Abstract
Background: Magnetic fluid hyperthermia is a kind of technology for treating tumors based on nanotechnology. It is suitable to various types of tumors. The purpose of this study was to prepare carboplatin-Fe@C-loaded chitosan nanoparticles with Fe@C as a magnetic core and to investigate efficacy of hyperthermia combined with chemotherapy for transplanted liver cancer in rats.
Methods: Fe@C nanopowder was treated with dilute hydrochloric acid to prepare Fe@C nanocage. Carboplatin-Fe@C-loaded chitosan nanoparticles were prepared by reverse microemulsion method with the nanocages as the magnetic cores, chitosan as the matrix. The shape, size, drug-loading rate, and in vitro cumulative release of the nanoparticles were observed and heat product under high frequency alternating electromagnetic field in vitro was explored. Eighty rats with transplanted liver cancer were randomly divided into 4 groups (group A: control group, group B: free carboplatin group, group C: nanoparticles with static magnetic field group, and group D: nanoparticles with static field and alternating magnetic field). Drug was injected into the hepatic artery. The therapeutic effect of hyperthermia combined with chemotherapy for tumor, toxicity and rat survival time were observed.
Results: Carboplatin-Fe@C-loaded chitosan nanoparticles were spherical in shape with an average size of (207 ± 21) nm and high saturation magnetization. The drug-loading rate of the nanoparticles was 11.0 ± 1.1%. The cumulative release percentage of carboplatin-Fe@C-loaded chitosan nanoparticles in vitro at different point time phase of 24 h, 48 h, 72 h, 96 h and 120 h were 51%, 68%, 80%, 87% and 91%, respectively. With an increase in carboplatin-Fe@C-loaded chitosan nanoparticle concentration and magnetic field strength, the heating rate and constant temperature of carboplatin-Fe@C-loaded chitosan nanoparticles dispersed in physiological saline were increased in an alternating magnetic field. In vivo experiments showed that after particle injection, tumor temperature reached 42.6° ± 0.2°C within 10 min in the alternating magnetic field; and the temperatures in the right hepatic lobes and the rectum were significantly lower than in the tumor and the constant temperature could last up to 30 min. The inhibition ratio of tumor weight in group D was significantly enhanced, no obviously toxic and side-effect occurred and survival time was prolonged.
Conclusion: Carboplatin-Fe@C-loaded chitosan nanoparticles possess good magnetic targeting and heat production properties. They can target liver cancer tissue by static magnetic field, and with the application of alternating magnetic field, effectively raise tumor tissue temperature and facilitate tumor apoptosis. The combination of chemotherapy and magnetic materials into nanoparticles as described herein demonstrates promising efficacy.
Introduction
Primary liver cancer is a common malignant tumor. It possesses high malignancy and poor prognosis. In the world, 560,000 patients with primary liver cancer occur every year and 53% of these patients are Chinese Citation[1]. The traditional therapies include surgical ablation, radiotherapy and chemotherapy Citation[2], which for early liver cancer may obtain satisfactory effectiveness. However, for advanced liver cancer with local infiltration and distant metastasis, the therapeutic effect is poor because surgery is not useful. Therefore, how to improve the therapeutic effect of liver cancer has been become an investigative focus. Superparamagnetic nanoparticles can serve as magnetic targetable drug carriers. The carrier can target drug to tumor, increase drug concentration in tumor, simplify administration method, reduce drug consumption, decrease treatment costs, and lessen toxicity and side effects of drugs on the body Citation[3]. At the same time, in the alternating magnetic field, the nanomagnetic drug carrier absorbs electromagnetic waves to produce heat, which not only has a direct cytotoxic effect on tumor cells, but also may enhance the therapeutic effects of chemotherapy and radiotherapy, may improve immune response and inhibit tumor metastasis Citation[4]. Conventional microwave hyperthermia, high intensity focused ultrasound, radiofrequency therapy and endogenic field hyperthermia are often not able to create the temperature suitable to treat deep tumor tissue without causing certain damage to normal tissue, which limits the application of hyperthermia in liver cancer Citation[5],Citation[6]. In our study, carboplatin-Fe@C-loaded chitosan nanoparticle was prepared with Fe@C as a magnetic core, chitosan as the matrix. It possesses dual physical drug-loaded properties and has thermogenic action in an alternating electromagnetic field. The purpose of this study was to evaluate the therapeutic effect of hyperthermia combined with chemotherapy of carboplatin-Fe@C-loaded chitosan nanoparticles for transplanted liver cancer in rats.
