Abstract
In this study, the therapeutic effect and the induced anti-tumor immunity through the alternating cooling and heating was investigated using 4T1 murine mammary carcinoma, a common model of human metastatic breast cancer. While fifteen of seventeen regular mice were cured, primary recurrence and metastasis caused death of all the nude mice within one month after the same treatment. Histological analyses showed that viable cells existed in the tumor debris after the treatment, indicating that the direct killing effect was not the only therapeutic mechanism. Further investigation found rejection of tumor upon re-challenge, and anti-tumor immune response was studied. Stronger cytotoxicity T-lymphocyte (CTL) and Th1 cytokines response as well as infiltration of immunocytes were observed in the treated mice in comparison to those after the surgical resection. The results showed that the alternating cooling and heat could stimulate anti-tumor immunologic response in vivo and the underlying mechanisms will be further investigated in the near future.
Introduction
In 2007, over 175,000 cases of female breast cancer were estimated in the United States, making it the most dangerous malignant cancer in women Citation[1]. General lumpectomy plus radiation was the common treatment for breast cancer in clinic. But this treatment led to poor prognosis, especially in young women with positive nodes Citation[2],Citation[3]. It was shown that metastatic recurrence was the major cause of the death even after the primary tumor was successfully removed Citation[3]. More importantly, previous studies also showed that surgery somewhat disturbed the balance of tumor and host, inducing angiogenesis and proliferation of distant dormant micrometastases. Sharp decreases in levels of antiangiogenesis factors and excessive release of tumor growth factors after surgery were considered to be the main cause Citation[4],Citation[5]. Moreover, surgical stress and decrease in lymphocytes induced by radiation could cause suppression of immunity, thus exacerbating tumor metastasis Citation[6],Citation[7]. Systemic treatment of primary tumor with distant micrometastases still remains as a difficult problem to solve.
At present, minimally invasive cancer therapy such as in situ thermophysical treatment which destroys tumor tissue by either heating or freezing is gaining more interest in breast cancer therapy. Hyperthermia, one of the heating methods, destroys tumor cells as well as tumor vessels based on the fact that tumor cells and vessels are more susceptible to thermal cytotoxic effect (40–44°C) in the tumor microenvironment (hypoxic, acidic) Citation[8]. Cryosurgery, on the other hand, uses extremely low temperature to directly injure tumor cells and vessels through ice formation Citation[9]. Not only do these techniques have advantages such as cosmetic conservation, minimal invasion, and low side effects, several reports have even showed that thermophysical treatment had immunological merits as well Citation[2],Citation[10–12]. Previous researches reported that heat could enhance immunogenicity of tumor cells and expression of heat shock proteins (HSP) Citation[13–15]. Hsps served as a vehicle to transfer tumor-specific antigen to professional antigen presenting cells (APC), and subsequently activate tumor-specific T cell response Citation[11],Citation[14],Citation[15]. On the other hand, cryosurgery caused tumor necrosis could initiate an inflammatory environment. The presence of tumor antigens in such an environment could induce cryoimmunity Citation[12],Citation[16],Citation[17].
Nevertheless certain problems have impeded further development of thermophysical treatment. Precisely controlling the damage region especially at the tumor edge is a difficult issue in cryosurgery, while hyperthermia is always accompanied by an increase of blood perfusion in the tumor peripheral region and thermotolerance is another issue leading to lower therapeutic effect Citation[9],Citation[18],Citation[19]. The increase in blood flow due to local hyperthermia might also have the danger of stimulating metastasis from the primary site Citation[20],Citation[21]. To enhance the effect of thermophysical treatment, Hoffmann et al. once described the idea of alternating treatment including both cryosurgery and heating Citation[22]. However, only normal tissue was studied and no significant synergistic effect was found. Since thermal sensitivity of tumor and normal tissues are rather different Citation[8], the effect of the alternating treatment in tumor region might not be the same as that in the normal region. Our previous work showed that the alternating treatment induced great damage to tumor vessels and might have stronger direct killing effect Citation[23],Citation[24]. No previous work has been reported on the immune response after such an alternating treatment. If anti-tumor immune response could be enhanced as well, then it has a great potential in treating breast cancers, and possibly those with distant dormant micrometastases.
