Abstract
Purpose: To analyse the treatment results of neo-adjuvant chemoradiation combined with regional hyperthermia in patients with resectable esophageal cancer.
Patients and methods: Between August 2003 and December 2004, 28 patients entered a phase II study combining chemoradiation over a 4.5-week period with five sessions of regional hyperthermia. Chemotherapy consisted of carboplatin (AUC = 2) and paclitaxel (50 mg/m2) and radiotherapy of 41.4 Gy in 1.8 Gy daily fractions. Locoregional hyperthermia was applied using the AMC phased array of four 70 MHz antennas, aiming at a stable tumor temperature of 41°C for one hour. Carboplatin was infused during the hyperthermia session. Esophageal resection was planned at 6–8 weeks after the end of radiotherapy. The majority of the patients had a T3 tumor (86%) and were cN+ (64%). Median follow-up for survivors was 37 months (range 31–46).
Results: Twenty-five patients (89%) completed the planned neo-adjuvant treatment and acute toxicity was generally mild. Twenty-six patients were operated on. A pathologically CR, PRmic, PR and SD were seen in 19%, 27%, 31% and 23% respectively. All patients had a R0 resection. In-field locoregional control during follow up for the operated patients was 100%. Quality of life was good for patients without disease progression. Survival rates at one, two and three years were 79%, 57% and 54% respectively.
Conclusion: Neo-adjuvant chemoradiation combined with regional hyperthermia followed by esophageal resection for patients with esophageal cancer resulted in good locoregional control and overall survival.
Introduction
The prognosis of esophageal cancer remains dismal, with a 2-year overall survival after surgery alone of 33–44% Citation[1–6]. The incidence of locoregional recurrences varied from 27 to 41% in six randomized trials Citation[1–6]. Although a statistically significant benefit in overall survival by neo-adjuvant radiochemotherapy (RCT) was observed in only one randomized trial, a meta-analysis did show a statistically significant advantage of RCT Citation[7]. Furthermore, neo-adjuvant RCT had a statistically significant effect of on pre-operative downstaging of the tumor Citation[7] and the incidence of locoregional recurrences decreased in five trials. However, even after a significant decrease by neo-adjuvant treatment, locoregional recurrences still occurred in 19–32% of the patients and are a major cause of treatment failure Citation[1], Citation[2], Citation[4–6], Citation[8]. Therefore, studies aiming at an improvement of locoregional control are still warranted.
Hyperthermia has proven to be a potent radiosensitizer Citation[9] and did improve local control significantly in randomized clinical studies for cervical cancer, breast cancer and melanoma Citation[10–12]. Hyperthermia has also shown to enhance the effect of chemotherapy. Phase II clinical studies were encouraging when heat was combined with cisplatinum (cDDP) in recurrent carcinoma of the uterine cervix and in soft tissue sarcomas Citation[13], Citation[14]. Clinical data on the effect of hyperthermia combined with cisplatinum in esophageal cancer are scarce. Sugimachi et al. showed in a small randomized trial an improvement of clinical and histological effects by intraluminal hyperthermia when added to chemoradiation Citation[15], Citation[16]. A disadvantage of this intraluminal heating technique is the limited penetration depth; the temperature rise drops to 50% at only 5 mm depth Citation[15] and thus seems to be insufficient to heat the entire esophageal tumor. Also no longitudinal power steering is possible, which could result in temperature inhomogeneity. Li et al. measured 25% temperature heterogeneity in the axial direction Citation[17]. Locoregional heating can overcome both problems. A recent phase I study on locoregional hyperthermia combined with chemotherapy confirmed the feasibility of this technique and a more homogeneous temperature distribution Citation[18].
Based on the promising results of neo-adjuvant RCT in esophageal cancer and the sensitization by hyperthermia of both treatment modalities we performed a phase II study combining the three treatment modalities as preoperative treatment in esophageal cancer in order to improve locoregional control.