Materials and methods
Materials and reagents
Fe@C nanopowder (average size 25 nm, content of iron 99.99%, carbon layer thickness 5 nm) were provided by Shenzhen Zunye Nano-materials (Shenzhen, China). Carboplatin (purity > 99%) was from Qilu Pharmaceutical Factory (Jinan, China), chitosan (MW 8291, deacetylated degree 95%) from Jinan Haidebei Ocean Biological Engineering (Jinan, China), aerosol OT (Aerosol OT, AOT) from Zhejiang Jiashanjufeng Chemical Plant (Jiaxing, China), 50% glutaral from Tianjin Kemiou Chemical Reagent Company (Tianjin, China) and normal heptane from Shantou Guanghua Chemical Plant (Shantou, China). Other reagents were of analytical grade and were used as received.
Preparation and characterization of Fe@C-loaded chitosan nanoparticles
Five hundred mg Fe@C nanopowder was put in 50 mL of dilute hydrochloric acid (0.1 mol/L); the reaction lasted 12 h at room temperature and Fe@C nanocages were prepared. The Fe@C nanocages were washed with distilled water until the cleaning solution pH was 7, then was freeze-dried for storage after magnetic separation. Twenty-five mg of Fe@C nanocages were soaked in saturated carboplatin aqueous solution for 24 h. After magnetic separation, the Fe@C nanocages were added to 5 mL of 1.5% chitosan-acetic acid solution, and then carboplatin was also added and mixed by sonication to form the aqueous phase. The surfactant, AOT, was dissolved in 100 mL of normal heptane to form the oil phase. Under sonication and stirring, the aqueous phase solution was added drop by drop into oil phase solution to form micro emulsion. After 30 min, 2 mL of cross-linking agent (50% glutaraldehyde solution) was added. The stirring and sonication were maintained for 1 h to allow solidification of the nanoparticles. The nanoparticles were washed with acetone, absolute alcohol and distilled water five times, respectively. After freeze drying, carboplatin-Fe@C-loaded chitosan nanoparticles were obtained. carboplatin-Fe@C-loaded chitosan nanoparticles were packed according to 5 mg per vial, and then sent to Guanzhou Radiation Center for cobalt radiation sterilization (radiation dosage 15 kGy). The appearance, structure and characterization of the carboplatin-Fe@C-loaded chitosan nanoparticles were observed and analyzed with transmission electron microscope and X-ray diffraction.
Drug content and encapsulation efficiency of carboplatin nanoparticles
Ten mg carboplatin-Fe@C-loaded chitosan nanoparticles were suspended in 10 mL of 1 M HCl by sonication. After 36 h, the supernatant was collected by centrifugation and magnetic separation, and then filtered through 0.22 µm filter membrane. The concentration of carboplatin in the supernatant was determined by complex spectrophotometry and the supernatant from the drug-unloaded nanoparticles was used as a contrast. The drug content of the nanoparticles was calculated by the following formula:
Ten mg carboplatin-Fe@C-loaded chitosan nanoparticles were suspended in 10 mL of distilled water by sonication, then centrifugated and underwent magnetic separation. The concentration of carboplatin in distilled water was determined, then was multiplied by the volume of distilled water to obtain the amount of free carboplatin. The encapsulation efficiency of the nanoparticle was calculated by the following formula:
In vitro release experiment
An aliquot of 100 mg carboplatin-Fe@C-loaded chitosan nanoparticles and corresponding quality of 11 mg carboplatin standard preparation (as control) were respectively suspended in 5 mL of distilled water, and then were sealed in a dialysis bag (molecular weight cut-off: 8000–12,000). The dialysis bag was incubated in 95 mL of distilled water at 37°C under horizontal agitation (100 rpm). An aliquot of 1 mL of the incubation medium was taken to measure the concentration of carboplatin at 24 h, 48 h, 72 h, 96 h and 120 h, respectively. The experiment above was repeated for 10 mL of bovine serum and 90 mL of physiological saline instead of distilled water. The carboplatin content was determined by complex spectrophotometry and the cumulative release percentage was calculated by the following formula:where W1 and W2 respectively represent carboplatin dissolution content and carboplatin total content in nanoparticles Citation[7].
