Abstract
Objective: The objective was to evaluate urethral tissue heat injury in a rabbit model. Histopathology analysis was used to determine the minimum temperature required to achieve urethral tissue injury following interstitial radiofrequency ablation (RFA).
Materials and methods: A total of 37 healthy rabbits were divided into 6 groups randomly and treated with interstitial RFA in the penial parenchyma. The temperatures of urethra were monitored and controlled to 48°, 49°, 50°, 51° 52° and 53°C respectively. The urethral tissue acute heat injury (48 h after heating) was assessed by HE stain, Terminal Deoxylnucleotidyl Transferase Mediated-dUTP Nick End Labeling (TUNEL) stain and quantitative analysis in tissue sections.
Results: Histologically, the main feature of acute heat damage was necrosis or vascular congestion or thrombosis of blood vessels of the urethral wall. This occurred only in one out of five cases at 49°C and 50°C heating, but in four out of five cases at 52° and 53°C. The percentage of necrosis was significantly different at tissue temperature of 52°C and above. Quantitative image analyses of TUNEL stain sections demonstrated a significant increase in the positive staining for apoptotic cell at tissue temperatures of 50°C and above. It indicated that the TUNEL stain to detect cell death was more sensitive than routine histology.
Conclusion: The results from this in vivo study indicate that 50°C and 5 min heating of rabbit urethra during interstitial RFA is the minimum temperature for heat injury of the normal rabbit urethra as measured at 48 h after treatment.
Introduction
Transperineal radiofrequency interstitial tumor ablation (RITA) of the prostate is a novel technology that enables precisely targeted low-level radiofrequency (RF) energy to heat and ablate tumor tissue. Recently, transrectal ultrasound (TRUS)-guided RITA therapy has been demonstrated to be effective in the treatment of patients with prostate cancer Citation[1–3]. RF lesions are created during ablation due to resistive heating as RF current passes through tissue. Targeted tissue is irreversibly destroyed by coagulative necrosis with temperature being the direct determinant of margins defining a thermal sphere of necrosis Citation[4]. However, RFA of tumors has the potential risk for thermal damage to nearby normal collateral tissues Citation[5]. Potential complications of RFA for prostate tumor include nerve, bowel, bladder, lower urinary tract, and vascular injury during ablation process. Perforation or fistulous connection with these nearby structures is possible Citation[6],Citation[7]. Thus, the goal of creating a sufficient area of tumor necrosis must be weighed against the risk for injury to collateral tissues, and mechanisms for limiting maximum temperatures of surrounding organs during ablation are necessary for patient safety Citation[8],Citation[9]. When temperature-sensitive structures are near the target lesion, thermometry could further increase the safety of this evolving minimally invasive procedure. It is important to measure the temperature that causes cell death of these structures first in order to effectively use this technique.
For hyperthermia, elevation of tissue temperature in a range of 50° to 60°C would induce irreversible cellular damage within minutes. With higher temperature elevation (60–100°C), the irreversible damage is essentially instantaneous Citation[10],Citation[11]. Different tissues have variable thermal thresholds Citation[12]. For instance, intentional nerve damage to treat pain indicates that 45°C is the threshold for RFA thermal damage for nervous tissue Citation[13–16], while myocardial injury occurs when a temperature of approximately 50°C has been reached Citation[7],Citation[17]. A temperature of 45°C has been used as the upper limit of heating for hyperthermia near the bowel Citation[18–20]. Unfortunately, no such threshold has been determined for RFA in vivo of urethral tissue which might be helpful for RFA treatment of prostate cancer.
Therefore, the objective of the present investigation was to evaluate heat injury of urethral tissue in a rabbit model. Histopathology analysis was used to determine the minimum temperature required to achieve urethral tissue heat injury following interstitial RFA. This limiting maximum temperature tolerance of urethra during ablation would provide guidance for RFA treatment to ensure patient safety.
Materials and methods
Animals
All the studies were conducted with the approval of the animal care committee of our country. The experiments were performed using 37 healthy New Zealand white rabbits (Experimental Animals Center, Shanghai, China) weighing 2.5–3.0 kg each. They were allowed to acclimatize for at least 5 days before they were used and kept in a closed system. Environmental temperature was regulated between 18 and 20°C and non-regulated relative air humidity was approximately 60%. The animals were allowed food and water ad libitum between the various procedures.
