Abstract
The goal of immune-based tumor therapies is the activation of immune cells reactive against a broad spectrum of tumor-expressed antigens. Vaccines based on chaperone proteins appear promising as these proteins naturally exist as complexes with various protein fragments including those derived from tumor-associated antigens. Multi-chaperone systems are expected to have highest polyvalency as different chaperones can carry distinct sets of antigenic fragments. A free-solution isoelectric focusing (FS-IEF) technique was established to generate chaperone-rich cell lysates (CRCL). Results from murine systems support the contention that CRCL induce superior anti-tumor responses than single chaperone vaccines. We established an in vitro model for human melanoma to evaluate the capacity of CRCL to transfer endogenously expressed tumor antigens to the cross-presentation pathway of dendritic cells (DC) for antigen-specific T cell stimulation. CRCL prepared from human melanoma lines contained the four major chaperone proteins Hsp/Hsc70, Hsp90, Grp94/gp96 and calreticulin. The chaperones within the melanoma cell-derived CRCL were functionally active in that they enhanced cross-presentation of exogenous peptides mixed into the CRCL preparation. Superior activity was observed for Hsp70-rich CRCL obtained from heat-stressed melanoma cells. Despite the presence of active chaperones, melanoma cell-derived CRCL failed to transfer endogenously expressed melanoma-associated antigens to DC for cross-presentation and cytotoxic T cell (CTL) recognition, even after increasing intracellular protein levels of tumor antigen or chaperones. These findings reveal limitations of the CRCL approach regarding cross-presentation of endogenously expressed melanoma-associated antigens. Yet, CRCL may be utilized as vehicles to enhance the delivery of exogenous antigens for DC-mediated cross-presentation and T cell stimulation.
Introduction
In the normal cellular environment, chaperone proteins perform their intracellular functions as multi-protein complexes consisting of chaperones, co-chaperones and substrate molecules Citation[1], Citation[2]. Purified tumor-derived chaperone proteins such as heat shock protein 70, 90 and 110 (Hsp70, Hsp90, Hsp110), Grp94/gp96 and calreticulin were successfully used as vaccines resulting in the generation of tumor-reactive T cell responses and protective anti-tumor immunity in numerous animal and human models Citation[3–12]. The antigenic potential was demonstrated to reside in the repertoire of antigenic, tumor-derived peptides carried by the chaperones and not in the proteins themselves Citation[10], Citation[13–15]. Recently we documented that human Hsp70 through complex formation with antigenic peptides acts as antigen delivery vehicle improving antigen cross-presentation and T cell stimulation Citation[16]. Yet other studies addressed the question whether multi-chaperone/co-chaperone vaccines would be more effective than single-component chaperone vaccines. One of the strategies utilized chaperone-rich cell lysates (CRCL) obtained from tumor homogenates using a free-solution isoelectric focusing technique (FS-IEF) Citation[17–24]. CRCL were shown to contain at least the four major immunogenic chaperone proteins Hsp70, Hsp90, Grp94/gp96 and calreticulin. CRCL are described as large, multi-member entities wherein chaperones are associated in high molecular complexes Citation[17]. CRCL derived from murine tumor material were capable of inducing innate and adaptive immune responses and protective anti-tumor responses in different models Citation[25]. So far, only one study assessed the immunostimulatory capacity of CRCL derived from human material Citation[26].
CRCL appear promising as vaccines for human immunotherapy for several reasons. First, the FS-IEF technique for enriching multiple chaperones from tumor lysate is technically feasible and yields more immunogenic material for clinical use than individual chaperone purification procedures Citation[18], Citation[19]. Second, results from murine model systems suggest that the efficacy of CRCL as an anti-cancer vaccine is not limited to tumors of particular histological origin or tumorgenicity or metastatic potential. Third, there is no need to know or to identify tumor-specific antigens of the tumor material used for CRCL preparations Citation[25]. These characteristics should allow a wide application of this vaccine strategy for different types of cancer. Because CRCL analyses using human tissues are to date limited to one study, we selected a human melanoma system to evaluate the efficacy of CRCL in the cross-presentation of tyrosinase and Melan-1/MART-1, both endogenously expressed, non-mutated tumor-associated differentiation antigens of low immunogenicity Citation[27], Citation[28]. In a previous study we have demonstrated that Hsp70 isolated from melanoma cell lines under conditions preserving chaperone-substrate complexes allowed the activation of tyrosinase-specific T cells after incubation of DC Citation[8]. The preparation of these Hsp70-complexes (Hsp70-PC) was labor intensive and had low yield. Since multi-chaperone CRCL preparations promised technical feasibility combined with equal or even better immunological efficacy we used this melanoma system for CRCL preparation and immunological evaluation.
Here we document that the CRCL technique held the promise of complex protein composition containing multiple chaperone proteins (Hsp70/Hsc70, Hsp90, Grp94/gp96, calreticulin). Detectable amounts of tumor-associated antigens (TAA) (tyrosinase, Melan-A/MART-1) as full length proteins were also present in CRCL from tyrosinase- and Melan-A/MART-1-positive cell lines. The chaperone proteins within the melanoma-derived CRCL preparation were active antigen delivery vehicles in that they significantly enhanced the cross-presentation of exogenous peptides with high affinity binding motifs for Hsp70 which were added to the CRCL preparation. Despite these characteristics, the CRCL did not deliver antigens (tyrosinase, Melan-A/MART-1) endogenously expressed by the melanoma cells from which the CRCL were prepared to immature DC (i-DC) thus failing to induce CTL activation. This failure was not due to inhibitory effects of CRCL on DC or T cells. Neither over-expressing the tumor antigens nor up-regulating the Hsp70 by heat treatment changed the outcome of CRCL-mediated cross-presentation. The results reveal limitations of the CRCL technique for the in vitro cross-presentation of endogenous tyrosinase and Melan-A/MART-1 antigens. However, the capacity of CRCL to facilitate the cross-presentation of exogenous peptides, in particular those with Hsp70-binding sequences, indicates the potential utilization of CRCL as a delivery vehicle for the generation of vaccines with defined antigen-specificity.
Material and methods
Peptides
The tyrosinase peptide Tyr368–376 (YMNGTMSQV) and the Melan-A/MART-1 peptide Melan-A26–35 (ELAGIGILTV) were synthesized and purified by the in-house facility. The pep70-Tyr hybrid peptide (biotin-GSG-HWDFAWPW-GSG-YMNGTMSQV) was purchased from Biosyntan GmbH (Berlin, Germany) and from the University of Munich, Gene Center (Munich, Germany). It consists of the nonameric HLA-A2 binding epitope from tyrosinase (YMNGTMSQV) and the pep70 sequence (HWDFAWPW) which confers high affinity binding to HSP70 family members.