Thermal effect in high frequency alternating electromagnetic field
Effects of magnetic fluid of different concentrations on thermogenesis
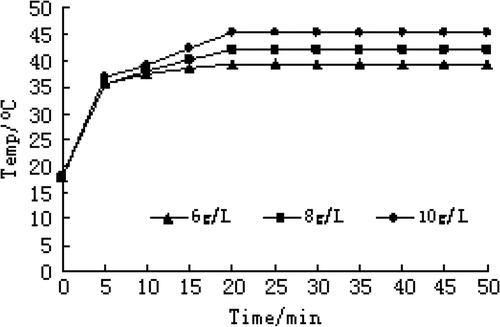
Carboplatin-Fe@C-loaded chitosan nanoparticles were diluted to 6 g/L, 8 g/L and 10 g/L of magnetic fluid with physiological saline. Aliquots of 2 mL magnetic fluid, 6 g/L, 8 g/L and 10 g/L were respectively put in an alternating electromagnetic field (frequency 180 KHz, output current 27 A, 4 coils, inside diameter 4 cm, magnetic density 0.027 T) at room temperature (20°C) to heat for 50 min and the temperature was measured every 5 min, which was repeated 3 times.
Effects of different electric field intensity on thermogenesis of magnetic fluid
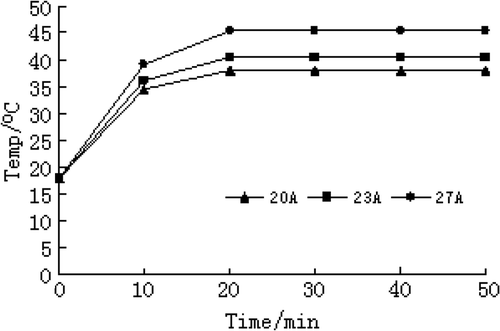
An aliquot of 20 mg Carboplatin-Fe@C-loaded chitosan nanoparticles was diluted with 2 mL of physiological saline to 10 g/L of magnetic fluid, then put in an alternating electromagnetic field (frequency 180 KHz; output current 27 A, 23 A and 20 A, respectively) to heat for 50 min and the temperature was measured every 5 min, which was repeated 3 times.
Establishment and treatment of transplanted liver cancer rat models
Transplanted liver cancer rat model and grouping
One hundred male SD rats weighing between 250 g and 270 g and 40 male SD infantile rats weighing between 80 g and 100 g were purchased from Guangdong Province Medical Experiment Animal Center (Animal certification number: SCXK2006-0002). The animals were treated according to animal ethics standards during experiment process. Rat Walker-256 carcinoma cell-line was purchased from China Center for Type Culture Collection in Wuhan University. Transplanted liver cancer rat models were prepared according to Schotman et al. Citation[8]. In brief, Walker-256 cells were inoculated into the peritoneal cavity of 20 infantile rats. After a week, the infantile rats were killed, then solid tumor was taken and cut into tumor pieces of 1 mm3, which was implanted beneath the left liver capsule in 100 rats. After 10 days, 80 transplanted liver cancer rat models were established and randomly divided. Eighty rat models were randomly divided into 4 groups (each group with 20 rats). Group A (control group) was given physiological saline, group B was given free carboplatin, group C was given Carboplatin-Fe@C-loaded chitosan nanoparticles and magnetic field, group D was given Carboplatin-Fe@C-loaded chitosan nanoparticles, magnetic field and hyperthermia.
Therapeutic methods and therapeutic effects
Therapeutic methods
After anesthesia, the peritoneal cavity of transplanted liver cancer rat models was opened. If the tumor grew well, the gastroduodenal artery was isolated, then its distal end was ligated and the common hepatic artery was blocked with vascular clamp. An incision was made on the gastroduodenal artery, a silica gel tube with 2 mm outside diameter was catheterized into the gastroduodenal artery and fixed, then the vascular clamp was removed and drug was given through the hepatic artery. To inject drug took 5 min per rat in the 4 groups. One mL of physiological saline was given in group A. Free carboplatin (10 mg/kg body weight (BW) of rats) was given in group B. Dispersal liquid of physiological saline of carboplatin-Fe@C-loaded chitosan nanoparticles (equivalently 10 mg/kg of carboplatin) was given, and then the tumor site was put in 5,000 Gs static magnetic field for 30 min followed by removing the magnet and closing the abdomen in group C. Particle administration and magnetic field in group D were the same as those in group C. After anesthesia by intraperitoneal injection of 0.5\% sodium pentobarbital (60 mg/kg BW), the hepatic site was put in the center coils of a high frequency induction heater (output frequency 180 KHz, output current 27 A) for 30 min in group D.