Experimental design
Thirty-seven rabbits were numbered using a random number table, one was the control animal, the other 36 rabbits were divided into 6 groups (6 rabbits per group), named group A, B, C, D, E and F, respectively. One animal in every group was chosen as the sham-treated rabbit. Rabbits were anesthetized by an intraperitoneal injection of 30 mg/kg of sodium pentobarbital (Shanghai Pharmacy, Shanghai, China). All hair of the animal's dorsal skin was removed. All the animals were kept in the supine position after a grounding dispersive electrode pad was placed on the dorsum. Unless otherwise noted, body (nasopharyngeal) temperature was maintained between 38.5° and 39.5°C by means of a heating blanket.
Radiofrequency ablation
All procedures were performed with the RITA 1500 generator (RITA Medical Systems, Mountain View, CA). The electrode used was the RITA Starburst XL, which has a 14-gauge diameter with one needle on which a thermistor is integrated into the tip. The circuit consisted of dispersive electrodes connected to the generator and electrodes delivering the RF current to the tissues.
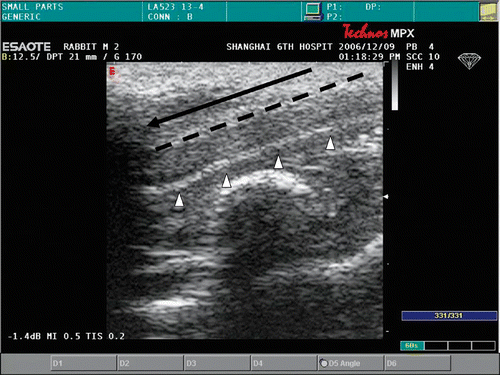
After sterilizing the perineal region of the rabbit, the needle was advanced into the penial parenchyma along its long axis, and the real-time temperature monitoring thermocouple sensor was placed parallel into the urethra (). Correspondence of the tip of the needle to the head of thermocouple sensor was assured by preoperative marking of the skin area above the root of the penis. The generator was switched to automatic H mode and a moderate power level of 30 W was used. The temperature of the radiofrequency generator was set to 48°C, 49°C, 50°C, 51°C, 52°C, and 53°C respectively according individual group, and the time for the required temperature to be maintained was set to 5 min. The temperature to be obtained in the urethral tissue was monitored by the thermocouple sensor. Sham-treated animals consisted of rabbits that were treated but without the application of ablation. The control animal was treated with nothing. Temperatures and impedances were continuously monitored through a real-time image displayed on the RITA generator. Measured parameters of the rabbits during the experiment were recorded.
Histologic analysis
The animals were sacrificed by administering a lethal dose (90 mg/kg) of intravenous sodium pentobarbital 48 h after the heating. The penises corresponding to the RFA levels were removed carefully. The mucosa surface of the urethra was examined externally for charring, pitting and perforation. The specimens were fixed in 10% phosphate-buffered formaldehyde for subsequent embedding in paraffin and histopathology. The serial histology sections (5 µm) were obtained in the transverse planes. Necrotic regions were determined based on the characteristic morphological features recognizable on hematoxylin and eosin (HE)-stained sections. Digital images of the regions including urethral mucosa, submucosa tissue and muscular layer surrounding the urethra were analyzed using the RF and temperzature measurement probes as reference points. Using a BX-50 microscope (Olympus, Tokyo), cells were classified into one of two categories by the following histological criteria at a magnification of ×400 : (1) intact cell – characterized by round to oval nuclei with delicately stippled chromatin, preserved spindled cell shape without cytoplasmic retraction, and pale eosinophilic cytoplasm; (2) coagulative necrosis – characterized by indistinct cellular detail with relatively preserved tissue structure, including pyknotic to karyorectic nuclei and shrunken hypereosinophilic cytoplasm. Tracing a necrotic cell by hand on the screen, and the percentage of necrosis was calculated by NIH Image software for every section. We evaluated the necrosis percentage of selected areas in the urethral mucosa, submucosa tissue and muscular layer tissue set as the region of interest. The thermal injury grade was obtained from all the different tissues in each group and the average of five different sections in each group was the represented result of the treatment group.