Cultivation of cells
The human melanoma cell line, 624.38-MEL (tyrosinase- and Melan-A/MART-1-positive; HLA-A*0201-positive) Citation[29], Citation[30], was a kind gift from M.C. Panelli (National Institutes of Health, Bethesda, MD, USA), and A375-MEL (tyrosinase- and Melan-A/MART-1-negative; HLA-A*0201-positive) was purchased from the American Type Culture Collection (Rockville, MD, USA). The cytotoxic T cell clone (TyrF8) Citation[31] (kindly provided by P. Schrier, Department of Clinical Oncology, Leiden University Hospital, The Netherlands) recognizes the HLA-A2-restricted tyrosinase peptide Tyr368–376 (YMNGTMSQV), the post-transcriptionally modified version YMDGTMSQV and the epitopes naturally processed and presented by melanoma cell lines Citation[32]. The cytotoxic T cell clone A42-MART-1 is specific for the HLA-A2-restricted Melan-A/MART-1 peptide Melan-A26–35 (ELAGIGILTV, AAGIGILTV and LAGIGILTV) Citation[27], Citation[33] (kindly provided by M. C. Panelli, National Institute of Health, Bethesda, MD, USA). Both clones were cultured as described Citation[32] and used between days 8 and 14 after the last restimulation.
All tumor cell lines were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/ml penicillin/streptomycin and 1 × non-essential amino acids (all chemicals from PAA, Pasching, Austria). Cell lines were routinely tested for mycoplasma using a commercial kit and manufacturer's instructions (Biochrom, Berlin, Germany).
Human monocytes were used to generate monocyte-derived DC. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood from healthy volunteers by density gradient centrifugation over Ficoll-Hypaque (PAA, Pasching, Austria). Monocytes were isolated from PBMC by positive immunomagnetic selection (anti-CD14 MACS beads; Miltenyi Biotech, Bergisch Gladbach, Germany). CD14+ cells were then plated in 6-well plates at a concentration of 1 × 106/well in RPMI 1640 medium, supplemented with 10% low endotoxin heat-inactivated FCS, 2 mM glutamine, 1 × non essential amino acids and 100 U/ml penicillin/streptomycin (all from PAA, Pasching, Austria). For serum-free cultivation, CD14+ cells were plated in 6-well plates at a concentration of 5 × 106 cells in 4 ml AIM-V (GibcoInvitrogen, Carlsbad, CA) per well. RhGM-CSF (800 U/ml) (Novartis, Nuernberg, Germany) and rhIL-4 (500 U/ml) (PromoCell, Heidelberg, Germany) were added to the monocyte cultures at day 0, day 4 and day 6. At day 6–8 immature DC (i-DC) were used for functional assays. When indicated, DC maturation was induced by the addition of a cytokine mixture (IL-1ß, 20 ng/ml; IL-6, 1000 U/ml; TNF-α, 500 U/ml; PGE2, 10 µM) (IL-1ß, IL-10 and TNF-α from R&D Systems, Minneapolis, MN; PGE2 from Sigma-Aldrich, Taufkirchen, Germany) for 24 h or by adding LPS (lipopolysaccharide) (1 µg/ml) (Sigma-Aldrich) for 24 h or 48 h. The institutional review board of Ludwig-Maximilians-University approved the use of blood donations for these studies. Informed consent was provided according to the Declaration of Helsinki.
Heat shock treatment of melanoma cells
Melanoma cells were plated in T175 cell culture flaks and were grown to 80–90% confluence. Heating was performed by directly immersing the cell culture flasks in a temperature-controlled water bath at 41.8°C for 2 h Citation[32]. After a recovery time of 20 h viable adherent cells were harvested, frozen and used for Western blot analysis and CRCL preparation.
Infection of melanoma cells with MVA-hTyr
MVA-hTyr is the stable, recombinant Modified Vaccinia Virus Ankara containing the cDNA for the human tyrosinase gene Citation[34]. 2 × 107 A375-MEL cells were plated in T175 cell culture flasks. For the infection with MVA-hTyr, cells were incubated with virus using a MOI of 5 U/ml for 2 h in RPMI 1640 +2% FCS, under occasional swaying. After infection, medium was exchanged for regular cultivation medium. 18–20 h after infection viable cells were harvested, frozen and used for Western blot analysis and CRCL preparation.
Preparation of chaperone-rich lysates of human melanoma cell lines
Free solution-isoelectric focusing (FS-IEF) preparation of chaperone-rich cell lysates (CRCL) from human melanoma cell lines was performed as described Citation[17], Citation[20], with some modifications. Following the published protocol, melanoma cells were homogenized in homogenization buffer (10 mM Tris/HCl, (pH 7.4)/10 mM NaCl, 1% CHAPS, (all from Sigma-Aldrich, Taufkirchen, Germany) including 2 µg/ml leupeptin, 0.8 mM Pefabloc, 1 µg/ml DNase and 1 Complete protease inhibitor cocktail tablet (all from Roche Molecular Biochemicals, Mannheim, Germany)) in a glass-teflon homogenizer at a ratio of 8–10 × 108 cells/50 ml buffer. The described detergent mixture containing TritonX-100, TritonX-114 and Igepal used for homogenization and FS-IEF was replaced by CHAPS. The homogenate was centrifuged in two steps at 4°C (10.000 × g for 30 min; 100.000 × g for 90 min), as described. The supernatant was dialyzed into sequentially lower concentrations of homogenization buffer, ending in water. The protein concentration was determined using colorimetric bicinchoninic acid assays (BCA Reagent, Pierce Endogen, Rockford, IL), and frozen in aliquots. For FS-IEF a mixture consisting of cell lysate (10–15 mg of total protein), acid/base pairs, urea and detergent was prepared (total volume 60 ml). Acid/base pairs in the pH ranges of 3.9–5.6 (400 mM MES and 400 mM Gly-Gly), 4.5–6.1 (400 mM MOPSO and 400 mM ß-alanine) and 5.1–6.8 (400 mM TAPS and 400 mM ϵ-amino-caproic acid), (all chemicals from Sigma-Aldrich, Taufkirchen, Germany) were used for pH gradient establishment (2.5 ml of each solution), as described. Compared to the published protocol, the concentration of urea (Fisher Scientific UK Ltd, Loughborough, UK) was increased to 7 M and CHAPS (Sigma-Aldrich) was used as detergent (see above) in a concentration of 0.15%. The isofocusing was performed in a Rotofor device (Biorad, Hercules, CA) with 15 W constant power for 5 h, as described. Twenty fractions were harvested and analysed by SDS-PAGE and subsequent Western blot probing with specific antibodies for the chaperones Hsp70/Hsc70, Hsp90, Grp94/gp96 and calreticulin. Hsp70/Hsc70-enriched fractions of 8–10 independent Rotofor runs were pooled and dialyzed against 2 M urea in 0.5 × PBS, pH 7.4, followed by dialysis into 0.5 × PBS. As described, the dialysate was concentrated using a centrifugal filter device (10 kDa cut off) (Millipore, Billerica, MA). For detergent removal, protein samples were applied to a column filled with 2 ml of Extracti-Gel™ detergent removing gel (Pierce Endogen, Rockford, IL, USA) and chromatography was performed per manufacturer's instructions. For endotoxin removal, protein samples were applied to a column filled with 1 ml Detoxi-Gel™ endotoxin removing gel (Pierce Endogen, Rockford, IL, USA). This procedure was repeated until the endotoxin read-out was less than 1 EU/mg protein using the Limulus Amebocyte Lysate assay (Cambrex Bio Science, Walkersville, MD). The final protein solution was reconstituted in 1 × HKM buffer (25 mM Hepes (pH 7.6), 50 mM KCl, 5 mM MgCl2) (all chemicals from Sigma-Aldrich), quantified (BCA protein assay), sterile filtered and stored at −70°C until use. A typical yield was 0.5–1.5 mg CRCL using 5 × 108 human melanoma cell lines. Toxicity of CRCL preparations was tested with one step MTT assay (CellTiter 96® Aqueous One Solution Cell Proliferation assay, Promega, Mannheim, Germany) using K562 cells. No toxicity of CRCL preparations in the range of 30–300 µg/ml was observed.
