presents one of the patients treated in Rotterdam last year. This then 76-year-old woman underwent excision of a small nodule on the tip of the nose in September 2007. Pathology showed basal cell carcinoma with adequate margins. There were wound-healing problems, however, and in January 2008 swelling and pain increased. New biopsies showed desmoplastic melanoma.
Figure 1. A patient with a desmoplastic melanoma of the nose before (A), and seven months after (B) treatment with radiotherapy (5 × 6 Gy) and hyperthermia (three treatments).
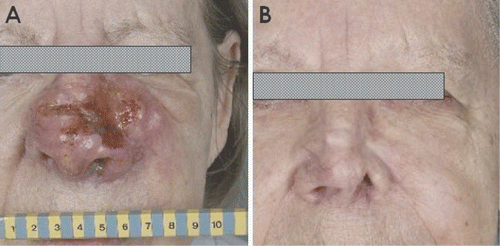
Staging studies showed enlarged lymph nodes in both sides of the neck, and metastases in both lungs. She was therefore treated with palliative intent. According to the guidelines of the Dutch malignant melanoma working group, the treatment consisted of radiotherapy and hyperthermia.
In May 2008 the tumour on the nose, extending to the left cheek (), was treated with radiotherapy, five fractions of 6 Gy twice weekly, and hyperthermia, three treatments of 60 min. The patient was referred again in December 2008, when the neck nodes started to cause complaints. The facial tumour then was in complete remission (). The neck nodes were also treated with radiotherapy (six fractions of 6 Gy) and hyperthermia (three treatments). The patient was seen again in February 2009 with some fibrosis at the site of the primary tumour without signs of tumour recurrence, and the neck nodes in ongoing regression. She had no problems of her lung metastases and has decided to refrain from systemic therapy for the moment.
In 1997, the Dutch malignant melanoma working group had included in their revised guidelines combined radiotherapy and hyperthermia for patients with irresectable tumours, especially when the aim of the treatment was long-duration local tumour control. In 2008, the Dutch Health Care Insurance Board had to advise about hyperthermia to be included in diagnosis-related groups: the system introduced in the Netherlands a few years ago to finance health care. One of the tumour entities that was accepted as standard indication for hyperthermia without further discussion was irresectable malignant melanoma when treated with radiotherapy. The decision of both the malignant melanoma working group and the Health Care Insurance Board was based on the level I evidence provided by the randomized study, published by Overgaard et al. in the Lancet in 1995 and, in more detail, in this journal in 1996 Citation[1],Citation[2].
In their paper Overgaard et al. describe the results of the multicentre randomized trial by the European Society for Hyperthermic Oncology (ESHO 3-85), in which the effect of radiotherapy plus hyperthermia was compared to that of radiotherapy alone in patients with malignant melanoma. The Lancet paper focuses on the local control data, while the International Journal of Hyperthermia paper presents a more detailed analysis of parameters related to the outcome of the study and a description of the quality of the hyperthermia treatment. The ESHO 3-85 trial was initiated after a number of previous studies had suggested a beneficial effect of hyperthermia in malignant melanoma. Several of these studies were performed in patients with multiple lesions Citation[3–8]. Per patient, the lesions were all treated with the same radiotherapy dose and in some of the lesions radiotherapy was combined with hyperthermia. All studies showed better complete response (CR) rates in the combined treated lesions. Also, some of the participants in ESHO 3-85 had previously published personal clinical experience with hyperthermia in malignant melanoma Citation[4],Citation[8],Citation[9–10]. Previous experimental and clinical work by Jens Overgaard formed the basis of the treatment schedule prescribed in ESHO 3-85 Citation[11],Citation[12].
For malignant melanoma, larger doses per fraction give higher response probabilities Citation[12]. In a mouse model, the effect of different treatment schedules of combined radiotherapy and hyperthermia on mammary carcinoma and its surrounding skin were studied. The results had shown that the thermal enhancement ratio for both tumour and skin was the highest when hyperthermia was given immediately after radiotherapy, but the thermal gain was highest with a time interval of 4 h between the two modalities. With this time interval, there was no enhancement of skin toxicity and still an enhancement of tumour effect. Another finding in this study was that when both radiotherapy and hyperthermia were given daily, and hyperthermia 4 h after radiotherapy, skin toxicity was moderately enhanced, which was not seen when the interval between two combined treatments was extended to 72 h. Apparently, a hyperthermia treatment can still have some radiosensitizing effect 24 h later.
In 12 patients with 49 cutaneous or lymph node melanoma metastases, similar differences were found between simultaneous or sequential combination of radiotherapy and hyperthermia: maximum thermal enhancement from the simultaneous combination, but maximum therapeutic gain with a time interval between the two treatments. On the basis of these results, simultaneous combination can only be recommended when the tumour is selectively heated.
