Abstract
Purpose: We have recently proposed utilizing alkali metal as powerful self heating seeds to thermally ablate tumor tissues. This study is dedicated to disclosing some fundamental mechanisms related to the anti-tumor effects of sodium and its controllability as an economic, safe and efficient thermochemical agent in targeted tumor treatment.
Materials and methods: EMT6 cell line was incubated under four situations: no treatment, NaOH solution treatment, hyperthermia treatment and a combined NaOH solution and hyperthermia treatment. Cell cytotoxicity was measured by a CASY cell counter and analyzer system. Breast EMT6 tumors in mice were subjected to treatment by NaOH solution, hyperthermia and encapsulated sodium. The changes of tumor volumes were continuously measured for 12 days after treatment. Tumors of another four mice were harvested immediately after treatment to assess viability.
Results: In vitro cell experiments suggested that thermal effect combined with chemical treatment produced a more significant cell cytotoxicity compared to thermal or chemical treatment alone. Encapsulated sodium demonstrated a slower and more continuous heat release than bare sodium. The sodium treatment produced a dramatic regression of tumors that lasted throughout the 12 days of the study. Histological sections showed complete necrosis in sodium-treated tumors, whereas control tumors and heat-treated tumors remained viable, and NaOH-treated tumors showed partial destruction.
Conclusions: The results suggest that sodium allows a minimally invasive treatment which could produce both thermal and chemical lethal injury to tumors. Using sodium to ablate target tumors is potentially an effective, safe and low cost way to treat malignant tumors.
Introduction
Hyperthermia is being gradually introduced in the treatment of prostate cancer Citation[1], Citation[2], breast cancer Citation[3], and liver tumors Citation[4–7] etc. Up to now, various thermal ablation apparatuses have been successfully developed. Some typical heat generation principles generally include high intensity focused ultrasound Citation[8], radiofrequency Citation[9], microwave Citation[10], laser Citation[11] etc. However, many external heating types of equipment often cause unnecessary burn to healthy tissues during the delivery of energy from outside to the deep target Citation[12]. They are also usually expensive and complicated to operate, which limits the clinical applications of these methods.
To resolve the problems as stated above, we recently proposed a new conceptual minimally invasive tumor hyperthermia therapy using alkali metals as powerful self heating seeds Citation[13] where the target tissue is treated with a direct transplantation of an extremely small amount of sodium. Reaction between sodium and interstitial water in the target tumor Citation[14] produces a fairly strong exothermic chemical process (see equation (1)). During the reaction, the temperature at the target area would exceed the tumor tolerance threshold of around 42 ∼ 43°C and can be sustained over a certain period of time, which would induce tumor coagulation necrosis. Simultaneously, a lot of OH− is generated, and the alkali environment thus formed would cause the membrane of the tumor cells to collapse. For the detailed principle of the sodium destruction of tissue please refer to reference Citation[13].(1) The sodium ablation method has been subjected to a preliminary proof-of-concept test before. Differing from conventional hyperthermia therapy, such treatment makes full use of the water content existing throughout various biological organisms. In particular, it has a highly localized self-heating feature which is rather safe and beneficial for targeted tumor ablation, since heat is released only at the target.
The aim of this study is to disclose some fundamental mechanisms for the procedure described above and to characterize the destructive effect of sodium on tumor cells and the pathological changes of tumor tissue it causes.
Materials and methods
Cell line
EMT6 breast tumor cell line (bought from Cancer Institute & Hospital, the Chinese Academy of Medical Sciences, Beijing, China) was cultured in RPMI 1640 medium with 10% fetal calf serum plus penicillin/streptomycin in a humidified 5% CO2-95% air atmosphere at 37°C. For cytotoxicity studies with NaOH solution, hyperthermia, and NaOH solution in combination with hyperthermia, respectively, cells were grown in 30-mm culture dishes.
Assessment of cytotoxicity
The EMT6 cell line was grown to near confluence in 30-mm culture dishes. Each dish had 3 ml media. For the NaOH solution treatment group, 400 µl 0.1 mol/l NaOH solution (pH = 13) was added to the media and the culture dishes were placed in a super clean bench for 1 h. For the heating treatment group, the bottom of the culture dishes was immersed in a 43 ± 0.5°C water bath for 1 h. For the combined treatment group, after 400 µl 0.1 mol/l NaOH solution was added to the media, the bottom of the culture dishes were immersed in a water bath for 1 h. For the control group, no treatment was done. After treatment, media was aspirated and fresh media was added to the cells. They were then incubated for an additional 18 h at 37°C. After 18 h, the media was aspirated, and the cells were washed with PBS, and the cells were harvested from the culture dishes by trypsinization. The cell pellet was re-suspended during gentle vortexing. Cell viability was assessed by adding 100 ul re-suspended cell to CASY cell counter system (Model TTC, CASY® Technology, Germany), and the cell diameter distribution and viability were assessed with its analyzer system. All experiments were performed in triplicate.