The removal of detergent and endotoxin from CRCL preparations was required to allow functional assays with human DC. After testing different detergents as well as detergent-free purification methods, CHAPS was used for homogenization and FS-IEF because of its higher micellar concentration, smaller size of micelle formation and the ability to be removed by dialysis. Endotoxin was removed to obtain low endotoxin CRCL preparations (<1 EU/mg protein). Sterile filtration of the end product concomitantly removed any precipitates, which often accumulated during the purification procedure.
Western blot analysis
Cell lysates of melanoma cells before/after heat treatment or infection with MVA-hTyr, fractions of Rotofor runs and CRCL preparations were analysed by Western blot using standard protocols Citation[32]. Cell lysates were prepared by lysing 1–2 × 106 melanoma cells in lysis buffer (25 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% NP40 containing 2 µg/ml leupeptin, 0.8 mM Pefabloc and 1 mM DNase (freshly added just before use) for 30 min at 4°C). After centrifugation supernatants were taken and protein amount was determined by Bradford method (Bradford reagent, Biorad, Hercules, CA).
Melanoma-associated antigens were detected with antibodies recognizing human tyrosinase (monoclonal mouse IgG2a T311, NeoMarkers, Labvision, Westinghouse Dr. Fremont, CA) and human Melan-A/MART-1 (monoclonal mouse IgG1 HMB45, DAKO, Glostrup, Denmark). Heat shock proteins were detected using antibodies against human Hsp70/Hsc70 (monoclonal mouse IgG1 BRM-22, Sigma-Aldrich, Taufkirchen, Germany), human Hsp70 (monoclonal rat IgG1 6B3, produced by our in-house facility Citation[8]), human Hsc70 (monoclonal mouse IgG2 1B5), human Hsp90 (monoclonal mouse IgG1 AC88), human calreticulin (whole rabbit serum) (all from StressGen Biotechnologies, Victoria, British Columbia, Canada) and human Grp94/gp96 (whole rabbit serum, kindly provided by Dr Nicchitta, Duke University Medical Center, NC). Equal protein loading was verified by immunodetection of ß-actin using a rabbit anti-serum against human ß-actin (Sigma-Aldrich).
Flow cytometry
The following antibodies were used: isotype controls (mouse IgG1, mouse IgG2a and mouse IgG2b; Immunotech, Hamburg, Germany), anti-human CD14, anti-human CD83 (Immunotech, Hamburg, Germany), anti-human CD86 (Caltag, Burlingame, CA, USA), anti-human CD40, anti-human HLA-DR, anti-human CD80, PE-labeled anti-human CD11c and FITC-labeled anti-human CD209 (BD Biosciences Pharmingen, Heidelberg, Germany). PE-conjugated goat anti-mouse IgG (Dako, Hamburg, Germany) was used as secondary reagent for unlabeled primary antibodies. 2 × 105 cells were stained using standard protocols. Dead cells were excluded by propidium-iodide staining. Cells were analysed using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences Pharmingen).
Analysis of macropinocytosis and endocytosis
Human i-DC (d6 or d7) (1 × 106 cells/2 ml AIM-V) were treated with different amounts of CRCL (20–200 µg/ml). Untreated cells and cells incubated with 1 µg/ml LPS served as controls. After 24 h cells were harvested and analysed for macropinocytosis and endocytosis. 2 × 105 treated DC were resuspended in 0.5 ml AIM-V. For macropinocytosis, FITC-conjugated albumin (Sigma-Aldrich) was added in a final concentration of 0.5 mg/ml and incubated for different times (30 to 90 min) at 37°C. For endocytosis analysis, FITC-conjugated Dextran (FD-70S, Sigma-Aldrich) was added at a final concentration of 10 µg/ml for 3.5 h at 37°C. After the incubation, cells were intensively washed and analysed immediately on a FACSCalibur flow cytometer with CellQuest software (BD Biosciences Pharmingen, Heidelberg, Germany). The mean fluorescence intensity (MFI) of the cells in FL1 was determined. The background MFI of cells incubated at 0°C was subtracted.
IL-12 secretion and surface marker expression of human DC
Human monocyte-derived i-DC (d6–d8) (1 × 106 cells/2 ml AIM-V) were treated with different concentrations of CRCL (20–200 µg/ml) for 48 h. Untreated cells and cells incubated with 1 µg/ml LPS served as controls. After incubation, supernatants were harvested for cytokine measurements and cells were used for analysis of surface markers by flow cytometry. The IL-12p40 content in the supernatants was quantified by standard ELISA (BD Biosciences Pharmingen).
Mixed-leukocyte reaction (MLR)
Human monocyte-derived i-DC (d6–d8) (1 × 106 cells/2 ml AIM-V) were cultured with 40 µg/ml CRCL. DC without CRCL from parallel cultures served as control cells. After 24 h, DC were harvested, washed, irradiated (40 Gy) and seeded into 96-well round-bottom plates at titrated concentrations. Allogeneic T cells (1 × 105/well) were added to each well resulting in DC to T cell ratios from 1 : 2 to 1 : 64. Control wells contained untreated and CRCL-treated DC without T cells and T cells without DC, respectively. T cell proliferation was assessed after 5 days of coculture by adding [3H]-thymidine (1 µCi/well (0.037 MBq)) (Hartmann Analytics, Braunschweig, Germany) during another 24 h. [3H]-thymidine incorporation was measured using a microBeta counter (Beckman Coulter, Fullerton, CA).
Cross-presentation assay
The amount of exogenous antigens cross-presented by DC was assessed by the capacity of DC to stimulate IFN-γ secretion of TyrF8 or A42-MART-1 CTL specific for antigens tyrosinase or Melan-A/MART-1, respectively, as described previously Citation[8]. Briefly, i-DC (d7) were seeded at a concentration of 2 × 104 cells in 100 µl per well in 96-well round-bottomed plates and indicated concentrations of CRCL were added. After 24 h, DC were induced to mature by the addition of a cytokine mixture (IL-1ß, 20 ng/ml; IL-6, 1000 U/ml; TNF-α, 500 U/ml; PGE2, 10 µM) for additional 24 h. TyrF8 cells or A42-MART-1 CTL were added (2 × 104 cells/well) in 100 µl of medium to give final concentrations of 25 U/ml IL-2, 5% FCS, and 5% human serum. As positive control, tyrosinase peptide (Tyr368–376) or Melan-A/MART-1 peptide (Melan-A26–35) were added at concentrations ranging from 1–10 µg/ml to m-DC shortly before the CTL were applied. After 24 h, supernatants were harvested and the content of IFN-γ was measured by ELISA (OptEIA, BD Biosciences Pharmingen). In some experiments, different incubation times with CRCL were used (2–24 h) and the maturation step of DC was omitted. Where indicated, titered amounts of recombinant human Hsp70 (rhuHsp70) were additionally added to the CRCL and coincubated with the DCs (1.5 × 104) for 2 h before addition of the T cells (4 × 103).
