Abstract
Sanazole has been tested clinically as a hypoxic cell radiosensitizer. The aim of the present study was to investigate whether sanazole enhances apoptosis induced by hyperthermia at 44°C for 20 min in human lymphoma U937 cells. Sanazole alone induced continuous increase in the intracellular superoxide generation in a time-dependent manner and transient increase in the peroxide formation, which further were enhanced at 1 hour after HT treatment. Moreover, when the cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h, a significant enhancement of HT-induced apoptosis was evidenced by DNA fragmentation, morphological changes and phosphatidylserine externalization. Studying the apoptotic pathways involved in this enhancement, we found that loss of the mitochondrial membrane potential, release of cytochrome c from mitochondria to cytosol, and activation of caspase-3 and caspase-8 was enhanced significantly in the U937 cells after the combined treatment. Moreover, this combination enhanced activation of Bid, and down regulation of Hsp70. In addition, an increase in the intracellular Ca2+ concentration ([Ca2+]i), and externalization of Fas were observed immediately after sanazole and HT treatment. Our data indicate that sanazole can enhance the hyperthermia induced-apoptosis through the Fas-caspase-8- and [Ca2+]i-dependent apoptotic pathways. In addition, the down regulation of Hsp70 contributed to this enhancement.
Introduction
A combination of hyperthermia (HT) with radio- or chemotherapy for various solid tumors has been significantly effective Citation[1–3]. However, eradication of tumor cells is not always sufficient due to biological and technical difficulties. To overcome these difficulties, chemical heat sensitizers that enhance HT-induced apoptosis should be urgently developed. The induction of apoptosis has been proposed as a mechanism for HT-induced cell killing. The intracellular oxidative stress caused by reactive oxygen species (ROS) plays a key role in the induction of apoptosis Citation[4],Citation[5]. Recently, we found that HT-induced apoptosis was enhanced in human lymphoma U937 cells by excess oxidative stress due to some superoxide generators Citation[6–8] and by an intracellular H2O2 generator, 6-formylpterin(6-FP) Citation[9].
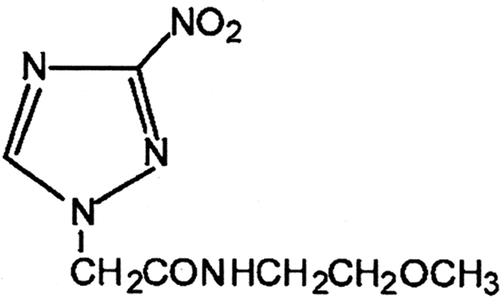
Sanazole (AK-2123) (N-2′-methoxy ethyl)-2-(3″-nitro-1″-triazolyl)acetamide () was presented as a new perspective hypoxic cell radiosensitizer due to its lower toxicity and higher radiosensitising effects Citation[10],Citation[11]. In aerobic conditions, sanazole may generate superoxide in the presence of the enzyme system NADPH/cytochrome P450 reductase Citation[12]. Some reports showed that sanazole can increase the efficiency of HT Citation[13],Citation[14]. However, little is known about how sanazole affects HT-induced apoptosis at cellular and molecular levels. In the present study, we aimed to examine the effect of sanazole in HT-induced (44°C for 20 min) apoptosis in U937 cells, and investigate the involved mechanisms in detail.
Materials and methods
Chemicals
Sanazole (AK-2123) was kindly provided as a gift by emeritus Professor Tsutomu Kagiya, Kyoto University. Sanazole was dissolved in RPMI 1640 medium to make desired concentrations for experimental use.
Cell line and cell culture
A human lymphoma cell line, U937, was obtained from the Human Sciences Research Resource Bank (Japan Human Sciences Foundation, Tokyo) and was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C in humidified air with 5% CO2.
Cell treatments
Cell suspension was first treated with sanazole for 40 min at 37°C. Then the cells were exposed to HT at 44°C for 20 min. After HT treatment, the cells were incubated at 37°C and harvested for the evaluation of apoptosis. Sanazole was kept in the cell suspension during HT and after HT. HT treatment was performed by immersing the plastic culture tubes containing culture medium (3 mL) in a water bath (NTT-1200, Eyela, Tokyo) at 44°C for 20 min. The temperature of the culture medium inside the test tube was monitored with a digital thermometer (#7563, Yokogawa, Tokyo) coupled with 0.8-mm diameter thermocouple during heating.