Temperature index
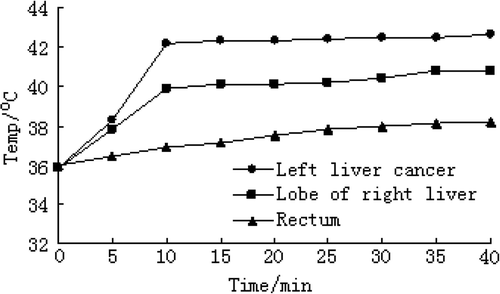
Temperatures in the left liver cancer, right hepatic lobe and the rectum were measured using 925 type single channel thermometer and plug-in probe (Lenzkirch, Germany) once every 5 min in group D when rats were receiving magnetic fluid hyperthermia.
Toxic effect on hepatic and renal function of rats
Ten rats were randomly taken from each group 7 days after treatment. Blood was collected by an orbital bleeding, and then blood serum was obtained by centrifugation to determined glutamate pyruvate transaminase (ALT), glutamic-oxalacetic transaminase (AST), total protein (TP), albumin (ALB), creatinine (Cr) and urea nitrogen (BUN).
Inhibition ratio of tumor weight
The ten rats whose blood was collected 7 days after treatment were killed by decapitation. Tumor tissue was isolated, and then weighed. Inhibition ratio of tumor weight was calculated according to the following formula.
Survival time of transplanted liver cancer rat models
Survival days were recorded in the rats with over 7 days of survival time. The observation on survival time lasted 60 days.
Statistical treatment
Life table method and logrank test were used in survival analysis. All measurement data were expressed as (χ ± s). Analysis of variance was used in multi-sample mean comparison. The comparison between two groups was performed by t-test. The statistical significance was established at P < 0.05.
Results
Shape and size of Fe@C and carboplatin-Fe@C-loaded chitosan nanoparticles
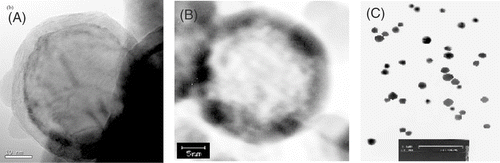
The core of Fe@C nanopowder was pure iron (purity 99.99%) and coated with 5 mm of active carbon (). After Fe@C nanopowder was treated with dilute hydrochloric acid, the pure iron in the center was dissolved to form a hollow nanocage (), and part of the active carbon of the inner layer was exposed. Carboplatin-Fe@C-loaded chitosan nanoparticles were fairly smooth and spherical in shape, and the average size was 210 ± 26 nm with a narrow distribution of 150–300 nm ().
Drug content and encapsulation efficiency of carboplatin-Fe@C-loaded chotosan nanoparticles
Drug content and encapsulation efficiency of carboplatin-Fe@C-loaded chitosan nanoparticles were 11.0% ± 1.1% and 91.28% ± 2.16%, respectively.
In vitro cumulative release percentage
When distilled water served as solvent, the release percentage of carboplatin standard preparation was 90% within 1 h, and in vitro cumulative release percentages of carboplatin-Fe@C-loaded chitosan nanoparticles in 24 h, 48 h, 72 h, 96 h and 120 h were 51%, 68%, 80%, 87% and 91%, respectively. When 10% bovine serum physiological saline served as solvent, the release percentage of carboplatin standard preparation was 91% within 1 h, and in vitro cumulative release percentages of carboplatin-Fe@C-loaded chitosan nanoparticles in 24 h, 48 h, 72 h and 96 h were 52%, 70%, 83% and 90%, respectively.
Influence of carboplatin-Fe@C-loaded chitosan nanoparticles of different concentrations on thermogenesis
Different concentrations of carboplatin-Fe@C-loaded chitosan nanoparticles, placed in a high-frequency alternating magnetic field for 20 min, can quickly reach the maximum temperature. The maximum temperature was constant and a balance between heat production and heat elimination developed. With the increase in magnetic fluid concentration, the rate of temperature rise was faster and constant temperature level was higher. Time-temperature relationship in magnetic fluid saline solution of different concentrations under current strength of 27 A is shown in .