Patients and methods
Patients
Between August 2003 and December 2004 28 patients with resectable cancer of the esophagus were included in the study. All patients were staged by endosonography of the esophagus, computed tomography (CT)-scan of the neck, chest and upper abdomen and external sonography of the cervical lymph node region. Tumor extension up to 2 cm in the cardia was accepted. Inclusion criteria were a histologically proven carcinoma of the esophagus, stage T2-3 N0-1 M0, and patients had to be medically fit for surgery. Prerequisites were an adequate renal function (glomular filtration rate [GFR] >60 ml/min), pre-treatment white blood count (WBC) >3.5 × 109/l and platelet count >100 × 109/l. Patients were excluded if the tumor was located above the level of the carina because of practical hyperthermia delivery reasons. The majority of the patients had a T3N1 tumor. Patient characteristics are shown in . The local ethical committee approved the protocol and from all patients a written informed consent was obtained.
Table I. Patient characteristics.
Treatment
Radiotherapy consisted of 41.4 Gy in daily fractions of 1.8 Gy, five times per week, administered with a four-field conformal planning technique. The planning target volume consisted of the gross tumor volume, including all pathological nodes plus a margin of 4.5 cm in cranio-caudal direction and of 1.5 cm in lateral direction. If the distal border of the tumor was situated at the esophagogastric junction, the caudal margin was reduced to 3 cm. Chemotherapy (5 cycles) was administered once a week during the radiotherapy period at the day of hyperthermia treatment and consisted of Paclitaxel 50 mg/m2 and Carboplatin AUC (area under the curve) = 2. Paclitaxel was given directly preceding or following the irradiation and always before the hyperthermia. Carboplatin was infused during the hyperthermia sessions over a period of 1.5 h. Time between radiation and onset of hyperthermia varied between 2 and 5 h.
Hyperthermia
An endoscopy was performed prior to each hyperthermia treatment under sedation with 5 mg midazolam intravenously. The tumor length and location were verified under fluoroscopy and marked on the skin with lead wires for positioning of the balloon catheter and tumor temperature monitoring. The latter involved two parallel 21-point T-type thermocouple probes (Ella CS, diameter 0.9 mm, spacing 1 cm, active length 20 cm) mounted on opposite sides of an inflatable balloon catheter (balloon length 80 mm, diameter 10 mm). Inflation of the balloon ensured adequate tissue contact and intraluminal fixation. Furthermore an e-field probe (diameter 1 mm) was mounted on the balloon adjacent to the tumor. For spinal cord temperature monitoring an intramuscular thermometry catheter at the level of the tumor was placed in the m. erector spinae before each treatment under local anesthetics. The insertion depth ranged between 3 and 5 cm. Temperature was recorded with a 14-point T-type thermocouple probe (Ella CS, diameter 0.9 mm, spacing 0.5 cm, active length 6.5 cm). For body temperature monitoring a rectal 14-point T-type thermocouple probe was used.
Locoregional hyperthermia was given using the home-made AMC (academical medical center) phased array of four 70 MHz antennas. This is a non-invasive heating system applying heat via four external microwave applicators arranged around the thorax. The power and phase of each applicator can be steered individually in order to get the optimal energy absorption in the target area, as measured with intraluminal E-field and thermal sensors in the esophagus. The patient was lying in prone position with his arms in elevated position to provide access for the two side-antennas. The other antennas were directed toward the back and belly. Between the antennas and patient four water bags were placed in front of each antenna, serving a dual purpose: to guide the EM-field into the patient and to provide skin cooling. The latter was achieved by circulating cold distilled water of approximately 13°C in the bags. The antenna power settings were 1 : 3 : 3 : 3 for the back : belly : left : right antenna. We endeavored not to exceed a maximum back temperature of 41°C. Therefore the contribution of the back antenna to the power was only 10% and further reduced if necessary. The antenna settings were optimized with the aid of the e-field probe to achieve a maximum field in the tumor. Total power was set at 800 W at the start of treatment. After a warming up period (maximum 30 minutes) the effective heating period of one hour started when a steady state tumor temperature was reached, aiming at a temperature of 41°C measured in at least one point in the tumor. Carboplatin infusion started at the beginning of the steady state temperature. Sedation with midazolam was continued during the hyperthermia treatment if necessary. The tumor temperature was recorded at the sensors corresponding to the tumor length and location as determined during endoscopy. Tumor temperatures were quantified as T10, T50 and T90, indicating the temperatures achieved in at least 10%, 50% and 90% of the sensors in the tumor, respectively.