To determine the capacity of CRCL as antigen carrier, 10 µM pep70-Tyr were mixed with CRCL (30–120 µg/ml) in 30 µl in a 96-well round-bottomed plate and the mixture was incubated for 2 h at RT to allow complex formation. 1.5 × 104 i-DC were added in 80 µl AIM-V and incubated for 1 h at 37°C/5% CO2. 4 × 103 TyrF8 CTL were added to yield a final volume of 210 µl consisting of 5% FCS, 5% human serum and 25 U/ml IL-2. After 24 h supernatants were harvested and the content of IFN-γ was measured by ELISA.
Statistical analysis
Data are expressed as the mean ± SD of triplicate samples. For calculation of significance the Wilcoxon rank sum test was employed.
Results
Composition of CRCL prepared from human melanoma cell lines
Human melanoma cell lines were used for CRCL preparations by FS-IEF. In order to reach compatibility with the human DC system, the method previously described for murine tumor material required some modifications, such as selecting a removable detergent, adjusting rotofor conditions and removing endotoxin (see material and methods).
As shown in , Hsp90, Hsp70/Hsc70, Grp94/gp96 and calreticulin of lysates of different human melanoma cell lines clustered along the pH gradient in fractions 9–13. These fractions were combined to yield the final CRCL preparations used for the study. The overall protein composition of CRCL preparations as determined by SDS-PAGE and subsequent Ponceau S-staining revealed a complex mixture that was similar in complexity to the starting lysates (). The chaperones Hsp70 and Hsc70 were strongly enriched in the CRCL compared to the cell lysate and Hsp90, Grp94/gp96 and calreticulin were present in roughly similar amounts (). The tumor-associated antigens, tyrosinase and Melan-A/MART-1, were present in the lysates of the antigen-positive melanoma line 624.38-MEL and still detectable in the respective CRCL preparation ().
Figure 1. Composition of CRCL prepared from human melanoma cell lines. (a) 15 mg of total protein from A375-MEL cell lysate were loaded onto a Rotofor device and FS-IEF was performed. Twenty fractions (each 2.5 ml) were harvested and aliquots of 15 µl were analysed by SDS-PAGE and Western blot for human Hsp90, Hsp70/Hsc70, Grp94/gp96 and calreticulin, using specific antibodies. Chaperones were detected in fractions 9-13, spanning a pH range from 5.4–6.5. (b) Fractions 9–13 of eight to ten independent Rotofor runs were combined to yield the CRCL. CRCL and cell lysates were analysed by SDS-PAGE and subsequent Western blot. Equal protein amounts of lysates and CRCL preparations, respectively, were loaded. Membranes stained with Ponceau S show the overall protein patterns of CRCL and cell lysates from melanoma cell lines A375-MEL and 624.38-MEL. (c) Membranes were developed using antibodies to chaperones, Hsp70/Hsc70, Hsp90, Grp94/gp96, calreticulin, and melanoma-associated antigens, tyrosinase and Melan-A/MART-1.
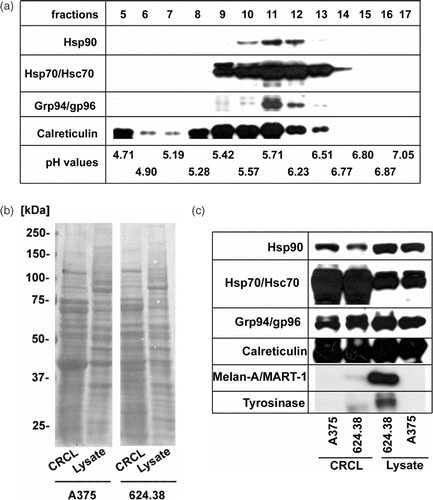
These results document that CRCL can be prepared from human melanoma cell lines with reproducible yields of 1–1.5 mg CRCL from 5 × 108 cells.
Melanoma cell-derived CRCL contain a chaperone system which can deliver exogenous peptides to the cross-presentation pathway of DC for CTL stimulation: Superior activity of CRCL from heat-treated melanoma cells
We previously documented that Hsp70 functions as a chaperone molecule and facilitates, through its ability to form complexes with peptides antigen cross-presentation by DC resulting in enhanced antigen-specific T cell stimulation Citation[16]. The melanoma cell-derived CRCL were highly enriched for Hsp70 compared to cell lysates (). Using a comparison against titered amounts of recombinant human Hsp70 in a fluorescent Western blot analysis we estimated that the amount of Hsp70 in CRCL preparations was around 5–10% of the total protein (data not shown). To evaluate the functional capacity of the CRCL as antigen delivery vehicle for exogenous peptides we utilized our previously established system which allows the quantification of the chaperone-related modulation of cross-presentation efficacy Citation[16]. Specifically, equal amounts of the hybrid pep70-Tyr peptide (10 µM) containing the tyrosinase-specific epitope and the Hsp70 binding sequence HWDFAWPW (here called pep70) were mixed with varied concentrations of CRCL and pre-incubated for complex formation. Then, i-DC from HLA-A2-positive donors and CTL were added to the CRCL/pep70-Tyr mixtures or to parallel settings containing an identical amount of hybrid peptide (10 µM) without CRCL. The extent of antigen transfer to DC was determined by measuring the amount of CTL-secreted IFN-. As shown in , the CTL IFN-γ response was higher when the DC were pulsed with pep70-Tyr/CRCL mixtures compared to DC pulsed with peptide only (1.93 fold at 90 µg/ml CRCL). The amount of CTL-secreted IFN-γ was higher when more CRCL was present (1.41 fold at 60 µg/ml CRCL and 1.93 fold at 90 µg/ml CRCL) (data not shown).
Figure 2. CRCL enhance cross-presentation of exogenous peptides, with heat-induced CRCL showing superior activity. (a) CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS) or CRCL(624.38-HS) respectively) (90 µg/ml) were mixed with the hybrid peptide pep70-Tyr (10 µM) and incubated for 2 h at RT. 1.5 × 104 DC were added for 1 h at 37°C/5% CO2 followed by the addition of 4 × 103 TyrF8 CTL. After 24 h IFN-γ in the supernatants was determined by ELISA. DC/CTL co-cultures with pep70-Tyr alone, with CRCL alone and without pep70-Tyr/CRCL served as controls. The relative IFN-γ production was calculated using IFN-γ of DC/CTL co-cultures with pep70-Tyr alone as reference value Citation[16]. Bars are the mean ± SD of three different experiments each performed in triplicate values. All cultures containing CRCL resulted in significantly higher CTL stimulation compared to controls with peptide alone (all p-values ≤0.05). (b) Chaperone and antigen levels of melanoma cells after heat treatment. 624.38-MEL cells were treated with sublethal heat shock (HS) (41.8°C for 2h) and allowed to recover for 20 h. Viable cells were harvested and analysed by Western blot for Hsp70, Hsc70, tyrosinase and Melan-1/MART-1. ß-actin served as loading control. (c) Chaperone content of CRCL derived from heat-treated melanoma cells. Equal amounts (10 µg) of CRCL(624.38 + HS) or CRCL(624.38 − HS) were analysed for Hsp90, Hsp70, Hsc70, Grp94/gp96 and calreticulin.