TUNEL stain analysis
The terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay was used to assess the heat injury independent of the morphological criteria. A TUNEL assay was carried out using an ‘in situ cell death detection kit’ (Roche, Applied Science). Formalin-fixed paraffin-embedded tissue sections were de-waxed by heating at 60°C followed by immersion in xylene and rehydrated through a graded series of ethanol and double-distilled water. Endogenous peroxidase was blocked by treating the sections with 0.45% H2O2 in methanol for 10 min at room temperature. TUNEL reaction mixture was prepared fresh according to the manufacturer's guidelines, by mixing the enzyme solution and label solution in the correct proportions. As negative control, only label solution was added to the tissue sections. Sections were incubated in a humidified chamber for 1 h at 37°C with 50 µl of TUNEL reaction mix or label only then washed in PBS for 15 min with two changes of buffer. Converter-POD was added to the sample and covered with a cover-slip, and slides were incubated in a humidified chamber for 30 min at 37°C. Slides were then washed with PBS as before. DAB chromagen/substrate (SIGMA: 1 × 10 mg tablet in 15 ml TBS, pH 7.6 with 1 µl 30% hydrogen peroxide) was added and slides incubated for 2 min at 25°C then rinsed in running tap water for 5 min and counterstained with Mayers hematoxylin.
The TUNEL stain images obtained were analyzed for positive staining at a magnification of 400 × using quantitative histochemical analysis software Image Pro Plus (Media Cybernetics, Baltimore, MD). First, two or three sections of each group were used for all RF-treated, sham-treated and control cases. All the selected cells found in the region of urethral mucosa, submucosa tissue and muscular layer between the RF and temperature measurement probes were sampled and all the structures identified as spheroids were also analyzed. The images obtained were captured on an image analysis system and analyzed with the Image Pro Plus software. Second, TUNEL stained intensity of dead cells was analyzed quantitatively among all the groups. We evaluated the integrated optical density (IOD) selected in the urethral mucosa, submucosa tissue and muscular layer tissue set as the region of interest. The ‘integrated’ refers to the sum of all the pixel intensity or density values in a given region Citation[21]. The numerical values obtained from the 10 different regions in each tissue were then averaged as representing the specified marker in the given tissue of each animal. The final value was the average of all the different tissues in each group. Higher values represent stronger staining.
Statistical analysis
Continuous data are presented as means plus or minus the standard deviation. The statistical analysis was performed using the SPSS statistical software (Version 10.0; SPSS Science, Chicago). Comparison of the data of individual groups was performed using one-way ANOVA and Student-Newman-Keuls (SNK) test for multiple comparisons. A probability of 0.05 was considered to be statistically significant.
Results
All of the 37 rabbits undergoing the experiment tolerated the procedure well and there was no mortality during the procedure. The measured parameters of the 37 rabbits in the experiment are also compiled in . There were no significant differences between the body temperatures before and after anesthesia used in the rabbits (P > 0.05). A total of 30 urethras were successfully ablated. The mucosa surface of the urethra was inspected and no visible chars were seen in the entire specimen. No complications, including crater lesions and perforations, were associated with any ablation.
Table I. Measured parameters of rabbits during experiment.