Measurement of DNA fragmentation
Quantitative DNA fragmentation assay was carried out according to the method of Sellins and Cohen Citation[15]. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Then the cells were lysed in lysis buffer (10 mM Tris, 1 mM EDTA and 0.2% Triton X-100, pH 7.5) and centrifuged at 13,000 g for 10 min. Subsequently, each DNA sample in the supernatant and the resulting pellet was precipitated in the 12.5% trichloroacetic acid (TCA) at 4°C and quantified using a diphenylamine reagent after hydrolysis in 5% TCA at 90°C for 20 min. The percentage of fragmented DNA in each sample was calculated as the amount of DNA in the supernatant divided by total DNA for that sample (supernatant plus pellet).
Morphological detection of apoptosis
The morphological changes in the cells were examined by Giemsa staining. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. The samples were harvested by centrifugation and washed by Dulbecco's phosphate-buffered saline (PBS). Then the cells were fixed with methanol and acetic acid (3 : 1) and spread on the glass slides. After drying, staining was performed with 5% Giemsa solution (pH 6.8) for 10 min. Cells featuring shrinkage and nuclear changes (condensation, fragmentation and apoptotic bodies) were considered as apoptotic cells Citation[8].
Apoptosis assay by flow cytomety
Flow cytometry was performed with propidium iodide (PI) and fluorescein isothiocyanate (FITC)-labelled annexin V (Immunotech, Marseilles, France) to detect phosphatidylserine (PtdSer) expression as an endpoint indicator of early apoptosis Citation[16]. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. The samples were washed with cooled PBS at 4°C and centrifuged at 1200 rpm for 3 min. The resulting pellets were adjusted to 105cells/mL with the binding buffer of the Annexin V-FITC kit. FITC-labelled annexin V (5 µL) and PI (5 µL) were added to 490 µL of the cell suspension and mixed gently. After incubation at 4°C for 10 min in the dark, the cells were analysed by flow cytometry (Epics XL, Beckman-Coulter, Miami, FL).
Measurement of intracellular superoxide
Dihydroethidine (DHE) (Molecular Probes, Eugene, OR) was employed to determine superoxide generation using the method of Gorman et al. Citation[17]. The DHE is a reduced non-fluorescent precursor. In the presence of superoxide anion species, DHE is oxidized into the fluorescent compound ethidium (Eth) that can be detected by virtue of its red fluorescence. Briefly, the cells were incubated with 5 µM DHE for 15 min at 37°C. Intracellular superoxide levels were assessed using flow cytometry (Epics XL) with excitation and emission settings of 488 and 600 nm, respectively.
Assessment of intracellular H2O2
Evaluation of intracellular H2O2 in U937 cells was performed by flow cytometry using the fluorescence generated in the cells loaded with the peroxide-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Molecular probes). H2DCF-DA reacts with H2O2 and/or peroxides, and is oxidized to a green fluorescent dichlorofluorescein (DCF). After treatment with sanazole, HT and the combination of sanazole and HT, H2DCF-DA was added to the cells at a final concentration of 5 µM, and then they were incubated at 37°C for 30 min. The cells were harvested and the fraction of DCF fluorescence positive cells was measured by flow cytometry for a total count of 1 × 104 cells (excitation at 488 nm; emission at 525 nm) as the proportion of cells containing intracellular H2O2 and/or peroxides Citation[18].
Mitochondria membrane potential (MMP)
A cationic fluorophore, tetramethylrhodamine methyl ester (TMRM) (Molecular Probes), accumulates electrophoretically in mitochondria in response to MMP and is released upon loss of MMP. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. The cells were collected, washed with PBS and centrifuged at 1200 rpm for 3 min. Then the cells were stained with 10 nM TMRM for 15 min at 37°C in 1 mL of PBS containing 1% fetal bovine serum followed by the immediate flow cytometry of red TMRM fluorescence (excitation at 488 nm; emission at 575 nm) Citation[8]. Percentage of cells showing low MMP cells was obtained from cell counts falling into a 0.1–12 low window of the TMRM log scale.
Assessment of intracellular caspase-3 and caspase-8 activity
The CaspGLOWTM Fluorescein Active Caspase-3 Staining Kit was used to monitor the intracellular caspase-3 activity according to the manufacturer's recommendations (MBL, Nagoya, Japan). The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Then, 300 µL of each of the samples and control cultures was aliquoted into micro tubes, and 1 µL of FITC-DEVD-FMK was added into each tube and incubate for 0.5 h at 37°C incubation with 5% CO2. Then samples were analysed by flow cytometry (Epics XL, Beckman-Coulter, Miami, FL) using the FL-1 channel.