![Figure 2. CRCL enhance cross-presentation of exogenous peptides, with heat-induced CRCL showing superior activity. (a) CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS) or CRCL(624.38-HS) respectively) (90 µg/ml) were mixed with the hybrid peptide pep70-Tyr (10 µM) and incubated for 2 h at RT. 1.5 × 104 DC were added for 1 h at 37°C/5% CO2 followed by the addition of 4 × 103 TyrF8 CTL. After 24 h IFN-γ in the supernatants was determined by ELISA. DC/CTL co-cultures with pep70-Tyr alone, with CRCL alone and without pep70-Tyr/CRCL served as controls. The relative IFN-γ production was calculated using IFN-γ of DC/CTL co-cultures with pep70-Tyr alone as reference value Citation[16]. Bars are the mean ± SD of three different experiments each performed in triplicate values. All cultures containing CRCL resulted in significantly higher CTL stimulation compared to controls with peptide alone (all p-values ≤0.05). (b) Chaperone and antigen levels of melanoma cells after heat treatment. 624.38-MEL cells were treated with sublethal heat shock (HS) (41.8°C for 2h) and allowed to recover for 20 h. Viable cells were harvested and analysed by Western blot for Hsp70, Hsc70, tyrosinase and Melan-1/MART-1. ß-actin served as loading control. (c) Chaperone content of CRCL derived from heat-treated melanoma cells. Equal amounts (10 µg) of CRCL(624.38 + HS) or CRCL(624.38 − HS) were analysed for Hsp90, Hsp70, Hsc70, Grp94/gp96 and calreticulin.](/cms/asset/7663e2d1-139a-4a2b-bb92-5afbda1014c3/ihyt_a_321505_f0002_b.gif)
CRCL(624.38+HS) derived from heat-treated 624.38-MEL cells, which had higher Hsp70 content (), were even better in delivering the pep70-Tyr peptide compared to CRCL(624.38-HS) derived from untreated cells (2.39 fold compared to 1.93 fold at 90 µg/ml CRCL) (). The results are in line with our previous demonstration that targeting peptides to form complexes with Hsp70 enhances their efficiency of being cross-presented by DC.
Together these results indicate that the melanoma cell-derived CRCL contain a chaperone system with the ability to deliver exogenous peptides with an Hsp70 binding motif to the cross-presentation pathway of DC for CTL stimulation. CRCL with higher content of Hsp70 show superior activity.
Melanoma-derived CRCL fail to transfer endogenously expressed tyrosinase or Melan-A/MART-1 to human DC for MHC class I-restricted CTL recognition
To determine the capacity of the CRCL to transfer antigens endogenously expressed by the melanoma cells from which they were derived, CRCL of tyrosinase- and Melan-1/MART-1-positive 624.38-MEL cells and antigen-negative A375-MEL cells, designated CRCL(624.38) and CRCL(A375), respectively, were added to human i-DC from HLA-A2-positive donors. Before addition of the T cells, CRCL-pulsed DC were matured with a cocktail of IL-1ß, IL-6, TNF-α and PGE2. CRCL-pulsed DC were assessed for their ability to stimulate IFN-γ release by CTL.
We used CRCL over a wide range (1–1000 µg/ml) and were unable to find evidence of antigen transfer and CTL stimulation by CRCL-pulsed DC (). The tested CRCL concentrations should cover an estimated range of 0.1 to 100 µg/ml of Hsp70, amounts which were active in cross-presentation when isolated as single chaperone HSP70-peptide complexes (see Citation[8], , half-maximal tyrosinase cross-presentation at 75 ng/ml HSP70-PC). Furthermore, lack of CTL stimulation was observed not only for the tyrosinase-recognizing CTL TyrF8, but also for the CTL A42-MART-1 recognizing Melan-A/MART-1. Both protein antigens were detected in CRCL preparations (see ), but apparently the peptide epitopes were not transferred to the DC MHC class I presentation pathway. Similarly, negative results were seen when the maturation cocktail was omitted from the DC cultures (data not shown).
Figure 3. Melanoma-derived CRCL do not transfer tyrosinase or Melan-A/MART-1 antigens endogenously expressed by melanoma cells to human DC for MHC class I-restricted CTL recognition. (a, b) Monocyte-derived i-DC were pulsed with titered amounts of CRCL from A375-MEL (antigen-negative) or 624.38-MEL (antigen-positive) for 16 h and then matured with a cocktail containing IL1-ß, IL-6, TNF-α and PGE2 for additional 24 h. Antigen-specific CTL (TyrF8 or A42-MART-1) were added to DC at a DC : T cell ratio of 1 : 1. After 24 h, supernatants were harvested and IFN-γ levels were analysed by ELISA. (c) To control the DC capacity for CTL activation and to detect inhibitory effects of CRCL, 10 µg/ml of tyrosinase or Melan-A/MART-1 peptides (Tyr368–376 or Melan-A/MART-126–35, respectively) were added to DC with or without A375-MEL-derived CRCL (250 µg/ml). One representative experiment of five is shown for each CTL (a and b). Controls (c) are shown exemplarily for TyrF8 CTL. SD are derived from triplicate values.
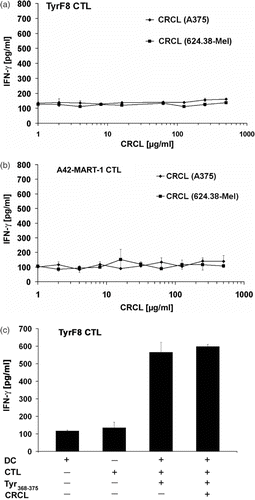
To exclude an inhibitory effect of CRCL on the ability of DC to present antigen for CTL stimulation, we added the tyrosinase-specific epitopes with or without CRCL to the DC/CTL co-cultures. As shown in , tyrosinase-specific CTL stimulation induced by exogenous peptide loading was identical in the presence or absence of CRCL excluding a general inhibitory activity of CRCL on DC antigen presentation or CTL stimulation. Identical results were obtained with the Melan-A/MART-1-specific epitope and the corresponding specific CTL A42-MART-1 (data not shown).
The negative outcome of cross-presentation of endogenously expressed melanoma associated antigens was seen independently of the melanoma cell line used for CRCL preparation or the detergent or the endotoxin removal step used for CRCL purification (data not shown).
Higher endogenous levels of Hsp70 or tyrosinase in human melanoma cell lines do not lead to CRCL with capacity to transfer endogenously expressed melanoma antigens to DC for CTL recognition
We previously demonstrated that Hsp70 isolated from 624.38-MEL cells under Hsp70-substrate-complex preserving conditions transferred tyrosinase to the cross-presentation pathway of DC Citation[8]. Because CRCL are a complex mixture of proteins (see ), we reasoned that Hsp70-tyrosinase-complexes, which should be capable to induce cross-presentation, may not be present in the CRCL preparation in sufficient amounts to achieve detectable cross-presentation by DC.
To generate a more favorable Hsp70 presence within the CRCL, we increased the Hsp70 level in 624.38-MEL cells by sublethal heat shock treatment (41.8°C for 2 h). As seen in , the amount of Hsp70 was significantly higher in heated melanoma cells while the Hsc70 protein level remained unchanged. The heat shock treatment did not alter the intracellular protein levels of tyrosinase and Melan-A/MART-1. CRCL from heat-treated 624.38-MEL cells, CRCL(624.38 + HS), had an estimated 2–3 fold higher Hsp70 content than CRCL of untreated cells, CRCL(624.38 − HS), reflecting the amount of Hsp70 in the 624.38-MEL cells (). The amount of Hsp90, another heat-inducible chaperone, was slightly increased in CRCL(624.38 + HS), while other not heat-inducible chaperones, such as Hsc70, Grp94/gp96 and calreticulin, were similar in CRCL(624.38 + HS) and CRCL(624.38 − HS) ().