Histological analysis
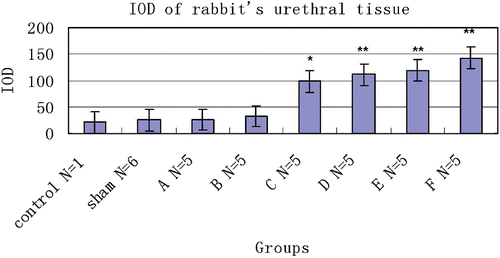
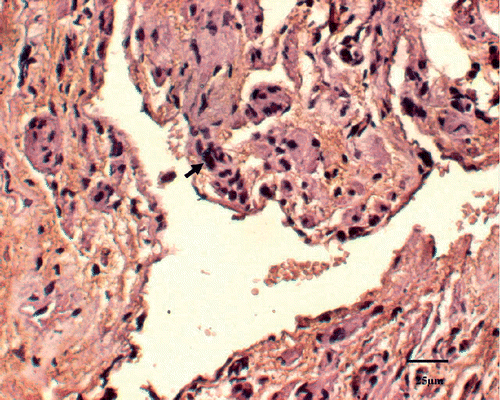
A histological examination was used to quantify tissue injury after thermal treatment. In the control animal, 1.52 ± 2.42% of the tissue had coagulative necrosis. Sham-treated tissue showed an insignificant percentage of necrosis (3.25 ± 1.36%) compared with that of the control animal (P > 0.05). Conversely, the percentage of coagulative necrosis progressively increased with RF ablation temperature after therapy and was significantly greater than in sham-treated tissues at both E(52°C) (P < 0.01) and F(53°C) (P < 0.01) groups. As the P values for percentage necrosis in Groups C and D in are very near significant, these values approached significance. The histological section showed the urethral mucosa, submucosa tissue and muscular layer surrounding the urethra were displayed clearly in the control tissue (). For treated groups, the main feature of acute heat damage was necrosis (mucosa, submucosa tissue and muscular layer) or vascular thrombosis of blood vessels at the urethral wall (). This occurred only in one out of five cases at 49°C and 50°C groups, but in four out of five cases at 52°C and 53°C groups. All lesions followed the general trend in which increasing temperatures were associated with proportionally higher percentages of necrosis (). The necrosis percentages of acute thermal damage for the treated groups were 1.52 ± 2.42%, 3.25 ± 1.36%, 4.71 ± 5.48%, 9.84 ± 5.07%, 20.59 ± 7.22%, 26.42 ± 3.48%, 61.39 ± 9.37% and 69.73 ± 11.74%, respectively (). The differences of necrosis percentages were significant at a tissue temperature of 52°C and above. But there were no significant changes among the tissues of control, sham treated, and A(48°C), B(49°C), C(50°C) and D(51°C) groups.
Figure 2. The histological section showed the urethral mucosa and submucosa tissue surrounding the urethra were displayed clearly in control tissue (▴). The intact cell is characterized by round to oval nuclei with delicately stippled chromatin, preserved spindled cell shape without cytoplasmic retraction, and pale eosinophilic cytoplasm (↑).
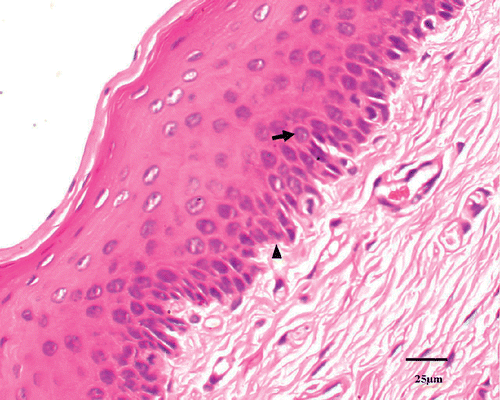
Figure 3. Coagulative necrosis is characterized by indistinct cellular detail with relatively preserved tissue structure, including pyknotic to karyorectic nuclei and shrunken hypereosinophilic cytoplasm (↑).
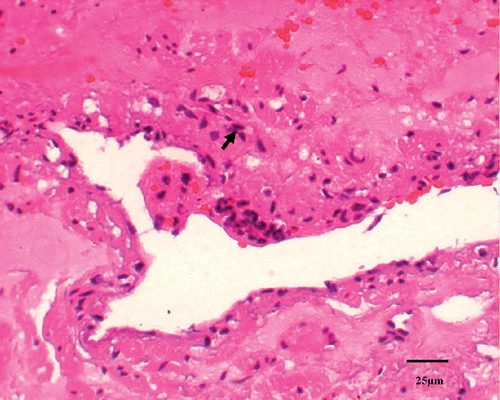
Figure 4. Mean necrosis percentages measured histologically in control (N = 1), sham-treated (N = 6) and RF-treated groups (N = 5/group). Also shown are results from untreated control animal (N = 1) for comparison. **P < 0.01.
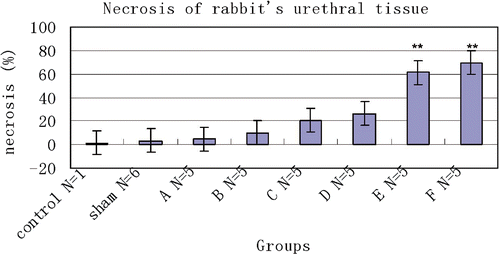
Table II. Necrosis and IOD of urethral tissue with different temperature after RFA.