To measure capase-8 activity, we employed a FLICE/Caspase-8 Colorimetric Protease Assay Kit (MBL). The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Then the cells were harvested, lysed, and the protein lysate was collected. The protein samples (100 µg) were mixed with 50 µL of 10 mM dithiothreitol (DTT) and IETD-pNA substrate was added at a final concentration of 200 µM. The mixtures were then incubated at 37°C for 2 h and then the activity was measured at 400 nm using a spectrophotometer (Beckman Instruments Inc., Fullerton, CA) Citation[19].
Western blot analysis
The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Then the cells were collected and washed with cold PBS. Cells were lysed at a density of 106 cells/50 µL of lysis buffer (1 M Tris-HCl, 5 M NaCl, 1% Nonidet P-40 (v/v), 1% sodium deoxycholate, 0.05% SDS, 1 mM phenylmethyl sulfonyl fluoride) for 20 min. After brief sonication, the lysates were centrifuged at 12,000 rpm for 10 min at 4°C, and the protein content in the supernatant was measured using a Bio-Rad Protein Assay reagent (Bio-Rad, Hercules, CA). The protein lysates (30 µg) were denatured at 96°C for 5 min after mixing with 5 µL SDS-loading buffer (62.5 mM Tris-HCl, 20% SDS, 5% 2-mercaptoethanol, 10% glycine, 0.001% bromophenol blue) and applied on SDS polyacrylamide gel (Daiichi Pure Chemicals, Tokyo) for electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences, Amersham, UK). Western blot analysis was carried out to detect expression of Bcl-2, Bax, Bid and β-actin using specific anti-Bcl-2 monoclonal antibody (mAb), anti-Bax polyclonal antibody (pAb), anti-Bid pAb, anti-Hsp70 mAb (Santa Cruz Biotech, Santa Cruz, CA), and anti-β-actin mAb (Sigma), respectively. Using the secondary HRP (horseradish peroxidase) conjugated anti-rabbit and anti-mouse IgGs, band signals were visualized on X-ray film by chemiluminescence ECL detection reagents (Amersham Biosciences) Citation[8].
For the preparation of cytosolic extracts, the cells were treated with HT or sanazole before they were incubated for 6 h at 37°C. Then the cells were harvested and suspended in an ice-cold solution containing 20 mM HEPES (pH 7.5), 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM phenylmethylsulphonyl fluoride, 10 µg/mL of leupeptin, 10 µg/mL aprotinin, 10 µg/mL pepstatin and 250 mM sucrose. The cells were disrupted using a Dounce homogenizer. Samples were centrifuged at 1500 g for 5 min at 4°C to remove nuclei and intact cells. The supernatant was centrifuged at 105,000 g for 30 min at 4°C. The resulting supernatant was used as the soluble cytosolic fraction Citation[9]. The protein content in the cytosolic fractions was determined as before. Following SDS-PAGE, Western blotting was performed to detect the cytochrome c release to the cytosol using anti-cytochrome c pAb (Santa Cruz Biotechnology) and anti-β-actin mAb (Sigma).
Flow cytometric detection of Fas on the cell surface
The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Then the cells (2 × 105) were washed twice with PBS, suspended in 20 µL of washing buffer containing 2.5 µg/mL of FITC-labelled anti-Fas monoclonal antibody (clone: UB3, MBL) and incubated for 30 min at 37°C and analysed with flow cytometry Citation[7].
Determination of intracellular concentration of calcium ion in single cells
The method has been described previously Citation[6]. The cells were collected by centrifugation and washed with Hepes-buffered Ringer (HR) buffer (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.13 mM MgCl2, 1 mM Na2HPO4, 5.5 mM glucose, 10 mM Hepes-KOH, pH 7.4). The supplemented HR buffer contained 0.2% bovine serum albumin (Sigma), Eagle's minimal essential amino acids (Flow Laboratories, Irvine, UK), and 2 mM L-glutamine. For dye loading, cells were suspended at a density of 1 × 105 cells in 1 mL of the supplemented HR, and loaded with 5 µM Fura-2-AM (Dojindo Lab) for 30 min at 25°C. The cells were transferred to a tube with or without sanazole. After washing, digital images of Fura-2 fluorescence were acquired and analysed by a digital image processor (Argus 50/Ca, Hamamatsu Photonics, Hamamatsu, Japan) coupled with an inverted fluorescent microscope. The fluorescence ratio (510 nm emission fluorescence at 340 nm excitation to that at 380 nm excitation), F(340/380), was used as an indicator of [Ca2+]i in single cells. Fura-2 color images of individual cells (see ) and the mean F(340/380) values were obtained immediately after HT.