Pulse rate and O2 saturation were continuously monitored during the hyperthermia treatment. Blood pressure was monitored at the start and end of the session.
Surgery
A transhiatal resection of the esophagus with esophagogastrostomy was planned at 6–8 weeks after the end of neo-adjuvant treatment. A lymph node dissection was performed of peritumoral lymph nodes (transhiatally) and nodes along the hepatic artery, the left gastric artery and the splenic artery. Histological response in the resection specimen was classified as complete response (CR) if no vital cells were present, microscopically partial response (PRmic) if only small microscopical foci of vital cells were present within large areas of necrotic tissue, partial response (PR) if the specimen consisted of vital fields within necrosis, and as stable disease (SD) when the majority of the tumor cells were vital.
Survival was calculated from date of first treatment, according to Kaplan Meier analysis. Inclusion for survival analysis was based on intention to treat. SPSS software was used for statistics. The log-rank test was used for analysis of prognostic factors.
Results
Feasibility and acute toxicity
Twenty-five patients (89%) completed the planned neo-adjuvant treatment and twenty-seven patients had at least four weeks of radiochemotherapy plus hyperthermia (96%). One patient refused hyperthermia at the start of treatment and was excluded from acute toxicity and early response scores. In two patients the last cycle of chemotherapy and hyperthermia was cancelled, in one patient because of massive esophageal bleeding in a large necrotic tumor area, which demonstrated a histologically complete remission after surgical resection. In the other patient thermochemotherapy was cancelled after twice developing severe fever in the hours following the hyperthermia treatment, probably hyperthermia induced. Radiation treatment was completed in this patient. Physical discomfort during hyperthermia was encountered in 71% of the patients, and was mainly mild. Only one session of hyperthermia had to be ended prematurely because of discomfort. Nausea was experienced in 24% of the patients, all ≤ grade 2. Hematological toxicity requiring dose adjustments was not observed. Weight loss of >2 kg was observed in 6 patients, a weight gain of >2 kg in 2 patients and in 70% of patients the body weight remained within 2 kg of their initial weight. Dysphagia improved, remained stable or worsened in 41%, 44% and 15% respectively according to the common toxicity scores. Two patients developed thrombo-embolism; one patient developed a femoral thrombosis and one patient suffered from a secondary pulmonary embolism, which was fatal.
Mean pulse rate increased from 82 p/min at the start to 110 p/min at the end of the hyperthermia session (N = 25, five sessions per patient). Mean diastolic blood pressure decreased from 93 to 88 mm Hg during hyperthermia treatment.
Late toxicity
A benign stenosis of the anastomosis occurred in seven patients during follow-up, which is in line with previous publications on surgery alone. Mean Karnofsky score at three years was 90% for surviving patients.
Hyperthermia results
Mean esophageal T10, T50 and T90 at tumor level were 39.9°C, 39.3°C and 38.7°C respectively. Mean CEM43°C T90 (cumulative equivalent minutes of 43°C) was 1.01 min. The intended temperature of at least 41°C in one probe at tumour level was reached in only half of the patients. The thermal dose was limited because of pain (sternal or shoulder) or general discomfort in seven patients and in two patients because of excessively high temperatures in the back probe. The thermal dose was limited in the majority of cases due to an inability to deliver enough power. There was a non-significant trend for better response when higher tumor temperatures were reached: Mean T90/CEM43°C T90 for CR, PRmic, PR and SD responders was 39.0°C/1.84 min, 38.7°C/0.77 min, 38.5°C/0.83 min and 38.5°C/0.82 min, respectively (p = 0.35). Mean systemic (rectal) temperature rise was 1.1°C (SD ± 0.4°C), which includes one patient who developed high fever in direct response to the hyperthermia for two sessions.