Influence of different electric field intensity on thermogenesis of magnetic fluid
The same concentration of carboplatin-Fe@C-loaded chitosan nanoparticles placed in high-frequency alternating magnetic field with different current strength for 20 min, can quickly reach the maximum temperature. The maximum temperature was constant and a balance between heat production and heat elimination developed. With the increase in current strength, the rate of temperature rise was faster and constant temperature level was higher. Time-temperature relationship in 10 g/L of magnetic fluid saline solution under different current strength is shown in .
Temperature in tumor and other sites during magnetic mediated hyperthermia
shows that the temperature in the tumor sites of group D can quickly rise to 42°C within 10 min, which was maintained by regulating magnetic intensity. After the end of magnetic induction heating, the temperature in the tumor sites might drop to 36°C within 15 min. Meanwhile, during magnetic mediated hyperthermia, the temperature in the right hepatic lobe and the rectum were 40.8° ± 0.18°C and 38.2° ± 0.19°C, respectively. Except the initial moment, the temperature in the tumor site was significantly higher than those in the right hepatic lobe and rectum (P < 0.05). The temperatures in tumor sites of groups A, B and C were at about 36°C all the time.
Toxic effect on rat hepatic and renal function
shows that there was no significant difference after administering a series of biochemical indicators between experimental groups and the control group (P > 0.05)
Table I. The biochemical indicator levels in rat serum of each group after administration (x ± s).
Inhibition ratio of tumor weight
shows that there was no significant difference in average weight of tumor and inhibition ratio of tumor weight between groups A and B; there was significant difference in average weight of tumor and inhibition ratio of tumor weight between group A and groups C and D (P < 0.01); compared with group C, average weight of tumor was reduced and inhibition ratio of tumor weight was increased in group D (P < 0.01).
Table II. Tumor average weight and inhibition ratio of tumor weight in each group 7 days after treatment.
Survival time of transplanted liver cancer rat models
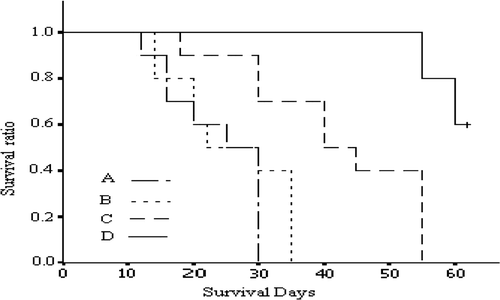
In group A the survival time was between 12 days and 30 days, with an average survival time of 22.6 days. In group B the survival time was between 15 days and 33 days with an average survival time of 23.5 days. In group C the survival time was between 30 days and 55 days with an average survival time of 39.4 days. In group D the shortest survival time was 52 days and 6 rats were still surviving at the observation deadline. Logrank analysis of survival time in the four groups showed that there was no statistical difference between groups A and B. Compared with groups A and B, the survival time in group C was significantly prolonged (P < 0.05 and P < 0.01); and compared with groups A, B and C, the survival time in group D was significantly prolonged (all P < 0.001) ().
Discussion
Carboplatin-Fe@C-loaded chitosan nanoparticles were fairly smooth and spherical in shape with an average size of 207 nm, and were coated with biodegradable, biocompatible and hydrophilic chitosan Citation[9]. Nanoparticles whose size is less than 300 nm can penetrate through the permeable neoplastic endothelium and stay in tumor to kill tumor cells. If its surface is hydrophilic, a nanoparticle (C-Fe@C-CN) can avoid phagocytosis of reticuloendothelial system and possesses the capability of long circulation Citation[10]. Therefore, after the nanoparticles entered the body by the feeding artery for the tumor or a peripheral vein, the nanoparticles could escape the rapid phagocytosis of the reticuloendothelial system and selectively stay in solid tumor by the guidance of the magnetic field. The nanoparticles are able to gather in tumor intercellular space through enlarged capillary endothelial intercellular space formed due to tumor tissue defect. Local drug concentration is increased and the duration of active drug is prolonged, so that the effectiveness of treatment is enhanced Citation[11].