TUNEL stain analysis
Dead cells by TUNEL stain were identified as those with a red-brown staining of the nucleus indicating the presence of nuclear DNA fragmentation. TUNEL staining on the samples demonstrated absence of apoptotic cells in the normal tissue (). There was minimal apoptotic activity in the tissues treated at 48°C and 49°C. However, in tissues treated for 50°C and above the positive stained apoptotic cells were more pronounced ().
Figure 5. TUNEL staining on the samples demonstrated absence of apoptotic cells in the normal tissue. The intact cell is characterized by round to oval nuclei without TUNEL-stained chromatin (↑).
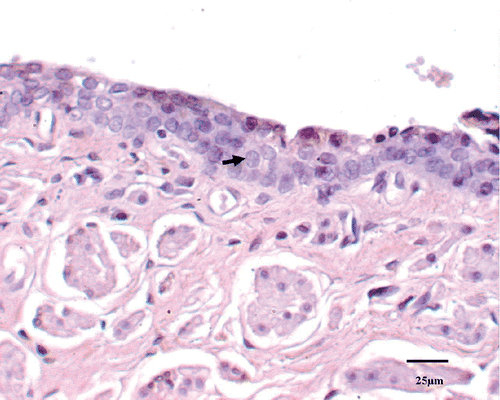
Figure 6. Dead cells by apoptosis stain were identified as those with a red-brown staining of the nucleus indicating the presence of nuclear DNA fragmentation (↑).
A steep increase in IOD distribution was observed between 48° and 53°C which suggests that this is the most critical temperature range. Quantitative image analyses demonstrated a significant increase in the positive staining of apoptotic cells at tissue temperatures of 50°C and above, which is significantly higher than that of control, sham-treated, 48°C and 49°C animals (). In groups A and B, no significant changes of IOD were seen compared with that of control and sham-treated groups. The IOD of positive staining cells was not significantly different statistically from group C(50°C), D(51°C), E(52°C), and F(53°C), but the increase in mean tissue IOD compared with sham-treated tissue was significant at each of 50°, 51°, 52° and 53°C groups (P < 0.05).
Discussion
RF energy has been employed extensively to treat benign prostatic hyperplasia (BPH) using the transurethral needle ablation (TUNA) device. Using a transurethral approach, coagulative necrosis is produced around the needles inserted into the prostate. However, in contrast to BPH, the therapy for prostate cancer (CaP) requires destruction of the entire organ, as CaP is frequently located in the peripheral zone and is by nature a multifocal disease. RFA, therefore performed through a transperineal approach, was more convenient and precise for localized CaP Citation[1].
RF energy produces ionic or molecular agitation and a collision of particles in accordance with the frequency of the energy wave generated. This produces heat in a much localized area around the point source, resulting in a central hot core. The continued delivery of RF energy supports the central core of heat, which then distributes by conduction to the surrounding tissue to produce the final lesion Citation[22]. However, the prostate is relatively small and surrounded by many important organs such as nerves, bladder, ureters, bowel, vessels and urethra, with the latter located in the center of the prostate. Therefore, potential complications induced by heat injury include nerve, bowel, bladder, lower urinary tract, and vascular injury during ablation of the prostate. Perforation or fistulous connection with these nearby structures is possible Citation[6],Citation[7]. Damage to the prostatic urethra may cause urinary incontinence or urinary fistula and secondary infection, a relatively major complication associated with transperineal prostate therapies.
Although some available RFA systems incorporate thermistors to evaluate tissue temperatures at the tip of the active RFA probe (Radionics, Burlington, MA), or near the margin and in between active tips (RITA Medical), these thermistors cannot be deployed separately from the probe itself. These thermistors are directed to the target tissue, but they are poorly positioned to provide precise temperature information about specific non-target tissue Citation[23],Citation[24]. So the use of thermometry to monitor the temperature changes near various vital structures is a safety mechanism to avoid complications of thermal damage in humans Citation[5].
In clinical therapy, to protect the urethra tissue from heat injury during RFA ablation of the prostate, doctors used a Foley catheter inserted into the urethra with cold saline infusion to cool the urethra. The cool-down cycle is a method to decrease the temperature of the urethral tissue in terms of dissipation of heat Citation[1], but the heat dissipated by conduction and convection through the Foley catheter is not an effective method for the heat non-heat-conductive silica gel wall. The urethral temperature was not measured during the process. So the mechanisms for limiting maximum temperatures of prostatic urethra during ablation are necessary for the patient's safety, and it is important to measure the temperature of cell death in order to effectively use ablation techniques.
