T cells Citation[10], Citation[40]. The role of IFN-α in creating cytotoxic CD8+ T lymphocytes has been demonstrated in the context of clearing viral antigens; it is additionally thought to be important in antitumor immunity Citation[41]. Daily, low-dose IFN-α also acts as an antiangiogenic drug Citation[9], Citation[12], Citation[42], as well as an antiproliferative agent Citation[42].
GM-CSF (sargramostim) was used to stimulate leucocyte proliferation rather than the more commonly used G-CSF (filgastrim) because GM-CSF is known to enhance dendritic cell maturation and migration Citation[43]. GM-CSF also induces Class II MHC expression and activation of macrophages Citation[44], and promotes the influx of inflammatory cells to the tumor Citation[45], Citation[46], thereby potentially increasing host immunity against tumor antigens.
Conclusion
We conclude that the MTD of cisplatin within the regimen was 60 mg/m2. Using lower than standard doses of chemotherapy drugs, the schedule-optimized thermo-chemo-biotherapy regimen was relatively well tolerated and exhibited antitumor activity in patients with heavily pretreated, chemotherapy-resistant metastatic cancers. The therapy is feasible, and appeared to benefit patients even with advanced, resistant disease. The regimen should be further tested in the most responsive tumor diagnoses (such as neuroendocrine, and pancreas cancers), as a randomized Phase II study; where one group will be given chemotherapy drugs with FR-WB-TT, and the other randomized group the same optimally scheduled chemotherapy drugs without heat.
Acknowlegements
This clinical investigation was carried out in the University of Texas Medical School at Houston and the study was supported in part by NIH grant #M01- RR 02558 through the University General Clinical Research Center (GCRC).
This study would not have been possible without the efforts of Mrs Madelene Jewell-Ottosen, RN, Director of the University of Texas Health Science Center at Houston Clinical Research Center. We also thank William Julian Morrell, RN in the Memorial Hermann Cancer Center for outpatient patient care, as well as Ms Julie Medina, pharmacy technician, who efficiently provided treatment medications. Additionally, we thank Mrs Sandra Seanez, and Jamey Boettcher for patient registration and treatment coordination, and Ms Maude Veech for editorial effort.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
Notes
1. The treatment facility (UCRC) was closed for more than nine weeks following flood damage.
References
- Wondergem J, Bulger RE, Strebel FR, Newman RA, Travis EL, Stephens LC, Bull JM. Effect of cis-diamminedichloroplatinum(II) combined with whole body hyperthermia on renal injury. Cancer Res 1988; 48: 440–446
- Ohno S, Siddik ZH, Baba H, Stephens LC, Strebel FR, Wondergem J, Khokhar AR, Bull JM. Effect of carboplatin combined with whole body hyperthermia on normal tissue and tumor in rats. Cancer Res 1991; 51: 2994–3000
- Baba H, Siddik ZH, Strebel FR, Jenkins GN, Bull JM. Increased therapeutic gain of combined cis-diamminedichloroplatinum(II) and whole body hyperthermia therapy by optimal heat/drug scheduling. Cancer Res 1989; 49: 7041–7044
- Bull J, Loo C, Strebel F, Jenkins G, Tomoda M, Rowe R. Treatment of a highly metastatic mammary adenocarcinoma with fever-range, long-duration, low temperature (40°C for 6 h) whole body hyperthermia (LL-WBH) combined with gemcitabine (dFdC) in rats. Proceedings of the 90th meeting of the American Association for Cancer Research, C Croce, Philadelphia, PA 1999; 3643
- Bull J, Strebel F, Jenkins G, Tomoda M, Rowe R. Sequence-dependency of antitumor effect of gemcitabine combined with cisplatin in the highly metastatic MTLN3 rat mammary adenocarcinoma. Proceedings of the 91st Annual Meeting of the American Association for Cancer Research, FJ Rauscher, San Francisco, CA 2000; 410
- Bull J, Strebel F, Rowe R, Deng W. The importance of schedule in whole-body thermochemotherapy. Int J Hyperthermia 2008; 2: 171–181
- Tannir NM, Cohen L, Wang X, Thall P, Mathew PF, Jonasch E, Siefker-Radtke A, Pagliaro LC, Ng CS, Logothetis C. Improved tolerability and quality of life with maintained efficacy using twice-daily low-dose interferon-alpha-2b: Results of a randomized phase II trial of low-dose versus intermediate-dose interferon-alpha-2b in patients with metastatic renal cell carcinoma. Cancer 2006; 107: 2254–2261
- Fidler I. Regulation of neoplastic angiogenesis. J Natl Cancer Inst Monogr 2001; 28: 10–14
- Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol 1992; 3: 89–96
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13: 119–134
- Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol 2002; 71: 565–581