In this paper, the synergistic effect and the induced anti-tumor immunity through the alternating cooling and heating was studied using 4T1 murine mammary carcinoma, a common model of human metastatic breast cancer Citation[25]. Due to its highly invasive and poorly immunogenic characters, the 4T1 tumor could have caused death even after the primary tumor was surgically removed Citation[26]. The treatment was carried out when micrometastases were well established. The therapeutic effect of the alternating cooling and heating therapy was studied. To investigate the immune factor in the whole process, we also performed the alternating treatment in nude mice with 4T1 tumors at the same stage. Further anti-tumor immune response in normal mice after the treatment was compared with that after the surgical resection. Since the 4T1 tumor could induce leukemoid reaction through releasing growth factors to suppress the immune system Citation[25], the splenic changes after the treatment were recorded as well.
Materials and methods
Animal model
Six- to eight-week-old female BALB/c mice (Animal Laboratory of Shanghai Medical College, China) and nude mice (Animal Centre, CAS, Shanghai, China) were housed in isolated cages and a 12-h light/dark cycle environment. They were fed sterile food and acidified water with the pH value kept at 2.5–2.8. The murine mammary carcinoma 4T1 cells were kindly provided by Shanghai First People's Hospital, China. Cells were grown in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum, plus 100 U/mL penicillin, and 100 g/mL streptomycin (Shanghai Sangon, China). To prepare the tumor-bearing mouse, approximately 1 × 105 cells were injected subcutaneously into the right femoral region of each mouse. Tumor sizes were measured every 3 days and its volume was estimated using the following formula:
Treatment procedures
Four weeks after tumor inoculation, when the tumor volume reached about 0.4 cm3, the mice were anesthetized with intraperitoneal injection of 4% chloral hydrate (1 ml/100 g). The tumor site was sanitized with alcohol and underwent different treatments respectively. A total of 41 tumor-bearing mice were used. No treatment was performed on six mice of the control group. In the surgical resection group, wide excision surgical resections were performed on six mice. After controlling of hemorrhage, the wound was closed with interrupted nylon sutures. All of the procedures were performed aseptically.
In order to investigate the direct therapeutic effect, seventeen mice were treated with the alternating cooling and heating. Nine mice were initially used and another eight were treated to repeat the experiment several months later. During the treatment, the tumor overlaying skin was forced into contact with the BCS 196 cryostage by tweezers (Linkam Scientific Instruments, UK). Thermal paste with thermal conductivity of 1.4 W/(m K) was used to ensure good contact. A thermocouple was inserted into the bottom of the tumor to monitor the local temperature at the distal end. The temperature of the cryostage was maintained at −64°C during the freezing process. Then the cryostage was turned off completely and achieved equilibrium with the environment. Finally the cryostage was set to be 52°C as the boundary in the heating process.
Heat transfer between the body and the tumor was described using the Pennes bioheat transfer equation with blood perfusion. In the recovery process, the natural convection boundary condition with the air temperature at 25°C was applied. At 20 mm deep inside the body, the constant core temperature at 35°C was used. At the outer surface of the tumor, the freezing and heating temperatures were set to be of −64°C and 52°C respectively. The blood perfusion rates in Pennes bioheat equation were chosen to be at 0.00233 ml/s/ml and 0.00126 ml/s/ml, for tumor and tissue respectively Citation[19].
At the beginning of the treatment, freezing was performed until the distal temperature in the tumor was below 0°C and the freezing was held for an additional 1 min. Numerical heat transfer analysis showed that an ice ball had formed through the whole tumor region during this procedure. The temperature within the tumor ranged from −12°C to −64°C when the freezing process ended. The cooling system was then shut off and the tumor was allowed to passively thaw for 10 min followed by contact heating at 52°C through the thermal stage for 20 min. According to the numerical simulation results, the tumor tissue temperatures ranged from 52°C to 43°C monitored by the thermocouple placed at the distal end during the heating process. The alternating cooling and heating was repeated for three times in 24-h intervals. Six mice were used in either the above mentioned cooling or heating treatment alone respectively. The treatments were also repeated once a day for three days. The same alternating treatment was also performed on six nude mice for comparison.