When tested for their cross-presentation capacity, CRCL(624.38 + HS), despite higher Hsp70 content, were still unable to stimulate cross-presentation ().
Figure 4. Inability to cross-present endogenous melanoma-associated antigens is independent of endogenous levels of Hsp70 or tyrosinase. (a) CRCL from Hsp70-enriched melanoma cells fail to cross-present endogenous tyrosinase. Monocyte-derived i-DC were incubated with titered concentrations of CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS), CRCL(624.38 − HS), or CRCL(A375), respectively) for 2 h. A42-MART-1 CTL were added to yield a DC to T cell ratio of 1 : 1. After 24 h supernatants were harvested and IFN-γ levels were determined by ELISA. One of three representative experiments is shown. SD derived from triplicates. (b) Tyrosinase-overexpressing A375 melanoma cells. A375-MEL cells were infected with MVA-hTyr (Modified Vaccinia Virus Ankara containing the cDNA of the human tyrosinase gene) using a MOI of 5 U/ml. Cells were harvested 18 h after infection and analysed for human tyrosinase by SDS-PAGE and Western blot in parallel with uninfected A375-MEL and 624.38-MEL cells, which express the tyrosinase endogenously. ß-actin and Hsp70/Hsc70 served as loading control. (c) CRCL from tyrosinase-overexpressing melanoma cells do not facilitate cross-presentation of endogenous tyrosinase. Monocyte-derived i-DC were pulsed with titrated amounts of CRCL(A375), CRCL(624.38) or CRCL(A375/MVA-hTyr) for 6 h. DC were then matured with a cocktail containing IL1-ß, IL-6, TNF-α and PGE2 for 18 h. TyrF8 CTL were added at a DC to T cell ratio of 1 : 1. After 24 h, supernatants were harvested and IFN-γ levels were determined by ELISA. One of three representative experiments is shown. SD derived from triplicates. (d) Indicated amounts of recombinant Hsp70 protein were mixed to 20 µg/ml CRCL(A375/MVA-hTyr) and co-incubated with DC for 2 h followed by the addition of tyrosinase-specific CTL TyrF8. After 24 h supernatants were harvested for IFN-γ measurements. Control incubation of DC with tyrosinase nonamer peptide (Tyr368-376) at 1, 5 and 50 µM yielded IFN-γ values of 70, 114 and 250 pg/ml attesting that the DC presentation and CTL response was functional. All values are derived from triplicate cultures.
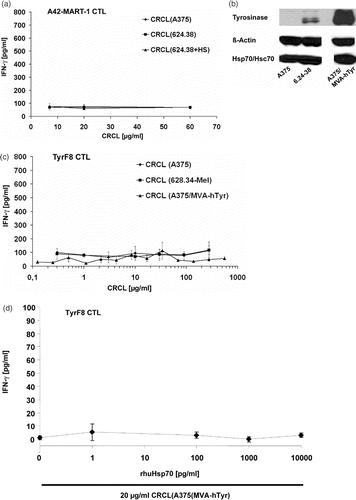
As another explanation for the failure to stimulate cross-presentation, we considered that the endogenous expression level of tyrosinase in melanoma cell lines could be too low to form sufficient Hsp70-complexes when distributed over multiple chaperones. To enhance the tyrosinase level in the melanoma cells, we induced overexpression using the modified vaccinia virus ankara (MVA) system. Infection of A375-MEL cells with recombinant MVA containing the cDNA of the human tyrosinase gene (MVA-hTyr) resulted in much higher intracellular tyrosinase levels than 624.38-MEL cells, which express the tyrosinase naturally (). Hsp70/Hsc70 protein levels were similar in infected and not infected A375-MEL cells (). Full-length tyrosinase protein was present in the CRCL of infected cells in similar amount as in CRCL from 624.38-MEL cells (data not shown). However, one has to keep in mind that the amount of full-length antigenic proteins was found not to be relevant for successful cross-presentation. Rather, protein fragments chaperoned by heat shock proteins were found to be the necessary and sufficient source of antigen for cross-presentation and priming of CD8+ T cell responses Citation[13]. When assessed for cross-presentation, the CRCL(A375/MVA-hTyr) still were unable to induce cross-presentation and TyrF8 CTL stimulation ().
Finally, we titered functionally active recombinant human Hsp70 to the CRCL(A375/MVA-hTyr) to yield improved Hsp70 chaperone concentrations. Yet again we failed to induce tyrosinase cross-presentation ().
Human melanoma cell line-derived CRCL do not change functional characteristics of i-DC, including phenotypic markers, IL-12 secretion, MLR activity and antigen uptake
Human i-DC were cultured with CRCL(624.38) or CRCL(A375) and 24 h later they were analysed for surface marker expression and IL-12 secretion. CRCL had no effect on the expression of CD83, CD80, CD86, CD40, HLA-DR and CD209 on human i-DC (). Furthermore, CRCL did not stimulate IL-12 secretion of i-DC () or change the capacity of i-DC to stimulate allogeneic T cell proliferation in a mixed lymphocyte reaction (MLR) (). Additionally, CRCL did not change the ability of i-DC for macropinocytosis or endocytosis as assessed by the incubation of treated DC with FITC-Albumin or FITC-Dextran (). LPS-treatment on the other hand strongly decreased the capacity of DC for macropinocytosis or endocytosis. These results are consistent with the observations that LPS induces characteristics associated with DC maturation while CRCL treatment did not.
Figure 5. Human melanoma-derived CRCL do not change IL-12 secretion, MLR activity and uptake characteristics of i-DC. (a) Monocyte-derived i-DC (d7) were incubated with 40 µg/ml CRCL(624.38) for 24 h (uptake assay, MLR) or for 48 h (IL-12 production). Untreated and LPS (1 µg/ml)-treated cells served as controls. The IL-12p40-content of DC culture supernatants was determined by ELISA. One representative experiment of five is shown. (b) For MLR, untreated and CRCL-treated i-DC were harvested, irradiated (40 Gy) and co-cultured with allogeneic T cells at indicated ratios for 6 days. T cell proliferation was assessed by measuring the uptake of [3H]-thymidine (1 µCi/well [0.037 MBq]) during the last 24 h of culture. The values represent the mean of triplicate samples ± SD of one experiment representative of three. (c) For uptake assays, DC were harvested 24 h after treatment. Macropinocytosis was assessed using FITC-conjugated albumin at a final concentration of 0.5 mg/ml for 30 min. (d) FITC-conjugated Dextran at a final concentration of 10 µg/ml for 3.5 h was used to determine endocytosis. DC were analysed by flow cytometry. The bars represent the difference in MFI of FITC-Albumin or FITC-Dextran (FL1) obtained by subtracting the background MFI of cells incubated at 0°C from the MFI of cells incubated at 37°C. One representative experiment of three is shown respectively (c, d).