Statistics
The results are expressed as the mean ± standard deviation. Significance was assessed with Student's t-test and was assumed for p-values <0.05. All experiments were performed in triplicate.
Results
Effects of sanazole on HT-induced apoptosis
To study the ability of sanazole to potentiate HT-induced apoptosis, DNA fragmentation was examined in the cells treated with sanazole (2, 5 and 10 mM), HT (44°C, 20 min) and the combination of sanazole and HT. We found that the combination of 10 mM sanazole and HT showed significant enhancement in the percentage of DNA fragmentation compared with HT alone at 6 h (). The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. Changes in cell morphology were examined by Giemsa staining under light microscope. The combination of sanazole and HT-induced typical morphological changes of apoptosis such as chromatin condensation and nuclear fragmentation were more prominent than in the cells treated with HT alone (). Flow cytometry using Annexin V-FITC and PI double staining revealed that the percentage of early apoptotic cells (Annexin V-FITC stained cells) was significantly higher after the combined treatment than that in the cells treated with HT alone at 6 h. In contrast, the percentage of secondary necrosis (Annexin V-FITC and PI stained cells) did not significantly change ().
Figure 2. The effect of sanazole, HT and the combination of sanazole and HT on DNA fragmentation in U937 cells. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. DNA fragmentation assay was carried out. The results are presented as the means ± SD (n = 3). *p < 0.05.
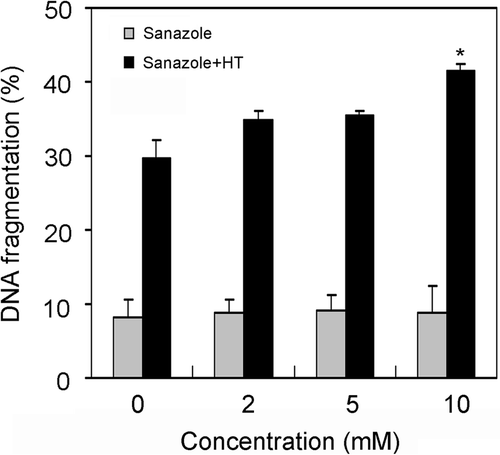
Figure 3. Assessment of apoptosis induced by sanazole, HT and the combination of sanazole and HT. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. (a) Apoptotic features in response to the combination with HT and sanazole in U937 cells. Signs of apoptosis were detected by Giemsa staining and then examined under a microscope at a magnification of ×400. The results are presented as the means ± SD (n = 3). (b) Cells were collected and stained with Annexin V-FITC and PI for flow cytometry. The results are presented as the means ± SD (n = 3). *p < 0.05.
Induction of ROS and oxidative stress by sanazole and HT
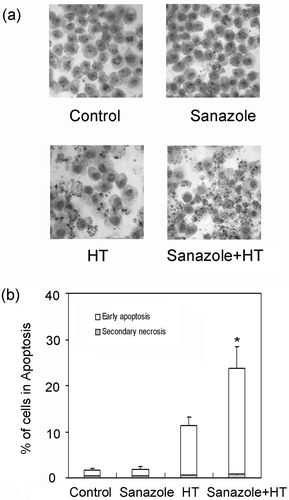
In the present study we evaluated intracellular levels of superoxide using DHE-staining. The results indicate that 10 mM sanazole induced higher generation of intracellular superoxide compared with 2 and 5 mM sanazole at 6 h (). 10 mM of sanazole induced elevated levels of intracellular superoxide in the cells in a time-dependent manner (). When the cells were treated with HT (44°C, 20 min) in the presence of sanazole and incubated at 37°C for 1 h, the fraction of cells with elevated superoxide anion radical levels increased significantly. The percentage of cells with high superoxide anion radical levels was 3.3 ± 1.9% in the control cells, 15.1 ± 4.1% in the cells treated with sanazole alone, 12.3 ± 2.2% in the cells treated with HT alone, and 41.3 ± 5.4% in the cells treated with the combination of HT and sanazole (). H2DCF-DA was used to evaluate the levels of intracellular H2O2. U937 cells were treated with 10 mM of sanazole for 1, 2, 3 and 6 h. The results showed sanazole alone can induce the generation of H2O2 at 1, 2 and 3 h (). And maximum level of intracellular H2O2 was reached at 2 h (). The combination of sanazole and HT significantly increased the level of H2O2 at 1 h after HT compared to sanazole alone (p = 0.05, ). N-acetyl-L-cysteine (NAC) was utilized to examine whether ROS production is an essential event for the enhancement of apoptosis due to the combination. We first treated cells with 5 mM NAC for 30 min and then co-treated with 10 mM sanazole for 40 min followed by HT for 20 min, and then incubated the cells at 37°C for 6 h. As shown in , 5 mM NAC significantly decreased DNA fragmentation induced by combined treatment with sanazole and HT (p = 0.05).