Re-challenge with the 4T1 tumors cells in mice cured by combination therapy
Another six mice undergoing the alternating treatment were challenged with the 4T1 cells two weeks after the treatment. Approximately 1 × 105 cells were transplanted subcutaneously into the left femoral region. In the control group the tumor cells were injected into six normal mice of the same age without treatment. The growth of tumor was observed with respect to time.
In vitro cytotoxicity T-lymphocyte (CTL) response assay
In vitro CTL assay was carried out three days after the alternating treatment and five days after the surgical resection. The normal mice of the same age were used in the control group, four mice in each group. The mice were sacrificed and the spleens were taken. The maximum length of the spleens was measured. Then the splenic lymphocytes were isolated and erythrocytes were depleted with 0.75% ammonium chloride. Cytotoxicity activity was determined using lactate dehydrogenase (LDH) releasing assay according to the manufacturer's instructions (Jiancheng Technology, Nanjing, China). Briefly, target cells (1 × 105 4T1 tumor cells) were mixed with different ratios of effector cells and co-cultured for 4 h at 37°C in an atmosphere containing 5% CO2. Release of LDH was then evaluated using a commercially available kit. Spontaneous and total LDH release was calculated from media plus targets only and targets plus 1% Trition-X-100, respectively. Cytotoxicity was calculated as follows. The variables were optical density (OD) of the solutions at 450 nm.
Serum cytokine levels
One Millilitre of blood were obtained from mice one day after the alternating treatment and four days after the surgery by orbital sinus. The specimens were centrifuged and the serum was collected. Then the serum was analyzed for IFN-gamma, interleukin (IL)-12 using commercial ELISA kits from Jingmei Biotech, China. A standard curve was established according to the manufacture's instruction. Experimental values were computed with the use of regression analysis. Normal mice were used as control, four mice in each group.
Immunohistochemical and histological staining of tumor debris
Tumor debris was immediately removed from another three mice while the alternating treatment was completed. Blood was flushed out with phosphate buffer and the samples were cut into two parts. One part was fixed with OCT (optimum cutting temperature) compound at −70°C for immunohistochemical staining. The frozen tumor tissues were cut along the sagittal direction of skin using a LEICA CM1900 at −20°C. Correlative histological sections of 10 µm thickness were prepared, air-dried for 30 min and fixed with cold acetone for 15 min. These sections were incubated with 5% skim milk for 30 min to block background staining. They were then incubated at 4°C overnight with murine FITC anti-mouse CD4 (clone GK1.5), PE/Cy5 anti-mouse CD8a (clone 53–6.7). All of the antibodies conjugated to fluorescent residues were purchased from BioLegend, San Diego, CA. Fluorescence images were collected at 500–530 nm (FITC) and 650 nm (PE/Cy5) by laser confocal microscopy LSM510 Meta (Zeiss, Germany). All of the sections were also stained with hematoxylin. To observe changes in the tumor region after the treatment, the tumor tissue was fixed with 10% formaldehyde for H&E stain. The samples were dehydrated and embedded in paraffin, and then 7 µm sections were cut using a LEICA RM2126 and stained with hematoxylin and eosin.
Statistical analysis
Data are given as means, standard deviations, and analyzed using the Student's t-tests. P values of 0.05 or less were considered significant.
Results
Alternating treatment induced great necrosis in tumor
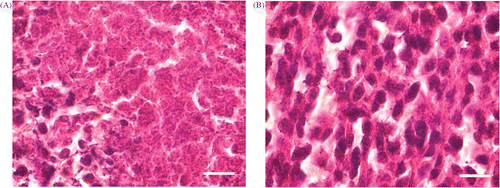
When the alternating treatment was completed, tumor debris was immediately taken for histological analysis (). Most of the tumor cells in the region were found to be necrotic. However, many viable tumor cells still existed especially in the peripheral area. Numerical analysis using the Dewey's equivalent heating time for the tumor volume has also indicated similar results.