![Figure 5. Human melanoma-derived CRCL do not change IL-12 secretion, MLR activity and uptake characteristics of i-DC. (a) Monocyte-derived i-DC (d7) were incubated with 40 µg/ml CRCL(624.38) for 24 h (uptake assay, MLR) or for 48 h (IL-12 production). Untreated and LPS (1 µg/ml)-treated cells served as controls. The IL-12p40-content of DC culture supernatants was determined by ELISA. One representative experiment of five is shown. (b) For MLR, untreated and CRCL-treated i-DC were harvested, irradiated (40 Gy) and co-cultured with allogeneic T cells at indicated ratios for 6 days. T cell proliferation was assessed by measuring the uptake of [3H]-thymidine (1 µCi/well [0.037 MBq]) during the last 24 h of culture. The values represent the mean of triplicate samples ± SD of one experiment representative of three. (c) For uptake assays, DC were harvested 24 h after treatment. Macropinocytosis was assessed using FITC-conjugated albumin at a final concentration of 0.5 mg/ml for 30 min. (d) FITC-conjugated Dextran at a final concentration of 10 µg/ml for 3.5 h was used to determine endocytosis. DC were analysed by flow cytometry. The bars represent the difference in MFI of FITC-Albumin or FITC-Dextran (FL1) obtained by subtracting the background MFI of cells incubated at 0°C from the MFI of cells incubated at 37°C. One representative experiment of three is shown respectively (c, d).](/cms/asset/6d3e3498-452e-4675-91e8-f9679b6f4ac5/ihyt_a_321505_f0005_b.gif)
Table I. Human melanoma-derived CRCL do not change phenotypic markers of i-DC.
Similar results were obtained with CRCL concentrations ranging from 20–200 µg/ml, different incubation times ranging from 8–48 h and CRCL derived from different melanoma cell lines (624.38-MEL cells without/with heat-treatment, A375-MEL cells without/with MVA-hTyr infection) (data not shown).
Discussion
Since the early 1980s chaperone proteins, such as Grp94/gp96, Hsp70 and calreticulin, have been recognized as tools for antigen-specific vaccination, as they were found to induce antigen-specific CD8+ T cell responses and innate immune activation, in particular maturation of DC Citation[10]. Recently, a FS-IEF method was established to generate chaperone-enriched cell lysates Citation[17]. For clinical immunotherapy, this method is an attractive approach, since it yields high amounts of protein to prepare material for multiple vaccination rounds from limited tumor sources. Furthermore, the short experimental preparation time will allow starting patient treatment quickly after surgery. Similar to the single chaperone vaccines there would be no need to identify specific antigens in the human tumor material. Based on the knowledge that different chaperones can carry distinct antigenic protein fragments Citation[36] it is also expected that CRCL should contain a broader spectrum of antigenic ligands compared to single chaperone vaccines. Indeed, mouse models support the contention that CRCL have superior anti-tumor activity than single chaperone vaccines Citation[25]. To date, only one published study assessed the CRCL approach in a human ovarian cancer model Citation[26].
We further investigated the capacity of human cell-derived CRCL to deliver endogenously expressed tumor antigens to DC for CTL recognition. We utilized a human melanoma system for which we had previously demonstrated that isolated Hsp70/Hsc70 transfer the endogenously expressed melanoma-associated antigen tyrosinase to DC for MHC class I-restricted presentation and CTL recognition Citation[8]. Based on this observation and the literature on CRCL, we hypothesized that CRCL prepared from these melanoma cell lines should also transfer tyrosinase and other melanoma cell-expressed antigens to DC, possibly with higher efficacy.
To utilize the CRCL together with human DC and CTL the published purification protocol required some modification. Because residual detergent regularly interfered with DC viability the TX-100 was exchanged for CHAPS which could be removed sufficiently using dialysis. Endotoxins were depleted to prevent chaperone-unrelated DC maturation and a filtration step was introduced to provide sterility to the preparation. This filtration step had the added consequence that precipitates, which regularly occurred during the purification procedure, were removed. Using the modified procedure, we reproducibly obtained CRCL preparations with undetectable detergent and low endotoxin content compatible with human in vitro DC/CTL co-cultures. Using this modified purification procedure CRCL isolated from different melanoma lines were highly enriched in the chaperones Hsp70 and Hsc70, and contained Grp94/gp96, Hsp90 and calreticulin.
The endotoxin-low CRCL had no detectable innate immune signaling function, judged by unaltered DC surface markers, unchanged cytokine profile and protein uptake capacity as well as unchanged capacity of CRCL-exposed DC to stimulate allogeneic T cell proliferation. These observations are in contrast to other reports Citation[20], Citation[22]. These studies mainly used murine tumor-derived CRCL in the context of murine bone marrow-derived DC, which may explain the differences. Support for the hypothesis that species-specific response patterns might exist can be derived from the single study using human material. In this study CRCL from ovarian tumor also did not induce changes in surface markers of human DC. Yet, the CRCL-exposed DC secreted IL-12 and had better allostimulatory capacity Citation[26]. Based on our data, it cannot be excluded that our CRCL may have DC-activating capacities that are not readily measurable among the current repertoire of specific cell-surface and secretion markers.
When we tested our CRCL for their capacity to transfer endogenously expressed tyrosinase or Melan-A/MART-1 antigens to DC for cross-presentation, we were unable to detect antigen transfer to the cross-presentation pathway. The failure of CRCL to cross-present endogenous tyrosinase was particularly surprising as we had previously isolated Hsp70-peptide complexes (HSP70-PC) from this melanoma system and achieved tyrosinase transfer and cross-presentation with as little 50 ng/ml of purified single chaperone HSP70-PC ( in Citation[8]). We estimated that Hsp70 represents about 5–10% of the total protein in the CRCL preparations. Therefore the tested range of CRCL (100 ng/ml–200 µg/ml) should have covered the ‘active’ Hsp70 concentration.
According to the biochemical analysis, the CRCL preparations contained Hsp70, Hsc70 and tyrosinase and Melan-A/MART-1 antigens. A previous study did already suggest that the detection of full-length antigenic proteins is not predictive for successful cross-presentation Citation[13]. Rather, protein fragments chaperoned by heat shock proteins were found to be the necessary and sufficient source of antigen for cross-presentation and priming of CD8+ T cell responses. Since this study clearly demonstrated that chaperone-antigen complexes are essential for chaperone-mediated cross-presentation, one explanation for the failure of our CRCL to cross-present endogenous antigens could be that the melanoma-derived CRCL did not contain enough chaperone-tyrosinase or chaperone-Melan-A/MART-1 complexes. Indeed, the CRCL procedure involves relatively strong denaturation steps and it is not clear which or how many antigen-chaperone complexes are maintained during the denaturing purification procedure, nor do we understand the nature of the possible re-association of antigen-chaperone complexes that may occur after returning to native buffer conditions Citation[17], Citation[22], Citation[37].
We considered that the amount of Hsp70 or Hsc70 within the complex mixtures of other proteins or the amount of endogenous antigens, in particular if it is distributed over multiple chaperones, could be too low to allow detectable cross-presentation by DC. Additionally, in melanoma-derived CRCL, the ratio between antigen-specific chaperone complexes/other proteins and unrelated complexes/proteins could be unfavorable for cross-presentation. We addressed these issues by increasing the amount of tyrosinase and Hsp70, which we knew from previous experiments, is capable of tyrosinase cross-presentation Citation[8]. Yet, even CRCL derived from Hsp70- or tyrosinase-overexpressing cells provided no evidence that endogenous antigen transfer did occur.