Treatment response
Regression of the tumor, as evaluated during the last endoscopic procedure in the fifth treatment week, was observed in 85% of the patients. Data on surgery were available for 26 patients. One patient died due to pulmonary embolism before surgery. Another patient developed hoarseness in the period between chemoradiation and surgery, which was considered as progressive disease. For that reason he was not operated on. The median interval between end of induction treatment and date of surgery was 49 days (range 26–75 days). Resection was microscopically radical in all 26 operated patients. Pathologically CR, PRmic, PR and SD were assessed in 19% (5/26), 27% (7/26), 31% (8/26) and 23% (6/26) respectively. Including the clinically progressive patient, there was a 74% (20/27) response rate with a 44% (12/27) complete response rate (inclusive nearly complete). Nodes at any site were histologically involved in 38%. CR or PRmic were observed in 5 out of 7 patients with a squamous cell carcinoma compared to 7 out of 20 patients with an adenocarcinoma.
Survival
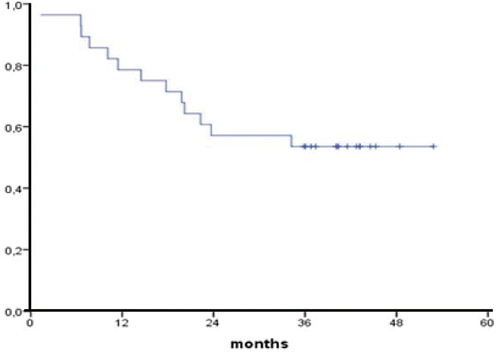
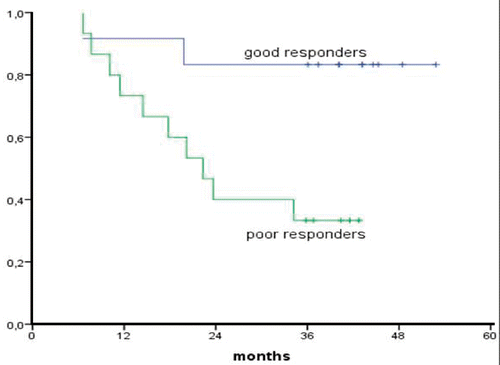
Median follow-up for survivors was 37 months (range 31–46 months). No patient was lost for follow up. Survival rates according to Kaplan Meier at one, two and three years were 79%, 57% and 54% respectively with no death events there after (). Good responders (CR and PRmic) had an actuarial 3-year survival of 83% compared to 33% for poor responders (PR and SD) (p = 0.004, ). Three-year survival of patients with squamous cell carcinoma was 70% (n = 7) compared to 43% for adenocarcinoma (n = 21) which was a non-significant difference (p = 0.34). Length of tumor, clinical N status and interval between neo-adjuvant treatment and surgery were not of significant prognostic value.
Local recurrences
Locoregional (mediastinal and anastomosis) control at 3 years was 96%. The only loco-regional recurrence occurred in the non-operated patient at 12 months. One patient developed a supraclavicular recurrence outside the irradiation field.
Discussion
This phase II study demonstrates that chemoradiation combined with external hyperthermia followed by esophageal resection is feasible and can result in an excellent locoregional disease control, a promising 3-year survival rate and a low toxicity profile. Most published phase III trials with preoperative chemoradiation demonstrated a significant decrease in locoregional failures, but still varied between 19 and 32% Citation[1], Citation[2], Citation[4], Citation[5] and thus higher than the current 4% in-field recurrence rate. In contrast to the high local control rate, the rate of histologically complete remissions in this study was comparable to previous results of preoperative chemoradiation Citation[4], Citation[8], Citation[19] and does not support an improved locoregional effect by hyperthermia. However, patients with massive necrosis and only small nests of vital cells (pPRmic) were not considered as complete remission. When those patients would be included in the CR group, this rate would be higher compared to other studies and would fit with the high local control rate.