Figure 1. (A) Histological analysis of tumor tissue after the alternating treatment. Immediately after the alternating cooling and heating treatment, the tumor debris was taken for H&E stain; (B) Histological analysis of tumor tissue without any treatment. The sections were viewed with 100× oil objective. (Scale bar, 10 µm) It showed that although the thermophysical treatment induced necrosis, there were still viable tumor cells remaining.
Of a given heating protocol, the Dewey's equivalent heating time 43°C was calculated by the following equations: Citation[27]where the empirical constant M was given by: Citation[27]
The cell survival rate after a given heating treatment was then obtained from the cellular survival rate curve in relation to the thermal dose. For quantification, the fitted curve is given as: Citation[28]
where Sheat is the total cell survival rate during heating.
Since the temperature within the tumor ranged from the distal end at about 43°C to the heating plate at 52°C, the equivalent heating time increases exponentially from 19 min to about 15,078 min. At the end of the treatment, the predicted cell survival rate was about 0.56 at the distal end although the cells were almost completely killed near the plate.
To further investigate the therapeutic effect, several mice were traced for a period time after the treatment to see whether recurrence happened. shows the therapeutic effects of different treatments. All of the treatments were carried out at the time when metastasis was well established and surgical resection of the 4T1 primary tumor could not cure any mice at all. Our results were consistent with the report of Pulaski et al. Citation[29]. Concerning thermophysical treatment, freezing alone could hardly inhibit the primary tumor growth. Although the primary tumor growth was somewhat inhibited by local hyperthermia treatment, five of six mice died from metastasis within one month after the treatment. The only one cured did not gain weight and died three months after the treatment. On the other hand, tumor regression was achieved completely in 15 of 17 mice after the alternating treatment. Only one mouse died from metastasis and another died during the treatment, possibly due to interindividual variability. One month later, the mice successfully treated began to gain weight as normal. No recurrence was observed over a period of five months. However, none of the six nude mice was cured by the same alternating treatment. Most of them died from primary recurrence and metastasis.
Table 1. Therapeutic results of the mice after different treatments.
Stimulation of anti-tumor immune response by the alternating treatment
To examine whether anti-tumor immune response was induced by the alternating cooling and heating, another six mice were re-challenged with the 4T1 tumor cells two weeks after the treatment. Approximately 1 × 105 cells were injected subcutaneously into the left femoral (untreated side). The 4T1 tumor growth in all treated mice was regressed, while progressive tumor growth was found in the six untreated mice, suggesting that the mice after the alternating treatment might have gained immunologic protection against tumor re-challenge.
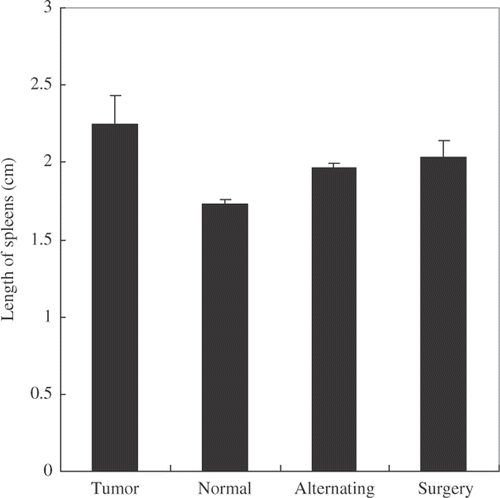
The 4T1 tumor could induce leukemoid reaction, which was characterized by bone marrow hyperplasia and splenomegaly. Immature myeloid cells (ImC) were accumulated in the spleen and peripheral blood and induced immune suppression in vivo Citation[30]. shows the size of spleen after different treatments. Both surgical resection and the alternating treatment could alleviate splenomegaly. However, there was no significant difference between these two groups in splenic morphology.
Figure 2. Spleen size changes after different treatments. Three days after the alternating treatment or five days after the surgical resection, the spleens were taken and their maximal length measured. Tumor bearing and normal mice were used as the controls. The results showed that both surgical resection and the alternating treatment could alleviate splenomegaly (p < 0.05, n = 4).