Package of sodium cylinder
Sodium cylinders were encapsulated by gelatin capsules for in vivo experiments. The inner diameter and the length of the capsule cap were 2.5 mm and 6 mm, respectively. The inner diameter and the length of the capsule body were 2.2 mm and 6 mm, respectively. The length of the locked capsule was 10 mm.
Thermal effect measurement of sodium capsules
The same quantity (0.01 ± 0.005 g) for bare sodium and encapsulated sodium was inserted into in vitro pork tissue respectively. A thermocouple was mounted on the external wall of the bare sodium and the sodium capsule, respectively. Their thermal responses were recorded and compared later. About 0.03 ± 0.005 g encapsulated sodium was applied in vivo to the tumor. A thermocouple was inserted into the tumor tissue attached to the sodium capsule.
The temperature sensors used in the experiments were the T-type copper-constantan thermocouples which were connected to an Agilent 34970 multimeter, USA. To prevent the chemical corrosion of sodium and water reactant, the copper-constantan thermocouple was packaged by a capillary stainless steel tube with 1.4 mm outer diameter and 0.8 mm inner diameter, in which one end of the capillary tube and the thermocouple head were sintered together. A 0.1 mm diameter copper–constantan thermocouple was chosen due to the fact that the thermocouple in the hyperthermia group was located in the heating needle. The thermocouple was calibrated in advance and an accuracy of ± 0.1°C was obtained. During the entire period of the experiment and the subsequent several minutes after treatment, the temperature was continuously recorded once every second.
Tumor-bearing animal model
The animal experiments performed complied with the International Laboratory Animals Care Convention. EMT6 tumor cells were grown in RPMI 1640 medium, the cells were harvested, washed, and re-suspended in sterile PBS. About 0.2 ml of the EMT6 tumor solution was injected into oxter of 2 female BALB/c mice (6 ∼ 8 weeks). When tumors in the oxter of the donor mice reached their largest dimension of 1.0–1.5 cm, the tumors were harvested under sterile conditions and cut into 1 mm cubes. After grinding and filtering, the tumor cells were re-suspended. About 0.2 ml of suspension was injected into the right oxter of 8 female BALB/c mice, weighing 15–20 g. Ten days later, these 8 mice with tumors ranging from 1.0 ∼ 1.5 cm were classified randomly into four groups. Two mice in the control group were given no therapy. Another two mice underwent an intratumoral injection of 1.5 ml NaOH solution (1 mol/l, pH = 14), which was injected into the center of the tumor. An additional two mice underwent sodium treatment, in which 0.03 ± 0.005 g encapsulated sodium was inserted into the tumor center along the horizontal direction. The temperature rise during reaction was recorded. In a final group, hyperthermia was induced by a 2.5 mm diameter electrical heating needle devised in our lab and the temperature and the time applied were the same as the thermal dosage in the sodium treatment group. The temperature of the electrical heating stick was controlled via input voltage and current.
Tumor size in each group was measured daily after the treatment according to the Carlsson formula Citation[15]: V = 1/2 a × b2, where a is the longest diameter (mm) and b is the shortest diameter (mm).
Histological sections
Tumor tissues at the treatment site of the remaining 4 mice were sacrificed and histologically examined by light microscopy immediately after treatment. Tumors were cut into 5μm frozen sections and prepared for histological assessment with hematoxylin and eosin staining.
Statistical analysis
Differences between experimental groups (with in vitro cell experiments performed in triplicate) were determined by t-test. A significant difference between groups was defined as p < 0.05.