It cannot be concluded from this small study whether it is the hyperthermia or the new chemoradiation schedule that accounts for the promising outcome. Sugimachi showed in a small randomized trial with neo-adjuvant chemoradiation with or without intraluminal hyperthermia that the clinical and histological effects were in favor of the hyperthermia arm, with comparable side effects Citation[15]. An overview of 294 patients who underwent preoperative chemoradiation or hyperthermochemoradiation in a non-randomized setting demonstrated a significant improved response rate and survival for the hyperthermia group Citation[20]. Currently, a phase III trial is being performed in the Netherlands comparing surgery alone versus preoperative chemoradiation with the same chemoradiation schedule as in the present study but without hyperthermia. Comparison of the results of the current hyperthermia study with those of the forthcoming phase III trial might generate a hypothesis as to the potential benefit of locoregional hyperthermia.
One important question is whether the temperatures achieved are adequate in this trimodality setting. Achieved tumor temperatures (T10 of 39.9°C and a mean CEM43°C T90 of 1.01 min) were lower than the goal temperature of 41°C and comparable to those in our previous phase I study Citation[18]. Enhancement of radiation or chemotherapy in clinical settings has been observed at measured temperatures of 40–41°C for one hour Citation[10], Citation[11], Citation[13], Citation[21]. A thermal dose-effect relationship has been shown in a prospective clinical study above 10 CEM43°C T90 in superficial tumors receiving radiation therapy only Citation[22]. However, data on the minimal thermal dose for clinically relevant enhancement of trimodality treatment are lacking. A recent in vitro study demonstrated that hyperthermia at 41°C was capable of enhancing cisplatin radiosensitivity in cisplatin sensitive cell lines, but enhancement of cisplatin resistant cell lines was only seen at 43°C Citation[23]. Other in vitro studies on trimodality treatment showed a synergistic interaction in both cisplatinum-resistant and cisplatinum-sensitive cell lines at 40°C, but hyperthermia was given for several hours Citation[24]. We do not consider several hours of hyperthermia clinically feasible with the presented treatment set-up. A trend towards a thermal dose effect was observed in this study, but numbers are small. Improving tumor temperatures to levels of proven radiochemosensitization thus still seems to be a goal to work on. The possibilities of improving external regional heating are limited due to several nearby major heat dissipaters such as heart, aorta and lungs. Combining external hyperthermia with intraluminal hyperthermia could be an option to increase the thermal dose. This combination would overcome the problem of limited heat penetration of an intraluminal applicator only Citation[25]. We are currently working on combining external heating with an intraluminal hot water balloon with or without a 434 MHz antenna or 27 MHz electrode. A combination with total body hyperthermia could also increase the temperature in esophageal tumors, but this treatment is medically and technically complex.
Preoperative chemoradiation plus hyperthermia is generally considered as a locoregional treatment. However, the encountered difference in survival between good and bad responders (83% versus 26%) in the current study cannot be explained by a locoregional effect, because long term locoregional control was equally good in both response groups. Although it can not be excluded that this difference is a result of tumor selection, it is more likely to be explained by a systemic effect of the trimodality treatment. Hyperthermia in combination with other therapies has been demonstrated to enhance several immunological factors in a number of studies, both in vivo and in vivo, especially in fever range temperatures Citation[26], Citation[27]. Further clinical research is needed to explore the systemic effect of locoregional trimodality treatment.
Conclusion
Preoperative chemoradiation combined with regional hyperthermia resulted in excellent locoregional tumor control and good overall survival and is therefore a promising therapy. Comparison of these data with results from a running phase III trial which uses the same chemoradiation schedule in a similar patient population but without hyperthermia could provide information on the specific role of hyperthermia in this treatment setting.