Figure 4. Generation of superoxide induced by sanazole, HT and the combination of sanazole and HT. (a) Dose-dependent changes in intracellular superoxide 6h after sanazole alone (2, 5 and 10 mM). (b) Time-dependent changes in intracellular superoxide (0, 1, 2, 3 and 6 h) after the treatment of 10 mM sanazole. (c) Induction of superoxide by sanazole and HT in U937 cells. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 1 h. The results are presented as the means ± SD (n = 3). *p < 0.05.
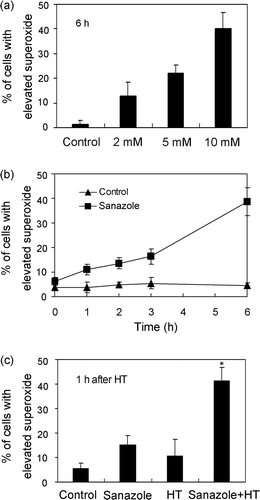
Figure 5. Effect of sanazole, HT and the combination of sanazole and HT on the generation of intracellular H2O2 in U937 cells. (a) Time-dependent changes in intracellular H2O2 induced by sanazole in U937 cells. (b) Induction of intracellular H2O2 by sanazole, HT and the combination of sanazole and HT. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 1 h. (c) Effects of NAC (5 mM) on DNA fragmentation induced by sanazole (10 mM), HT (44°C, 20 min) and the combination of sanazole and HT. The results are presented as the means ± SD (n = 3). *p < 0.05.
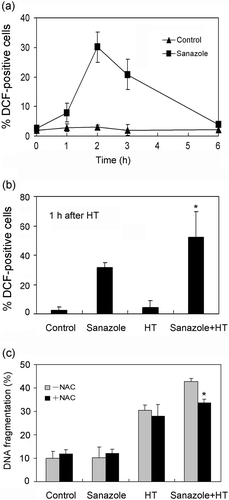
Detection of caspase-dependent mitochondrial pathway
Dysfunction of mitochondria and the activation of caspase have been known to play important roles in apoptosis Citation[20]. To examine whether the caspase-dependent mitochondrial pathway for apoptosis is related to the enhancement of HT-induced apoptosis by sanazole, we measured the MMP change, the intracellular caspase-3 and caspase-8 activities. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. The results showed that the combination of sanazole and HT significantly enhanced the loss of MMP compared to HT alone (). The increased intracellular caspase-8 and caspase-3 activity due to HT treatment was significantly enhanced by the combined treatment of sanazole and HT ().
Figure 6. Loss of MMP and activation of caspase-8 and -3 induced by sanazole, HT and the combination of sanazole and HT. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. (a) Loss of MMP was noted in the cells with HT treatment with or without sanazole as measured by flow cytometry using TMRM staining. (b) Activation of caspase-8 was measured by FLICE/Caspase-8 colorimetric protease assay kit. (c) Activation of caspase-3 was measured by flow cytometry using FITC-DEVD-FMK staining. The results are presented as the means ± SD (n = 3). *p < 0.05.
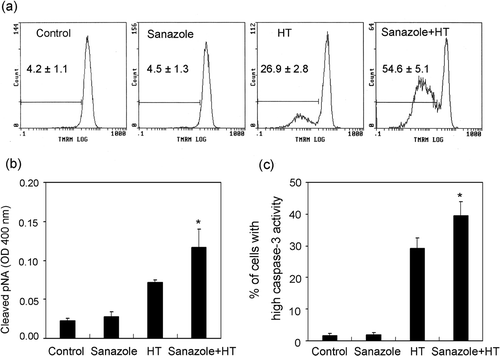
The Bcl-2 family proteins that have anti-apoptotic or pro-apoptotic functions can control the release of mitochondrial apoptosis factors including cytochrome c and apoptosis inducing factor (AIF) Citation[21]. The Western blot analyses revealed that the expression of Bid was significantly decreased after the combined treatment of sanazole and HT (). However, no changes in the expression of Bax and Bcl-2 were observed (). The release of cytochrome c from mitochondria to cytosol, which was induced by HT, was enhanced by sanazole (). These results suggest that the enhancement of HT-induced apoptosis by sanazole is associated with mitochondrial dysfunction, the activation of Bid, and the subsequent activation of caspases.