Results
Thermal effect of sodium capsules on in vitro tissue
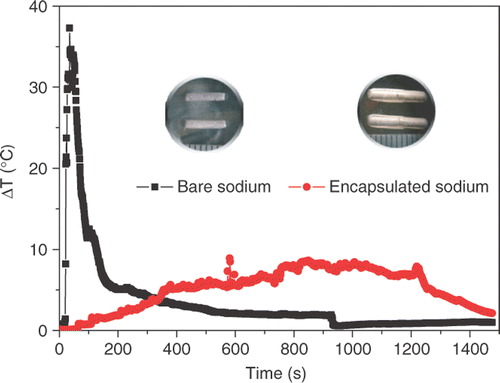
Sodium capsules were chosen to conduct tumor ablation due to the fact that bare sodium dissolved quickly in in vitro pork, while sodium capsules appeared slow. This phenomenon can also be revealed through comparison of the temperature responses between bare and encapsulated sodium in in vitro tissue (). The temperature rise induced by bare sodium was quickly elevated to 35°C and then decreased via a rapid rate. The temperature rise of the sodium capsules case was relatively gentle, staying at 5 ∼ 8°C from 400 s to 1200 s. Clearly, sodium capsules play an important role in controllable heat release during reaction with water.
Cytotoxicity comparison of cancer cells in vitro
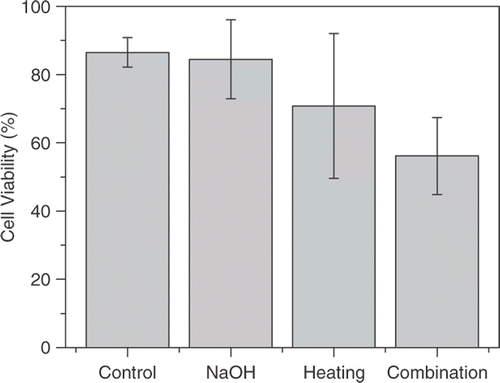
We tested the use of NaOH solution, heating and NaOH solution combined with heating to produce cancer cell cytotoxicity on breast tumor cell line. The CASY analyzer system revealed that cell viability for the control group, the 0.1 mol/l NaOH solution treatment group, the heating group and the NaOH in combination with the heating group was (86.5 ± 4.28%), (84.47 ± 11.55%), (70.8 ± 21.24%) and (56.17 ± 11.29%) respectively (). No significant difference in cytotoxicity was observed between the NaOH group and the control group (p > 0.05). The same is true for the heating group and the control group (p > 0.05). However, cell viability that had been treated with 0.1 mol/l NaOH solution in a 43°C water bath for 1 h was significantly lower (p < 0.05 compared to the control group).
Figure 2. Cell viability of the control group, NaOH group, heating group and combination group in in vitro EMT6 breast cells, respectively. The values represent the mean ± standard error of the mean for experiments performed in triplicate. The combination group show significantly different viability compared to the control group, while the NaOH group and the heating group do not.
Sodium-induced thermal effect on malignant breast tumor in vivo
BALB/c female mice bearing EMT6 tumors ranging in size from 1.0–1.5 cm underwent a direct intratumoral NaOH solution treatment, sodium capsule treatment and heating needle treatment, respectively. Under anaesthesia, the mice body temperature fell below normal temperature. In order to maintain their temperature around normal body temperature, the subject mice were placed on a 37°C thermostatic plate. The experiments were started after the mice and environmental air (22°C) had reached heat balance.
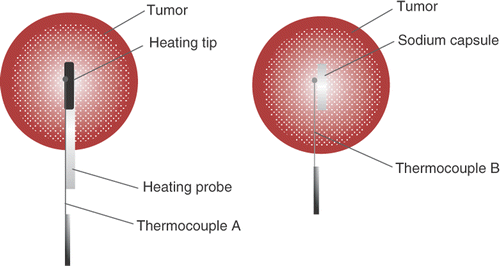
The thermocouple measurement positions during the experiment are schematically depicted by the bold solid point in . The thermocouple A was mounted on the internal wall of the heating probe and the distance from the tip was about 2 mm. The pin-like thermocouple B is inserted into the tissue along the external surface of the sodium capsule, the distance from the capsule top was about 2 mm.
Figure 3. Schematic for positioning the thermocouple in the heat treatment group and the sodium treatment group, respectively.
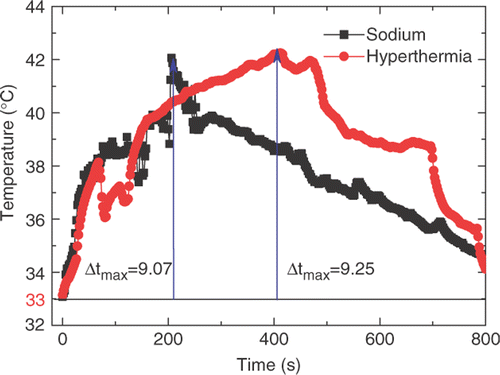
depicts temperature rises of the hyperthermia and sodium treatment groups, respectively. The initial body temperature of the mice was 33°C. Then a gradual increase to about 42°C was obtained for these two groups, followed by a slow decrease until coming back to the initial temperature. The temperature in the heating group was slightly higher. The thermocouple in the heating group was fixed at the internal wall of the heating needle while the thermocouple in the sodium group was placed between the capsule and the tissue.