Acknowledgement
Part of this research was supported by the Dutch Cancer society no. UVA 2002-2622.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimor J, Ester N, Haller DG, Ajani J, Kaha W, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998; 339: 1979–1984
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomized controlled trial. Lancet 2002; 359: 1727–1733
- Hulscher JBF, van Sandwick JW, de Boer AGEM, Wijhoven BPL, Tijssen JGP, Fockms P, Stalmeier PFM, Fen Kate FJW, van Dekken H, Obertop H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Eng J Med 2002; 347: 1662–1669
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinomas. J Clin Oncol 2001; 19: 305–313
- Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J, et al. Surgery alone versus chemoradiotherapy followed by surgery for respectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 2005; 6: 659–668
- Le Prise F, Etienne PL, Meunier B, Maddern G, Ben Hassel M, Gedouin D, Boutin D, Campion JP, Lanois B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994; 73: 1779–1784
- Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, Falchi AM, Cvaxi A, Camma C. Preoperative chemoradiotherapy for oesophageal cancer: A systemic review and meta-analysis. Gut 2004; 53: 925–930
- Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Olliet JC, Pavy JJ, Mercier M, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997; 337: 161–167
- Hildebrandt B, Wust P. The biologic rationale of hyperthermia. Cancer Treat Res 2007; 134: 171–81
- van der Zee J, Gonzalez D. The Dutch Deep Hyperthermia trial: Results in cervical cancer. Int J Hyperthermia 2002; 18: 1–12
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Acangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastastic malignant melanoma. A multicentre randomized trial by the European Society of Hyperthermic Oncology. Int J Hyperthermia 1996; 12: 3–20
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Rietbroek RC, Schilthuis MS, Bakker PJM, van Dijk JD, Postma AJ, Gonzalez Gonzalez D, Bakker AJ, van der Velden J, Helmerhort TJ, Veenhof CH. Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with a previously irradiated recurrent carcinoma of the uterine cervix. Cancer 1997; 79: 935–943
- Issels RD, Abdel-Rahman S, Wendtner C, Falk MH, Kurze V, Sauer H, Aydemir U, Hiddemann W. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: Long-term results of a phase II study. Eur J Cancer 2001; 37: 1587–1589
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with esophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 4: 485–493
- Kitamura K, Kuwano H, Araki K, Egashira A, Kawaguchi H, Saeki H, Morita M, Ohno S, Sugimachi K. Clinicopathologic features of patients with oesophageal cancer obtaining a histological complete response for preoperative hyperthermo-chemo-radiotherapy. Int J Hyperthermia 1998; 14: 233–243
- Li DJ, Hou BS. Preliminary report on the treatment of esophageal cancer by intraluminal microwave hyperthermia and chemotherapy. Cancer Treat Rep 1987; 71: 1013–1019
- Albregts M, Hulshof MC, Zum vorde sive vording PJ, van Lanschot JJ, Richel DJ, Crezee H, Fockens P, van Dijk JD, Gonzalez Gonzalez D. A feasibility study in oesophageal carcinoma using deep locoregional hyperthermia combined with concurrent chemotherapy followed by surgery. Int J Hyperthermia 2004; 20: 647–659
- Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Henessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–467
- Saeki H, Kawaguchi H, Kitmura K, Ohno S, Sugimachi K. Recent advances in preoperative hyperthermochemoradiotherapy for patients with esophageal cancer. J Surg Oncol 1998; 69: 224–229
- Dahl O. Interaction of heat and drugs in vitro and in vivo. Thermoradiotherapy and Thermochemotherapy. Biology, Physiology and Physics, MH Seegenschmidt, P Fessenden, CC Vernon. Springer, Berlin 1995; 1: 103–121
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005; 23: 3079–3085
- Bergs JWJ, Haveman J, ten Cate R, Medema JP, Franken NAP, van Bree C. Effect of 41°C and 43°C on Cisplatinum-radiosensitization in two human carcinoma cell lines with different sensitivities for cisplatin. Oncol Rep 2007; 18: 219–226
- Raaphorst GP, Miao J, Ng CE. Cisplatin and mild hyperthermia in radiosensitization to low dose rate irradiation in human ovarian carcinoma cells. Anticancer Res 1997; 17: 3469–3472
- Kok HP, van Haaren PM, van de Kamer JB, Crezee J. Theoretical comparison of intraluminal heating techniques. Int J Hyperthermia 2007; 23: 395–411
- Dayance BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia 2008; 24: 41–56
- Repasky E, Issels R. Phyiologicial consequences of hyperthermia: Heat, heat shock proteins and the immune response. Int J Hyperthermia 2002; 18: 486–489