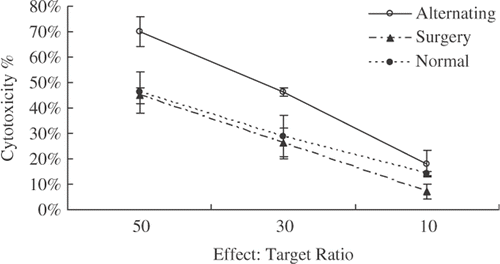
Since the activity of T-lymphocyte cells was inhibited in the 4T1 tumor model Citation[26], we measured the CTL response against 4T1 cells to investigate whether the suppression was relieved by the alternating treatment. Mice were sacrificed on the third day after the treatment, and the splenic lymphocytes were harvested and CTL response was measured using LDH releasing assay. The results are shown in . Significantly higher CTL activity was observed in the mice as compared with those in either surgical or normal groups (p < 0.05). The cytotoxicity of splenic lymphocytes against 4T1 tumor in the surgical group decreased drastically. It demonstrated that the alternating cooling and heating treatment enhanced T-lymphocytes activity against the 4T1 tumor cells.
Figure 3. Increased activity of CTL response in the mice cured by the alternating cooling and heating treatment. Three days after the alternating treatment or five days after the surgical resection, the splenic cells were taken. The CTL activity against 4T1 tumors (target cells) was examined using releasing LDH release assay (p < 0.05, n = 4).
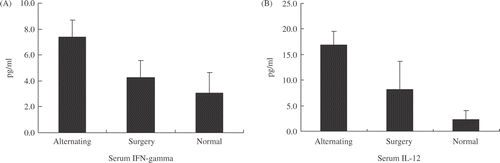
On the other hand, blood samples were collected one day after the alternating treatment and serum levels of Th1 (IL-12, IFN-gamma) cytokines were measured. The results are summarized in . Significant increase of serum IFN-gamma and IL-12 was found associated with the alternating treatment as compared with those that had undergone surgical resection.
Figure 4. Serum Th1 cytokines levels after the alternating treatment or surgical excision. Twenty-four h after the alternating treatment, a significantly higher level of both IFN-gamma and IL-12 was recorded as compared with surgical resection and normal control groups (p < 0.05, n = 4).
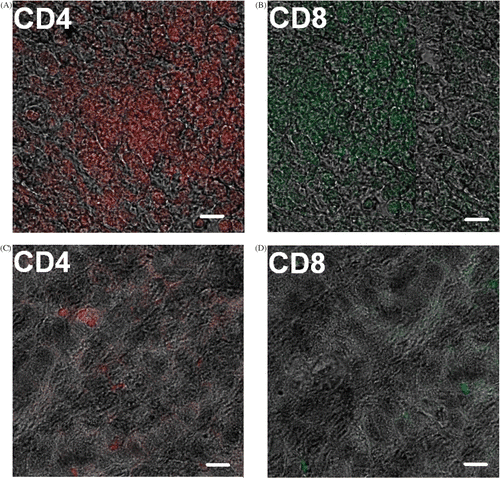
Further investigation was performed on immunocytes’ infiltration into the tumor region. Immunohistochemical staining was performed to investigate T cell distribution in tumor debris. As shown in , more CD4+, CD8+ T cells were detected in the tumor tissues after the alternating treatment as compared with that in the untreated tumor tissues. It showed that the treatment attracted immunocytes into the tumor region to clean up the tumor debris.
Figure 5. Immunohistochemical staining for immunocytes. Anti-CD4+, CD8+ cells antibodies conjugated to different fluorescent residues were used to observe the infiltration of T cells into the tumor debris. (A), (B): tumor debris after the alternating cooling and heating treatment; (C), (D): tumor without any treatment (Scale bar, 5 µm).
Discussion
Minimally invasive surgery has been widely used to treat cancers in recent years. In this study, using low-dose hyperthermia with pre-freezing, most of the surrounding normal tissues were kept intact while the thawing phase was extended to enhance vascular damage and tumor necrosis. To investigate whether cooling and heating had synergistic therapeutic effect, mice bearing a 4T1 tumor were treated with heating for 20 min after pre-freezing. Tumor growth was found inhibited by the treatment while neither freezing nor heating alone could achieve similar effects. Hyperthermia only inhibited primary tumor growth to certain degree, most of the mice died from metastasis within one month afterward. Low-dose freezing used in this study did not inhibit primary tumor growth. However, after the alternating treatment, great vascular damage was achieved and cryo-induced blood stagnation might prevent tumor cells from metastasis.