Fe@C nanopowder possesses a certain ability of absorption and slow release because it has high specific surface area and active carbon Citation[12]. After dilute hydrochloric acid treatment, the pure iron in the center of Fe@C nanopowder was dissolved to form a hollow nanocage, and the partially active carbon of the inner layer was exposed, increasing specific surface and pore volume. The nanoparticles possess dual physical drug-loaded mechanisms (matrix encapsulation and adsorption of nanocage). In our study, the drug content and encapsulation efficiency of carboplatin-Fe@C-loaded chitosan nanoparticles were 11.0% and 91.28%, respectively. In vitro drug release of the carboplatin-Fe@C-loaded chitosan nanoparticles lasted 5 days. While the carboplatin release from the nanoparticles was not measured, the in vitro release data led us to speculate that the initially rapid release might reach effective concentration, and then steady and slow release may serve to maintain drug concentration and to extend drug action time, showing a good drug-released ability Citation[13]. There are various kinds of enzymes in blood, so that the biological degradation of chitosan and drug release are faster than in distilled water Citation[14]. In our study, the release percentage of carboplatin-Fe@C-loaded chitosan nanoparticles in 10% bovine serum physiological saline reached 90% on the fourth day, which is in line with the result above. The combination of the iron core and facile carboplatin loading produce a particularly useful particle that is capable of magnetic targeting to tumors and effective drug release once in tumors.
Carboplatin-Fe@C-loaded chitosan nanoparticles with different concentrations were placed in a high-frequency alternating magnetic field. When the current strength of the magnetic field was unchanged, with the increase in the concentration of magnetic fluid, the temperature rose faster and reached the maximum level after 20 min, and then the temperature was constant at the maximum level, showing that the temperature control and the balance between heat production and heat elimination may be realized by controlling the concentration of magnetic fluid, which is consistent with the results reported by others Citation[15]. When the carboplatin-Fe@C-loaded chitosan nanoparticles with constant concentration was placed in the magnetic field with different current strengths, with the increase in current strength the rate of temperature rise was faster, and the temperature level was in direct proportion to current strength, demonstrating that temperature control may come true by controlling the current strength of the magnetic field, which is consistent with the results reported by others Citation[16],Citation[17]. Carboplatin-Fe@C-loaded chitosan nanoparticles have good drug control release ability and can strongly absorb electromagnetic energy to produce heat, and the tumor temperature may be controlled by adjusting nanoparticle concentration and magnetic field current strength, so that carboplatin-Fe@C-loaded chitosan nanoparticles have a dual role–drug carrier and hyperthermia.
The inner cores of carboplatin-Fe@C-loaded chitosan nanoparticles are pure iron and their magnetic intensity is 2.6 times the magnetic intensity of saturated magnetic Fe3O4.Therefore, they possess higher saturation magnetization. Carboplatin-Fe@C-loaded chitosan nanoparticles were injected into the hepatic region through the hepatic artery and accumulated in the tumor site on the left side of the liver, showing good magnetic targeting ability. After carboplatin-Fe@C-loaded chitosan nanoparticles in the tumor tissue were put in an alternating magnetic field to heat, the temperature in the tumor site of the left liver rapidly rose to effective therapeutic temperature (42°C) which was maintained for 30 min by controlling the current strength of the magnetic strength. At the same time, the temperatures in right hepatic lobe and the rectum were measured. We found that the temperature in the left liver cancer was significantly higher than those in right hepatic lobe and the rectum (P < 0.05), demonstrating that most of the carboplatin-Fe@C-loaded chitosan nanoparticles had gathered in the tumor site. Although a few carboplatin-Fe@C-loaded chitosan nanoparticles gathered in normal liver tissue, the heat produced by the nanoparticles was rapidly cooled by greater blood flow of the portal venous system to avoid heat damage to normal liver tissue, showing no significant temperature change Citation[18]. After hepatic arterial injection of the Carboplatin-Fe@C-loaded chitosan nanoparticles, there were no significant differences in biochemical indicators of blood serum between each group and the control group, indicating that the administered carboplatin dose would not cause obvious side effects on normal tissues.