Sodium-induced anti-effect on malignant breast tumor in vivo
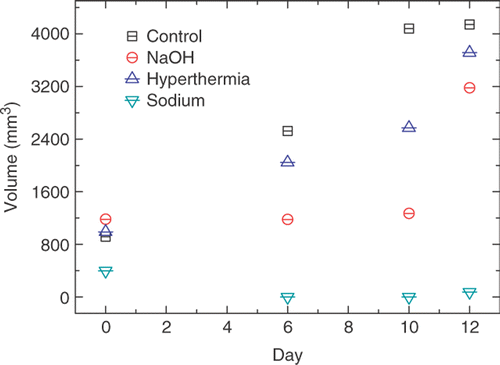
Tumor volume for each group was recorded daily after the treatment, and the results were depicted in . Strong necrosis of tumor occurred in NaOH and sodium treatment group after treatment. No significant necrosis occurred in hyperthermia group. On the sixth day and the tenth day, the tumor had disappeared in the sodium group.
Figure 5. Tumor volume change in four groups during the 12 days after treatment. Mice in the control group, NaOH group, heating group and sodium group underwent no treatment, an intratumoral injection of NaOH solution, sodium capsule therapy and heating needle therapy. There was a notable regression in the sodium group compared to the control group, NaOH group and heating group, respectively.
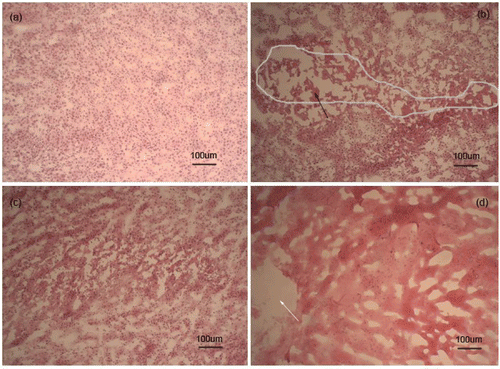
Histological evaluation
Untreated tumors showed an infiltrating growth trend (a). There was a partial destruction area in the NaOH solution therapy, with the grey line demonstrating necrotic tumor cells (b). In the hyperthermia group, tumors around the heating site showed no evidence of tumor cell death in the histological sections (c). Sodium-treated tumors showed that the cell structure completely disappeared and its nucleus was dissolved around the injection zone (d).
Figure 6. Hematoxylin/eosin staining of EMT6 tumor immediately after treatment for control group and three treatment groups, respectively. (a) The control group showed an infiltrating growth trend. (b) The injecting NaOH solution group with the black arrow indicating necrotic tumor cells. (c) The hyperthermia group showing no significant damage. (d) The sodium group with the white arrow demonstrating the injection zone of sodium and cells showing necrosis around the injection zone.
Discussion
From a clinical perspective, tumor cells generally are more sensitive to heat-induced damage and apoptosis than normal cells Citation[16]. This advantage has been exploited in conducting tumor hyperthermia therapy. The chemical mechanism of sodium treatment is similar to cathode reaction (see equation (2)) in an electrochemical therapy. At the cathode, sodium hydroxide and hydrogen gas are released (see equation (2)). The tissue proteins become denatured and the cell structure collapses and eventually dies off due to extreme pH values Citation[17]. Nowadays, electrochemical therapy has been adopted in clinical practice in China to treat malignant tumors Citation[18]. However, there are fewer thermal effects during electrochemical therapy and the treatment generally lasts about 1 ∼ 3 h. It is reported that the anti-tumor effect of electrochemical therapy in combination with an electrothermal needle is more effective than that of electrochemical treatment or hyperthermia alone Citation[19].(2) In this study, the fact that heated tumor cells in an alkaline environment can generate significant damage also proves that the thermochemical effect is not a simple superposition of thermal effect and chemical effect.
The alkali metal-based ablation therapy has both thermal and chemical effects during treatment which could result in significant tumor necrosis. Here, the effect of sodium therapy was compared with moderate temperature ablation. It demonstrates stronger damage than thermal ablation alone. More important still is the fact that no additional surgical equipment but only an extremely small amount of sodium is needed to achieve this powerful destruction.