- Marler JJ, Rubin JB, Trede NS, Connors S, Grier H, Upton J, Mulliken JB, Folkman J. Successful antiangiogenic therapy of giant cell angioblastoma with interferon alfa 2b: Report of 2 cases. Pediatrics 2002; 109: E37
- Matsuda H, Strebel FR, Kaneko T, Danhauser LL, Jenkins GN, Toyota N, Bull JM. Long duration-mild whole body hyperthermia of up to 12 hours in rats: Feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: Implications for adjuvant therapy. Int J Hyperthermia 1997; 13: 89–98
- Bull JMC, Strebel F, Jenkins G, Nagle V. A preclinical breast cancer protocol combining fever-range whole-body hyperthermia with Doxil. Proceedings of the 16th Annual San Antonio Conference on Breast Cancer, K Osborne, San Antonio, TX 1998; 32
- Pritchard MT, Ostberg JR, Evans SS, Burd R, Kraybill W, Bull JM, Repasky EA. Protocols for simulating the thermal component of fever: Preclinical and clinical experience. Methods 2004; 32: 54–62
- Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol 1984; 2: 187–193
- Tuma RS. Sometimes size doesn't matter: Reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst 2006; 98: 1272–1274
- Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer 2006; 42: 1031–1039
- Folkman J. Angiogenesis research: From laboratory to clinic. Forum (Genoa, Italy) 1999; 9: 59–62
- Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med 1995; 14: 1149–1161
- Ahn C. An evaluation of phase I cancer clinical trial designs. Stat Med 1998; 17: 1537–1549
- Wolff RA, Evans DB, Gravel DM, Lenzi R, Pisters PW, Lee JE, Janjan NA, Charnsangavej C, Abbruzzese JL. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res 2001; 7: 2246–2253
- Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 2003; 21: 3402–3408
- Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol 1997; 24: S7–2-7
- Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2′,2′-difluorodeoxycytidine 5'-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer chemotherapy and pharmacology 1991; 27: 258–262
- Kraybill WG, Olenki T, Evans SS, Ostberg JR, O'Leary KA, Gibbs JF, Repasky EA. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: Correlation with mouse models. Int J Hyperthermia 2002; 18: 253–266
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–181
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc 1958; 53: 457–481
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiation research 2001; 155: 515–528
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–767
- Jain RK. Delivery of novel therapeutic agents in tumors: Physiological barriers and strategies. J Natl Cancer Inst 1989; 81: 570–576
- Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res 1991; 51: 6691–6694
- Jain RK. The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation 1997; 4: 1–23
- Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007; 25: 2607–2615
- Haddock MG, Swaminathan R, Foster NR, Hauge MD, Martenson JA, Camoriano JK, Stella PJ, Tenglin RC, Schaefer PL, Jr Moore DF, et al. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: Results of the North Central Cancer Treatment Group Phase II Study N9942. J Clin Oncol 2007; 25: 2567–2572
- Ostberg JR, Repasky EA. Use of mild, whole body hyperthermia in cancer therapy. Immunol Invest 2000; 29: 139–142
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998; 177: 137–147
- Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, von Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nature immunology 2006; 7: 1299–1308
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother 2006; 55: 292–298
- Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid \dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 2006; 114: 2482–2489
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8 + T cells. Immunological reviews 2006; 211: 81–92
- Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res 1999; 5: 2726–2734
- Pillarisetty VG, Miller G, Shah AB, DeMatteo RP. GM-CSF expands dendritic cells and their progenitors in mouse liver. Hepatology (Baltimore, Md) 2003; 37: 641–652
- Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8 + Tc1 responses in vivo. Eur J Immun 2004; 34: 66–73
- Gomez-Cambronero J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS letters 2003; 550: 94–100
- Shen L, Smith JM, Shen Z, Hussey SB, Wira CR, Fanger MW. Differential regulation of neutrophil chemotaxis to IL-8 and fMLP by GM-CSF: Lack of direct effect of oestradiol. Immunology 2006; 117: 205–212
- Davidson SE, Trotti A, Ataman OU, Seong J, Lau FN, da Motta NW, Jeremic B. Improving the capture of adverse event data in clinical trials: The role of the international atomic energy agency. Int J Radiat Oncol Biol Phys 2007; 69: 1218–1213