The second generation carboplatin anti-tumor drug is a cell cycle non-specific drug, which can cause the cross-linking between and in DNA strands to prevent DNA melting, breaking DNA structure, inhibiting tumor cell proliferation and directly killing cancer cells Citation[19]. Inhibition ratio of tumor weight in group D was 93% 7 days after treatment, which was 1.9 times that in group C, decreasing tumor growth velocity and extending survival time. The main reason is that carboplatin-Fe@C-loaded chitosan nanoparticles serve as both energy converter to heat the tumor and drug carrier to bring drug to the tumor, enabling hyperthermia and chemotherapy to act together in anti-tumor activity. The temperature at 42°–43°C both effectively kills tumor cells outright and increases the tumor sensitivity to chemotherapeutics Citation[20]. The combination of hyperthermia and chemotherapy mediated by carboplatin-Fe@C-loaded chitosan nanoparticles may increase therapeutic effect, which is mainly associated with the following factors.
Hyperthermia may change tumor plasmalemmal permeability, which make it easier for drugs to enter cells Citation[21].
Hyperthermia may change capillary blood flow and drug distribution in tissue, which influences drug absorption and metabolism Citation[22].
Hyperthermia can facilitate the cross-linking of anti-cancer drugs and tumor cells, and inhibit DNA repair enzyme activity of tumor cells after chemotherapy Citation[23].
Hyperthermia can reduce or prevent occurrence of drug resistance. It is reported that the sensitivity of the tumor cells which previously had resistance to chemotherapy is greatly enhanced after hyperthermia Citation[24].
The tumor cells in the center of the tumor are not sensitive to chemotherapy due to hypoxia, but are sensitive to hyperthermia due to low pH value. In contrast, the tumor cells in the tumor periphery are sensitive to chemotherapy. The combination of these complementary mechanisms in a single particle may improve therapeutic outcomes Citation[25].
Compared with group B, the average weight of tumor in group C was significantly decreased, inhibition ratio of tumor weight in group C was significantly increased and survival time in group C was significantly extended, which is mainly due to the slow release of carboplatin in carboplatin-Fe@C-loaded chitosan nanoparticles, the increase in concentration of chemotherapeutic drug in the tumor site and nanoparticle vascular embolization Citation[26]. Additionally, the therapeutic effect in group B was worse than that in group C, because the half-life period of free carboplatin and the duration of active blood drug level in the tumor site were short. There were no significant differences in tumor average weight, inhibition ratio of tumor weight and survival time between group B and the control group.
Our results indicated that the therapeutic effect in group D was better than those in group C and group B, further demonstrating that carboplatin-Fe@C-loaded chitosan nanoparticles given by the hepatic artery and put in an alternating magnetic field may facilitate the synergistic effect of hyperthermia and chemotherapy, and effectively inhibit the growth of liver cancer.
Conclusion
Carboplatin-Fe@C-loaded chitosan nanoparticles prepared by us have demonstrated tumor-targeting capability and can precisely heat the target site to reach effective therapeutic temperature without injuring the tissue around the target site. Carboplatin-Fe@C-loaded chitosan nanoparticles have good controlled release ability and can greatly increase therapeutic effect. Carboplatin-Fe@C-loaded chitosan nanoparticles are a potential nanomagnetic targeting hyperthermia drug carrier.
Acknowledgements
This work was partly supported by the Natural Science Foundation of Guangdong Province, China, No. 04006966. The authors thank Professor Qiang Guo for his excellent technical assistance in transmission electron microscopy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parkin DM, Bray F, Ferlay J. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55(2)74–108
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134(6)1752–1763
- Reddy LH. Drug delivery to tumours: Recent strategies. J Pharma Pharmacol 2005; 57(10)1231–1242