But the same alternating treatment could hardly cure nude mice with 4T1 tumor at the same stage. On the other hand, histological analyses revealed that some viable tumor cells still existed immediately after the alternating treatment. Interestingly, in regular mice, the viable tumor debris disappeared later while primary recurrence and metastasis caused death of nude mice within one month after the treatment. These results suggested that direct killing effect was not the only reason for the excellent prognosis after the alternating treatment and T-cell dependent immunologic factor was vital for the cure process. The rejection of tumor upon re-challenge further demonstrated that an immunologic protection was strongly induced by the treatment.
In order to analyze the mechanisms for different prognosis achieved by the alternating cooling and heating treatment and surgical resection, the CTL response against 4T1 cells was measured. Our results showed that CTL response was still blocked after surgical resection, but the splenic lymphocytes taken from mice that underwent the alternating treatment had strong activity against the 4T1 cells in vitro. Further cytokine analysis showed that serum levels of Th1 cyokines (IFN-gamma and IL-12) increased after the treatment, suggesting that inflammatory cytokines involved in cell-mediated immunity were promoted. Apart from induction of CTL, release of IFN-gamma and IL-12 could increase natural killer (NK) cell activity and IL-12 induced antiangiogenesis effect might inhibit the recovery of tumor vessels Citation[31], thus enhancing the therapeutic effect by repeating the alternating treatment.
In addition, to investigate whether immunocytes migrated into the tumor region after the alternating treatment, infiltration of immunocytes in the tumor region was measured by immunohistochemical staining. Increased infiltration of both CD4+ and CD8+ T cells into the tumor site was observed. T-lymphocytes could be activated when they interacted with tumor specific antigen presented by APC such as dendritic cells (DC) or macrophages with co-stimulatory signal. Then T-lymphocytes could turn CD8+ cells into CTL to eliminate the remaining tumor cells Citation[32].
This study shows that the alternating cooling and heating treatment might induce an active T-cell immune response and a strong protective effect. According to some previous work, either heating or freezing alone would also induce anti-tumor immune response, but not strong enough to exert a protective effect Citation[33]. Our experiments were carried out when metastasis was well established. While applying heat treatment alone or using surgical resection, most of the mice died from metastasis even when the primary site was cured. However, low dose and local hyperthermia with pre-freezing achieved excellent prognosis. Moreover, the novel alternating treatment has been suggested to induce some immune effect and prevent the potential metastasis.
Concerning how anti-tumor immune response was activated, heat shock proteins whose expression might be dramatically increased after hyperthermia and great inflammatory environment created by freezing could play an important role during the whole process. After the alternating treatment, intracellular HSPs greatly increased and tumor cells became necrotic, which favored formation of HSP-antigen complex and its release into the surrounding media Citation[15],Citation[17],Citation[34]. On the other hand, freezing initiated an inflammatory environment, in which many different cytokines were released Citation[16],Citation[17]. In addition, both heat and cryosurgery could induce maturation of dendritic cells (DC), enhancing their antigen uptake, migration and T cell stimulatory activity Citation[14],Citation[33],Citation[35]. The alternating cooling and heating treatment might cause maturation of the circulating ImC into mature APC. Mature APC then accepts the HSP-antigen complex in the medium and presents the tumor specific antigen to T cells with co-stimulatory effect. Subsequently, the suppression of T cells is relieved and the number of tumor-specific effect T-cells increased. More effect T cells could be attracted to the tumor site and CTL be formed to clean up tumor debris as well as metastasis. Although our study showed that the immune response after the alternating treatment was strong and protective, further research on the mechanisms of immune response is needed. Application of this new treatment protocol may provide an effective and systemic treatment for cancer, possibly for those with distant micro-metastasis in clinic.