Two distinct types of cell death are involved in tissue injury: necrosis and apoptosis (programmed cell death) Citation[25],Citation[26]. Although many mechanical and traumatic injuries are characterized predominantly by necrotic cell death, it is now recognized that apoptosis constitutes an important part of many pathological and physiological processes including heat-induced injury, ischemia, organ development, and tissue remodeling Citation[25],Citation[26].
The mechanism of tissue destruction by focal hyperthermia appears to be multifactorial and not purely related to direct thermal effects. There is increasing evidence that focal hyperthermia injury occurs in two distinct phases. There is an initial direct injury followed by a progressive phase of tissue damage occurring on cessation of the initial stimulus Citation[27]. This progressive injury may involve a balance of several factors, including apoptosis, microvascular damage, ischemia–reperfusion injury, Kupffer cell activation, altered cytokine expression, and alterations in the immune response Citation[27],Citation[28]. The exact mechanism of this process is undetermined. The full extent of tissue damage becomes evident 1 to 7 days after focal hyperthermia application, depending on the model used Citation[27],Citation[29],Citation[30]. In order to avoid secondary infection and other complications associated with urethra injury, we assessed the acute injury results of urethral tissue 48 h after the RFA.
Thermal injury can be better understood by examining the behavior of cellular components at supraphysiological temperatures. High temperatures cause alteration in conformations often leading to irreversible processes (denaturation), which affect cell viability and trigger cell death. The two cell death processes, necrosis and apoptosis, were distinguished from each other based on histological criteria and staining in the tissue specimens examined. Apoptosis can also be intensely induced by pathological stimuli, especially heat stress. However, routine histology to access the extent of immediate focal hyperthermic injury after heat application is generally inaccurate because morphological changes are a poor indicator of cell vitality at this stage Citation[31]. Alternatively, nuclear DNA fragmentation occurs relatively early in the process of apoptosis, preceding significant disruption of the nuclear membrane. It is a relatively late event in necrosis, occurring when there is already severe nuclear and/or cellular membrane disruption. The TUNEL method can provide an early indication that DNA fragmentation is occurring. Higher values represent stronger staining. The TUNEL staining labels fragmented DNA which is not specific for either apoptosis or necrosis Citation[32]. Therefore, the presence of cells positively stained in the TUNEL assay indicated apoptosis and necrotic tissue together.
In the present study, cell death by both apoptosis and necrosis was observed in different degrees in all tissue specimens examined. Differences observed in the degrees of cell death were found to be related to tissue temperature during RFA. Although some degree of tissue injury was observed at all temperatures studied, the higher temperatures (52°C and 53°C) appeared to generate a more severe degree of tissue injury than did the lower temperatures (48°C to 51°C). It has been indicated that as the distance increased from the RF needle, the predominantly necrotic area transitioned gradually to apoptosis Citation[33]. We found similar results that the more necrotic regions occurred in tissue at the higher temperature. In addition, histological sections revealed no significant heat damage features occurring at the treated areas of the groups at 51°C and below. Vessel thrombosis and congestion at the treated region were evident in some sections at 52°C and above. Conversely, TUNEL stain revealed significant heat damage at 50°C and above indicating that the TUNEL stain to detect cell death was more sensitive than routine histology. However, in our study the acute heat injury was observed 48 h after tissue RFA. The histological appearance of the necrotic and the apoptotic cells deserves further observation over a long time period. In addition, the temperature necessary to produce necrosis is completely dependent on the duration of heating. In this study we used different temperatures and a fixed heating time of only 5 min. Studies of thermal doses at different temperatures and heating times should be researched further.
The knowledge concerning the mechanisms of RF and characteristics of biological tissue are, however, largely derived from in vitro experiments. This may not be directly applicable to focal hyperthermia in vivo effects Citation[34]. So, in our study we performed the experiment in vivo on the model of rabbit. During the RFA process, we heated the urethral tissue near the root of the penis as far as possible, but not near the bladder because of osteoid tissue between the penis and bladder. In addition, RFA for 5 min is enough for a coagulative necrosis region with about 1 cm in diameter from our previous experiment, so the heating time of 5 min is also similar to that applied on humans.