However, safety should always be the first priority for patients and clinicians when performing such treatment in the near future. In this article, bare sodium was packed to ensure its operation safety and to avoid thermal damage to surrounding healthy tissue along the path of inserting the alkali metal. What is more, reaction between bare sodium and tissue is rather strong and rapid, while the sodium capsule provides a gentle heat release process.
Direct intratumoral injection of sodium capsules in vivo is a minimally invasive approach. It was observed that tumor damage district showed a complete necrosis around the sodium capsule insertion zone. Incomplete destruction of the malignant cells also is possible due to the fact that tumor shape is usually irregular and the sodium capsule does not possess cell-specific targeting function. Therefore, appropriate dosage and conformal treatment is needed to minimize damage to normal tissue while maximizing the thermochemically-induced cancer cell cytotoxicity.
Conclusion
The new finding that sodium is effective against tumor cells and tissues is encouraging. Thermochemical therapy has a better inhibitory effect on tumor growth compared to the growth inhibition rendered by thermal or chemical treatment alone. Sodium therapy produced localized tumor death that lasted throughout the 12-day study. Furthermore, histological examination revealed complete thermochemical destruction of tumor tissues, demonstrating that sodium could be used as a therapeutic agent to destroy malignant tumors.
However, using sodium as a thermochemical ablation agent is still at its early stage. Research efforts will be continued both in device improvement and thermal field analysis in the near future.
Acknowledgment
This research was supported by the NSFC Grants 50776097 and 50436030.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Madersbacher S, Pedevilla M, Vingers L, Susani M, Marberger M. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res 1995; 55: 3346–3351
- Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldöfner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 2006; 52: 1653–1661
- Hilger I, Hergt R, Kaiser W. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEEE Proc Nanobiotechnol 2005; 152: 33–39
- Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. Mr-guided laser-induced thermotherapy (litt) of liver tumours: Experimental and clinical data. Int J Hyperthermia 2004; 20: 713–724
- Liang P, Dong B, Yu X, Yu D, Cheng Z, Su L, Peng J, Nan Q, Wang H. Computer-aided dynamic simulation of microwave-induced thermal distribution in coagulation of liver cancer. IEEE Trans Biomed Eng 2001; 48: 821–829
- Rhim H, Dodd GD. Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound 1999; 27: 221–229
- Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000; 217: 119–126
- Curiel L, Chavrier F, Souchon R, Birer A, Chapelon JY. 1.5-d high intensity focused ultrasound array for non-invasive prostate cancer surgery. IEEE Trans Ultrason Ferr 2002; 49: 231–242
- McGhana JP, Dodd GD. Radiofrequency ablation of the liver: Current status. Am J Roentgenol 2001; 176: 3–16
- Orth K, Russ D, Durr J, Hibst R, Steiner R, Beger HG. Thermo-controlled device for inducing deep coagulation in the liver with the nd:Yag laser. Laser Surg Med 1998; 20: 149–156
- Sterzer F. Microwave medical devices. IEEE Microw Mag 2002; 3: 65–70
- Kato H, Koyama T, Nikawa Y, Saito M. Research and development of hyperthermia machines for present and future clinical needs. Int J Hyperthermia 1998; 14: 1–11
- Rao W, Liu J. Tumor thermal ablation therapy using alkali metals as powerful self heating seeds. Min Invas Ther 2008; 17: 43–49
- Gullino PM, Grantham FH, Smith SH. The interstitial water space of tumors. Cancer Res 1965; 25: 727–731
- Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas–an experimental study in rats. J Cancer Res Clin 1983; 105: 20–23
- Dewey WC. Arrhenius relationship from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Wemyss-Holden SA, Robertson GSM, Dennison AR, Hall PD, Fothergill JC, Jones B, Maddern GJ. Electrochemical lesions in the rat liver support its potential for treatment of liver tumors. J Surg Res 2000; 93: 55–62
- Nilsson E, von Euler H, Berendson J, Thorne A, Wersall P, Naslund I, Lagerstedt AS, Narfstrom K, Olsson JM. Electrochemical treatment of tumours. Bioelectrochemistry 2000; 51: 1–11
- Xie L, Sun CJ. A new local ablation for solid malignant tumours: Anti-tumour effect of hyperthermal and electrochemical therapy on transplantable mouse cancer. Int J Hyperthermia 2006; 22: 607–612