Acknowledgements
We thank Dr Huang's lab of the Shanghai First People's Hospital for the kind provision of the 4T1 tumor cell line. We also appreciate Chao Chen in our lab for his help in the numerical heat transfer analysis of the thermophysical treatment. This work has been supported by National Natural Science Foundation of China (50436030, 10705020).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43–66
- Sabel MS, Edge SB. In-situ ablation of breast cancer. Breast Dis 2001; 12: 131–140
- Baum M, Demicheli R, Hrushesky W, Retsky M. Does surgery unfavourably perturb the ‘natural history’ of early breast cancer by accelerating the appearance of distant metastases?. Eur J Cancer 2005; 41: 508–515
- Pinsolle V, Ravaud A, Baudet J. [Does surgery promote the development of metastasis in melanoma?]. Ann Chir Plast Esthet 2000; 45: 485–493
- Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res 1989; 49: 1996–2001
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 1999; 80: 880–888
- Hirai T, Matsumoto H, Yamashita K, Urakami A, Iki K, Yamamura M, Tsunoda T. Surgical oncotaxis – Excessive surgical stress and postoperative complications contribute to enhancing tumor metastasis, resulting in a poor prognosis for cancer patients. Ann Thorac Cardiovasc Surg 2005; 11: 4–6
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44: 4721s–4730s
- Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002; 60: 40–49
- Shah SA. Participation of the immune system in regression of a rat Mc7 sarcoma by hyperthermia. Cancer Res 1981; 41: 1742–1747
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of 'heat-controlled necrosis' with heat shock protein expression. Cancer Immunol Immunother 2006; 55: 320–328
- Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat 2005; 90: 97–104
- Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother 2001; 50: 515–522
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: An integrated view. Immunol Today 2000; 21: 129–132
- Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins?. Int J Hyperthermia 2005; 21: 713–716
- Johnson JP. Immunologic aspects of cryosurgery: Potential modulation of immune recognition and effector cell maturation. Clin Dermatol 1990; 8: 39–47
- Gazzaniga S, Bravo A, Goldszmid SR, Maschi F, Martinelli J, Mordoh J, Wainstok R. Inflammatory changes after cryosurgery-induced necrosis in human melanoma xenografted in nude mice. J Invest Dermatol 2001; 116: 664–671
- Lepock JR. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int J Hyperthermia 2003; 19: 252–266
- Song CW, Kang MS, Rhee JG, Levitt SH. The effect of hyperthermia on vascular function, pH, and cell survival. Radiology 1980; 137: 795–803
- Greenstein A, Koontz WW, Jr. Does local hyperthermia affect metastasis of a human prostate carcinoma grown in athymic nude mice?. Int J Hyperthermia 2002; 18: 285–291
- Salsbury AJ. The significance of the circulating cancer cell. Cancer Treat Rev 1975; 2: 55–72
- Hoffmann NE, Chao BH, Bischof JC. Cryo, Hyper or Both? Investigating Combination Cyro/hyperthermia in the Dorsal Skin Flap Chamber. Proc ASME Advances Heat Mass Transfer Biotech 2000; 47: 157–159
- Sun J, Luo X, Zhang A, Xu LX. A new thermal system for tumor treatment. IEEE, ShanghaiChina 2005, Piscataway, NJ
- Shen Y, Liu P, Zhang A, Xu LX. Tumor microvasculature response to alternated cold and heat treatment. Shanghai, China 2005, Piscataway, NJ: IEEE
- DuPre SA, Hunter KW, Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp Mol Pathol 2007; 82: 12–24
- Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: A balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother 2005; 54: 1137–1142
- Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977; 123: 463–474
- Sun J, Zhang A, Xu LX. Evaluation of alternate cooling and heating for tumor treatment. Int J Heat Mass Trans 2008, in press
- Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res 2000; 60: 2710–2715
- Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother 2002; 51: 293–298
- Shi X, Cao S, Mitsuhashi M, Xiang Z, Ma X. Genome-wide analysis of molecular changes in IL-12-induced control of mammary carcinoma via IFN-gamma-independent mechanisms. J Immunol 2004; 172: 4111–4122
- Adam JK, Odhav B, Bhoola KD. Immune responses in cancer. Pharmacol Ther 2003; 99: 113–132
- den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006; 95: 896–905
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 2000; 12: 1539–1546
- Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer 2005; 116: 624–633