With regard to basal anesthesia, we have to consider whether pentobarbital might have resulted in hypothermia, and perhaps alter the response of the tissues which is likely to be somewhat different from tissues under normal conditions. However, in a previous study, pentobarbital had only a limited effect on cerebrovascular tone in isolated canine blood vessels Citation[35]. Also, research has confirmed that pentobarbital did not significantly affect body temperature Citation[36],Citation[37]. In addition, from our results, it has almost no significant difference in the temperatures between pentobarbital treated and normo-thermic animals.
Limitations
This study was preliminary in nature and has limitations. First, we examined only the acute effects of heat injury during RF ablation. No conclusions about long-term implications can be reached without additional study. Second, the study was performed in a rabbit model. The thinner walls of the penis may have limited the complete assessment of the depth of lesion formation. Third, from the result of our previous study the prostate of a rabbit was confirmed to be a cystoid structure, and prostate tissue may not equate with penile parenchyma at all. As the penile parenchyma is full of blood vessels, the interstitial RFA of rabbit's prostate is impossible to be similar with the interstitial RFA of human prostate. In addition, the relatively high normal body temperature of rabbits in comparison to that of humans deserves some comments with regard to how our results apply to human disease. These limitations notwithstanding, knowledge of heat injury temperature of urethra may aid in the avoidance of urethra injury during RFA of the prostate.
Conclusion
We performed an experiment to compare urethra tissue temperatures of irreversible tissue injury with different RF energy delivery. The results from this in vivo study indicate that 50°C for 5 min of the urethra of the rabbit during interstitial RFA is the minimum heat injury temperature as measured 48 h after treatment. The placement of a urethra temperature probe affords a convenient method of temperature monitoring during ablation within the prostate.
Acknowledgements
This work was financially supported by the Research Program of the Shanghai Technology Committee and National Natural Science Funds of Chin (30770562). This study was supported by the hospital and school of medicine where the authors work. We thank Dr Y. Tian for helpful comments on statistical analyses and B.H.'s friend Ashley for sincere encouragement and careful examination on in this paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Djavan B, Zlotta AR, Susani M, Heinz G, Shariat S, Silverman DE, Schulman CC, Marberger M. Transperineal radiofrequency interstitial tumor ablation of the prostate: Correlation of magnetic resonance imaging with histopathologic examination. J Urol 1997; 50: 986–993
- Zlotta AR, Djavan B, Matos JC, Noel JC, Peny MO, Silverman DE, Marberger M, Schulman CC. Percutaneous transperineal radiofrequency ablation of prostate tumor: safety, feasibility and pathological effects on human prostate cancer. Br J Urol 1998; 81: 265–275
- Selli C, Scott CA, Garbagnati F, De Antoni P, Moro U, Crisci A, Eossi S. Transurethral radiofrequency thermal ablation of prostatic tissue: A feasibility study in humans. J Urol 2001; 57: 78–82
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005; 21: 755–760
- Diehn FE, Neeman Z, Hvizda JL, Wood BJ. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol 2003; 14: 1569–1576
- Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: Challenges and opportunities – Part II. J Vasc Interv Radiol 2001; 12: 1135–1148
- Jindal G, Friedman M, Locklin J, Wood BJ. Palliative radiofrequency ablation for recurrent prostate cancer. Cardiovasc Intervent Radiol 2006; 29: 482–485
- Haines DE. The biophysics of radiofrequency catheter ablation in the heart: The importance of temperature monitoring. Pacing Clin Electrophysiol 1993; 16: 586–591
- Dinerman JL, Berger RD, Calkins H. Temperature monitoring during radiofrequency ablation. J Cardiovasc Electrophysiol 1996; 7: 163–173
- Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: Challenges and opportunities – Part I. J Vasc Interv Radiol 2001; 12: 1021–1032
- Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer 2002; 94: 443–451