In ESHO 3-85, radiotherapy was given in three fractions of 8 or 9 Gy with four days interval and hyperthermia was given after each fraction. The protocol prescribed that normal tissue heating should be avoided. A total of 134 metastatic lesions in 70 patients were included. In patients with multiple lesions, the lesions were given a number prior to randomization. If the first was randomized to radiation, the second would be given combined treatment and vice versa, while both lesions would receive the same radiation dose. Overall, the study showed that the combined treatment resulted in significantly better CR and local control (LC) rates than radiotherapy alone. The CR rate was 35% and 62% in the radiotherapy alone and the combined arm, respectively, and LC control probability after two years and longer 28% and 46%. The analysis on patients with multiple lesions further showed that in paired tumours from the same patients, all heated lesions with one exception responded equally or better than lesions given radiation alone. There was no difference in acute and late toxicity between the two treatment arms.
Of 168/170 hyperthermia treatments, the applied hyperthermia dose in equivalent minutes at 43°C (Eqt43) was available. As in all other studies, the prescribed minimum dose of 1 h at 43°C per treatment was not reached in the vast majority of treatments. There was a strong relationship between minimum and maximum Eqt43. An analysis of hyperthermia dose-effect relationship with CR or two years local control probability showed that the maximum Eqt43 averaged over treatments was the strongest hyperthermia treatment parameter significantly related to clinical outcome.
This study is one fine example of what can be achieved with combined efforts. Eleven European institutes participated in the trial. In spite of the relatively large number of institutes participating in this trial, it still took six years to complete the study. Whether a trial can be successful depends on several factors. The study treatment should fit in with what is standard practice in the participating institutes, the participating clinicians have to believe in the concept and the trial coordinator should put energy into motivating the participant to enter patients. This worked out well for the ESHO 3-85 trial, which is one of the studies that gave us a basis to continue hyperthermia research. We need more successful trials. The founding of the ‘Atzelsberg Circle’, the Scientific Study Group for Hyperthermia in Radiation Oncology and Medical Oncology under the leadership of Professor Rolf Sauer, is therefore a positive development. In this group a number of institutes are working together in the design of phase II and III trials. At present, studies on the use of hyperthermia in recurrent rectal cancer, anal cancer, peritoneal metastases of ovarian cancer, pancreatic cancer and prostate cancer are under development and/or close to being started. The enthusiasm of the participants gives hope that we will be able to demonstrate more of the therapeutic gain from adding hyperthermia to other treatment modalities, which will allow us to use it in the benefit of an increasing number of patients in the future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Overgaard J, González González D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995; 345: 540–543
- Overgaard J, González González D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996; 12: 3–20
- Scott RS, Johnson RJR, Kowal H, Krishnamsetty M, Story K, Clay L. Hyperthermia in combination with radiotherapy: A review of five years experience in the treatment of superficial tumors. Int J Radiat Oncol Biol Phys 1983; 9: 1327–1333
- González González D, Van Dijk JDP, Blank LECM, Rümke Ph. Combined treatment with radiation and hyperthermia in metastatic malignant melanoma. Radiother Oncol 1986; 6: 105–113
- Marchal C, Bey P. Study of parameters influencing tumour response after thermoradiotherapy. Paper presented at the ninth ESHO Meeting, Cardiff, July 1987
- Howard GCW, Sathiaseelan S, Freedman L, Bleehen NM. Hyperthermia and radiation in the treatment of superficial malignancy: An analysis of treatment parameters, response and toxicity. Int J Hyperthermia 1987; 3: 1–8
- Kim JH, Hahn EW, Ahmed SA. Combination hyperthermia and radiation therapy for malignant melanoma. Cancer 1982; 50: 478–482
- Overgaard J, Overgaard M. Hyperthermia as an adjuvant to radiotherapy in the treatment of malignant melanoma. Int J Hyperthermia 1987; 3: 483–501
- Arcangeli G, Nervi C, Cividalli A, Lovisolo GA. Problem of sequence and fractionation in the clinical application of combined heat and radiation. Cancer Research 1984; 44: 4857s–4863s
- Dunlop PRC, Hand JW, Dickinson RJ, Field SB. An assessment of local hyperthermia in clinical practice. Int J Hyperthermia 1986; 2: 39–50
- Overgaard J. Fractionated radiation and hyperthermia: Experimental and clinical studies. Cancer 1981; 48: 1116–1123
- Overgaard J. The role of radiotherapy in recurrent and metastatic malignant melanoma: A clinical radiobiological study. Int J Radiat Oncol Biol Phys 1986; 12: 867–872