We also considered that the denaturation steps of the CRCL preparations resulted in defective chaperone proteins. However, when mixing Hsp70-binding tyrosinase peptides into melanoma cell-derived CRCL, CRCL enhanced the transfer of the exogenous peptides to the DC resulting in better cross-presentation and tyrosinase-specific T cell stimulation compared to DC pulsed with peptides in the absence of CRCL. The CRCL mediated enhancement was comparable to that of recombinant Hsp70 which was demonstrated previously Citation[16]. Thus, human melanoma-derived CRCL contain chaperones which are active as delivery vehicles for exogenous peptides. This observation complements recent results which had used murine liver-derived CRCL mixed with antigenic epitopes to document the induction of peptide-specific immunity Citation[37]. In our system we used the pep70-tyrosinase hybrid peptide, which contained the nonameric tyrosinase epitope and the octameric sequence HWDFAWPW, which is known to bind with high affinity to the endoplasmic reticulum resident Hsp70 homolog (BiP) Citation[36], Citation[38]. In line with targeting the peptide to form complexes with Hsp70 we observed that Hsp70-enriched CRCL, isolated from heat-stressed cells, had superior activity to cross-present pep70-peptides compared to CRCL from non-stressed cells.
In summary, the failure of CRCL from human melanoma cells to transfer and cross-present melanoma-associated antigens (tyrosinase and Melan-A/MART-1) in vitro suggests that the CRCL approach may have limitations regarding the antigenic spectrum which can be chaperoned for cross-presentation and T cell stimulation. Regardless, CRCL preparations can be exploited as delivery vehicle for the cross-presentation of exogenously added antigenic peptides.
Heat treatment was found to be an efficient and easy way to yield CRCL with superior capacity to cross-present exogenous antigenic sequences, particularly those peptides containing a targeting sequence for Hsp70. CRCL, generated with Hsp70-enriched CRCL mixed with Hsp70-binding peptides, may represent a new and superior vaccine to redirect T cell immunity to epitopes which would classically be ignored by T cells. The capacity of chaperones to catch exogenous antigens provides a working hypothesis for therapies, like clinical hyperthermia, which cause Hsp70 upregulation and release through induced tumor damage. In line with the in vitro observations, locally released chaperones could act as scavengers to ‘capture’ and cross-present antigens for immune activation in vivo Citation[6]. Thus, chaperone-based vaccines may be able to provide the benefit of the downstream effects of hyperthermia, but may also be personalized or intensified for additional immune advantage.
Acknowledgements
Elke Bleifuss and Henriette Bendz contributed equally to this work. Rolf-Dieter Issels and Elfriede Noessner are equal senior authors of this work. We would like to thank Dr Nicchitta, (Duke University Medical Center, Durham, NC, USA) for providing the anti-Grp94/gp96 antiserum.
The work was supported by grants SFB 455/project C5 (to H.B., E.N., RD.I.), SFB 456/project B7 (to I.D.), Duke University Brain Cancer SPORE Career Development Award (SPORE 5 P50 CA108786-02) and the Southeast Brain Tumor Foundation (both to M.W.G.).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci 2005; 62: 670–684
- Young JC, Barral JM, Ulrich Hartl F. More than folding: Localized functions of cytosolic chaperones. Trends Biochem Sci 2003; 28: 541–547
- Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res 2001; 61: 222–227
- Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: Biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother 2004; 53: 227–233
- Hoos A, Levey DL. Vaccination with heat shock protein-peptide complexes: From basic science to clinical applications. Expert Rev Vaccines 2003; 2: 369–379
- Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, Gastpar R, Issels RD. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int J Hyperthermia 2002; 18: 563–575
- Nair S, Wearsch PA, Mitchell DA, Wassenberg JJ, Gilboa E, Nicchitta CV. Calreticulin displays in vivo peptide-binding activity and can elicit CTL responses against bound peptides. J Immunol 1999; 162: 6426–6432
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol 2002; 169: 5424–5432
- Parmiani G, Testori A, Maio M, Castelli C, Rivoltini L, Pilla L, Belli F, Mazzaferro V, Coppa J, Patuzzo R, et al. Heat shock proteins and their use as anticancer vaccines. Clin Cancer Res 2004; 10: 8142–8146
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2002; 2: 185–194
- Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med 1993; 178: 1391–1396
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol 1994; 152: 5398–5403
- Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol 2005; 6: 593–599
- Breloer M, Marti T, Fleischer B, von Bonin A. Isolation of processed, H-2Kb-binding ovalbumin-derived peptides associated with the stress proteins HSP70 and gp96. Eur J Immunol 1998; 28: 1016–1021
- Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol 2002; 14: 45–51
- Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 2007; 282: 31688–31702
- Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother 2000; 49: 476–484
- Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res 2000; 6: 909–915
- Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003; 52: 226–234
- Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood 2003; 101: 4485–4491
- Zeng Y, Graner MW, Feng H, Li G, Katsanis E. Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat bcr-abl+ murine leukemia. Int J Cancer 2004; 110: 251–259
- Zeng Y, Graner MW, Thompson S, Marron M, Katsanis E. Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells. Blood 2005; 105: 2016–2022
- Zeng Y, Chen X, Larmonier N, Larmonier C, Li G, Sepassi M, Marron M, Andreansky S, Katsanis E. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer 2006; 119: 2624–2631
- Larmonier N, Cantrell J, Lacasse C, Li G, Janikashvili N, Situ E, Sepassi M, Andreansky S, Katsanis E. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol Jan 3, 2008, [Epub ahead of print]
- Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother 2006; 55: 329–338
- Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, Andreansky S, Brewer MA, Katsanis E. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol 2007; 148: 136–145
- Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 1994; 91: 3515–3519
- Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM, Melief CJ, Oseroff C, Yuan L, Ruppert J, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol 1994; 153: 5586–5592
- Riker AI, Kammula US, Panelli MC, Wang E, Ohnmacht GA, Steinberg SM, Rosenberg SA, Marincola FM. Threshold levels of gene expression of the melanoma antigen gp100 correlate with tumor cell recognition by cytotoxic T lymphocytes. Int J Cancer 2000; 86: 818–826
- Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res 1995; 55: 3149–3157
- Visseren MJ, van Elsas A, van der Voort EI, Ressing ME, Kast WM, Schrier PI, Melief CJ. CTL specific for the tyrosinase autoantigen can be induced from healthy donor blood to lyse melanoma cells. J Immunol 1995; 154: 3991–3998
- Milani V, Frankenberger B, Heinz O, Brandl A, Ruhland S, Issels RD, Noessner E. Melanoma-associated antigen tyrosinase but not Melan-A/MART-1 expression and presentation dissociate during the heat shock response. Int Immunol 2005; 17: 257–268
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 1994; 180: 347–352
- Drexler I, Antunes E, Schmitz M, Wolfel T, Huber C, Erfle V, Rieber P, Theobald M, Sutter G. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: Induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res 1999; 59: 4955–4963
- Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature 1991; 353: 726–730
- Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med 2000; 191: 1957–1964
- Kislin KL, Marron MT, Li G, Graner MW, Katsanis E. Chaperone-rich cell lysate embedded with BCR-ABL peptide demonstrates enhanced anti-tumor activity against a murine BCR-ABL positive leukemia. Faseb J 2007; 21: 2173–2184
- Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, Rothman JE, Houghton AN. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA 2000; 97: 3485–3490