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Froese G, Das RM, Dunscombe PB. The sensitivity of the thoracolumbar spinal cord of the mouse to hyperthermia. Radiat Res 1991; 125: 173–180
- Yamane T, Tateishi A, Cho S, Manabe S, Yamanashi M, Dezawa A, Yasukouchi H, Ishioka K. The effects of hyperthermia on the spinal cord. Spine 1992; 17: 1386–1391
- Sminia P, van der Zee J, Wondergem J, Haveman J. Effect of hyperthermia on the central nervous system: A review. Int J Hyperthermia 1994; 10: 1–30
- Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: Temperature distribution in the spinal canal. Am J Roentgenol 2000; 175: 1263–1266
- Tungjitkusolmun S, Woo EJ, Cao H, Tsai JZ, Vorperian VR, Webster JG. Finite element analyses of uniform current density electrodes for radio-frequency cardiac ablation. IEEE Trans Biomed Eng 2000; 47: 32–40
- Li DJ, Qiu SL, Zhou SL, Liu HL. Acute heat injury to the normal swine rectum. Int J Hyperthermia 1988; 4: 191–201
- Sugarbaker PH, Sugarbaker C, Stephens AD, Chang D. Radiofrequency hyperthermia in the palliative treatment of mucinous carcinomatosis of appendiceal origin: Optimizing and monitoring heat delivery in western patients. Int J Hyperthermia 2000; 16: 429–441
- Larson BT, Bostwick DG, Corica AG, Larson TR. Histological changes of minimally invasive procedures for the treatment of benign prostatic hyperplasia and prostate cancer. Clinical implications J Urol 2003; 170: 12–19
- Mendonca DM, Chimelli L, Martinez AM. Quantitative evidence for neurofilament heavy subunit aggregation in motor neurons of spinal cords of patients with amyotrophic lateral sclerosis. Braz J Med Biol Res 2005; 38: 933
- Organ LW. Electophysiologic principles of radiofrequency lesion making. Appl Neurophysio1 1976–1977; 39: 69–76
- Eick OJ, Wittkampf FH, Bronneberg T, Schumacher B. The LETR-Principle: A novel method to assess electrode-tissue contact in radiofrequency ablation. J Cardiovasc Electrophysiol 1998; 9: 1180–1185
- Nakagawa H, Wittkampf FH, Yamanashi WS, Pitha JV, Imai S, Campbell B, Arruda M, Lazzara R, Jackman WM. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation 1998; 98: 458–465
- Teodiczyk-Injeyyan JA, Cembrzynska-Nowak M, Lalani S, Peters WJ, Mills GB. Immune deficiency following thermal trauma is associated with apoptotic cell death. J Clin Immunol 1995; 15: 318–328
- Allen RT, Hunter WJ, III, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods 1997; 37: 215–228
- Nikfarjam M, Malcontenti-Wilson C, Christophi C. Focal hyperthermia produces progressive tumor necrosis independent of the initial thermal effects. J Gastrointest Surg 2005; 9: 410–417
- Nikfarjam M, Muralidharan V, Su K, Malcontenti-Wilson C, Christophi C. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperthermia. 2005; 21: 319–332
- Whayne JG, Nath S, Haines DE. Microwave catheter ablation of myocardium in vitro: Assessment of the characteristics of tissue heating and injury. Circulation 1994; 89: 2390–2395
- Wiersinga WJ, Jansen MC, Straatsburg IH, Davids PH, Klaase JM, Gouma DJ, van Gulik TM. Lesion progression with time and the effect of vascular occlusion following radiofrequency ablation of the liver. Br J Surg 2003; 90: 306–312
- Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, Christophi C. Progressive microvascular injury in liver and colorectal liver metastases following laser induced focal hyperthermia therapy. Lasers Surg Med 2005; 37: 64–73
- Perry SW, Epstein LG, Gelbard HA. Simultaneous in situ detection of apoptosis and necrosis in monolayer cultures by TUNEL and trypan blue staining. Biotechniques 1997; 22: 1102–1106
- Fung LC, Mingin GC, Massicotte M, Felsen D, Poppas DP. Effects of temperature on tissue thermal injury and wound strength after photothermal wound closure. Lasers Surg Med 1999; 25: 285–290
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res 2005; 127: 208–223
- Hatano Y, Nakamura K, Moriyama S, Mori K, Toda N. The contractile responses of isolated dog cerebral and extracerebral arteries to oxybarbiturates and thiobarbiturates. Anesthesiology 1989; 71: 80–86
- Small PM, Tauber MG, Hackbarth CJ, Sande MA. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun 1986; 52: 484–487
- Iida H, Iida M, Ohata H, Nagase K, Dohi S. Hypothermia attenuates the vasodilator effects of dexmedetomidine on pial vessels in rabbits in vivo. Anesth Analg 2004; 98: 477–482