- Wust P, Gneveckow U, Johannsen M, Böhmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy–Feasibility, tolerance and achieved temperatures. Int J Hyperthermia 2006; 22(8)673–685
- Lindegaard JC, Nielsen OS, Overgaard J. A comparison between the effect of step-down heating in a tumour and a normal tissue in vivo. Int J Hyperthermia 1991; 7(3)519–526
- Adachi Y, Mizuno S, Yoshida M. Efficient large-scale purification of non-histone chromosomal proteins HMG1 and HMG2 by using Polybuffer-exchanger PBE94. J Chromatogr 1990; 530(1)39–46
- Arica B, Caliş S, Kaş H, Sargon M. Hincal A. 5-Fluorouracil encapsulated alginate beads for the treatment of breast cancer. Int J Pharm 2002; 242(1–2)267–269
- Schotman SN, Schraa EO, Marquet RL, Zondervan PE, Ijzermans JN. Hepatocellular carcinoma and liver transplantation: An animal model. Transpl Int 1998; 11(Suppl.1)S201–205
- Vinsova J, Vavrikova E. Recent advances in drugs and prodrugs design of chitosan. Curr Pharm Des 2008; 14(13)1311–1326
- Orel VE, Dzyatkovskaya NN, Romanov AV, Kozarenko TM. The effect of electromagnetic field and local inductive hyperthermia on nonlinear dynamics of the growth of transplanted animal tumors. Exp Oncol 2007; 29(2)156–158
- Santhi K, Dhanaraj SA, Rajendran SD, Raja K, Ponnusankar S, Suresh B. Nonliposomal approach-A study of preparation of egg albumin nanospheres containing amphotericin-B. Drug Dev Ind Pharm 1999; 25(4)547–551
- Cao H, Gan J, Wang S, Xuan S, Wu Q, Li C, Wu C, Hu C, Huang G. Novel silica-coated iron-carbon composite particles and their targeting effect as a drug carrier. J Biomed Mater Res A 2008; 86(3)671–677
- Cao H, Gan J, Wang S, Xuan S, Wu Q, Li C, Wu C, Hu C, Huang G. Novel silica-coated iron-carbon composite particles and their targeting effect as a drug carrier. J Biomed Mater Res A 2008; 86(3)671–677
- Wang Q, Zhang N, Hu X, Yang J, Du Y. Chitosan/starch fibers and their properties for drug controlled release. Eur J Pharm Biopharm 2007; 66(3)398–404
- Chen JH, Ling R, Yao Q, Wang L, Ma Z, Li Y, Wang Z, Xu H. Enhanced antitumor efficacy on hepatoma-bearing rats with adriamycin-loaded nanoparticles administered into hepatic artery. World J Gastroenterol 2004; 10(13)1989–1991
- Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother 2004; 53(1)26–32
- Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, Saida T, Kobayashi T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003; 94(3)308–313
- Jones SK, Winter J, Gray BN. Treatment of experimental rabbit liver tumors by selectively targeted hyperthermia. Int J Hypertherma 2002; 8(2)17–28
- Schneider BJ, El-Rayes B, Muler JH, Philip PA, Kalemkerian GP, Griffith KA, Zalupski MM. Phase II trial of carboplatin, gemcitabine, and capecitabine in patients with carcinoma of unknown primary site. Cancer 2007; 110(4)770–775
- Yan S, Zhang D, Gu N, Zheng J, Ding A, Wang Z, Xing B, Ma M, Zhang Y. Therapeutic effect of Fe2O3 nanoparticles combined with magnetic fluid hyperthermia on cultured liver cancer cells and xenograft liver cancers. J Nanosci Nanotechnol 2005; 5(8)1185–1192
- Gallo JM, Gupta PK, Hung CT, Perriet DG. Evaluation of drug delivery following the administration of magnetic albumin microspheres containing adriamycin to the rat. J Pharm Sci 1989; 78(3)190–194
- Ohtsubo T, Igawa H, Saito T, Matsumoto H, Park H, Song CW, Kano E, Saito H. Enhancement of cell killing by induction of apoptosis after treatment with mild hyperthermia at 42°C and cisplatin. Radiat Res 2001; 156(1)103–109
- von Ardenne M, Chaplain RA, Reitnauer PG. Selective injury to cancer cells by a combined attack with acidification, heat, vitamin A, dimethyl sulfoxide and other agents facilitating the release of lysosomal enzymes. Arch Geschwulstforsch 1969; 33(4)331–344
- Ohtsubo T, Saito H, Tanaka N, Matsumoto H, Sugimoto C, Saito T, Hayashi S, Kano E. Enhancement of cisplatin sensitivity and platinum uptake by 40°C hyperthermia in resistant cells. Cancer Lett 1997; 119(1)47–52
- Toyota N, Strebel FR, Stephens LC, Rowe W, Matsuda H, Oshiro T, Jenkins GN, Bull JM. Effect of altered duration of 41.5°C whole body hyperthermia in combination with cis-diamminedichloroplatinum (II) on tumor and normal tissue apoptosis and tumor response in rats. Oncol Rep 1998; 5(5)1231–1236
- Häfeli UO. Magnetically modulated therapeutic systems. Int J Pharm 2004; 277(1–2)19–24