Figure 7. Changes in the expression of apoptosis-related proteins induced by sanazole, HT and the combination of sanazole and HT. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. (a) The changes of Bcl-2 family proteins. (b) Expression of cytochrome c in cytosolic fraction.
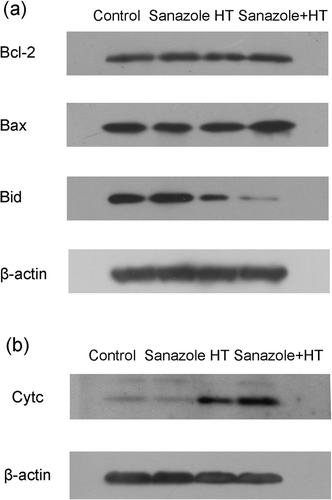
Expression of Hsp70 and externalization of Fas
Here the induction of Hsp70 was studied, since it attenuates cell death at the post-mitochondrial level Citation[22],Citation[23]. In U937 cells, Hsp70 was markedly induced 6 h after HT, whereas it was decreased after the combination of sanazole and HT (). The externalization of Fas was remarkably augmented 15 ± 3.1% 6 h after the combined treatment with HT and sanazole as compared with that after HT alone 10.9 ± 2% ().
Figure 8. Effects of sanazole, HT and the combination of sanazole and HT on expression of Hsp70 and externalization of Fas. The cells were treated first with 10 mM sanazole for 40 min, exposed to HT at 44°C for 20 min and the cells were further treated with the drug at 37°C for 6 h. (a) The expression of Hsp70 is measured by Western blot. (b) The externalization of Fas was measured by flow cytometry using anti-Fas FITC-conjugated antibody. The results are presented as the means ± SD (n = 3). *p < 0.05.
Figure 9. Effects of sanazole, HT and the combination of sanazole and HT on [Ca2+]i. U937 cells were stained with Fura-2/AM as described in Materials and methods immediately after treatment with HT in the presence or absence of sanazole, and [Ca2+]i was then measured. (a–d) Digital images of Fura-2 fluorescence. (e–f) The histograms of [Ca2+]i. (a, e) Control; (b, f) 10 mM of sanazole alone; (c, g) HT alone; (d, h) Combination of HT and sanazole.
![Figure 9. Effects of sanazole, HT and the combination of sanazole and HT on [Ca2+]i. U937 cells were stained with Fura-2/AM as described in Materials and methods immediately after treatment with HT in the presence or absence of sanazole, and [Ca2+]i was then measured. (a–d) Digital images of Fura-2 fluorescence. (e–f) The histograms of [Ca2+]i. (a, e) Control; (b, f) 10 mM of sanazole alone; (c, g) HT alone; (d, h) Combination of HT and sanazole.](/cms/asset/2e159192-3e31-4711-9302-3cc9d7a4ca94/ihyt_a_396913_f0009_b.gif)
Analysis of the Ca2+-dependent pathway
We carried out digital imaging of Fura-2 fluorescence to examine the change in [Ca2+]i immediately after the treatment of HT combined with or without sanazole. No difference in [Ca2+]i was observed in control and sanazole treated cells only (). However, after HT, the number of cells showing higher [Ca2+]i increased (). In the presence of sanazole during heat treatment, the cells exhibited much higher [Ca2+]i (). Histograms of [Ca2+]i after heat treatment with or without sanazole in 100 randomly selected cells from three independent experiments are shown in . The right shift of distribution in the histogram means increase in the number of cells containing higher [Ca2+]i.
Discussion
Previous studies have demonstrated that HT-induced apoptosis is affected by intracellular oxidative stress. The increase of intracellular ROS by HT is considered to be the major mechanism for HT-induced cell injury and the principle of hyperthermic treatment in cancer therapy Citation[24]. There is some evidence that HT induces increased levels of intracellular ROS such as superoxide, H2O2 and nitric oxide in various cell lines and tumor tissues Citation[25–28]. Among these ROS, H2O2 plays the key role in the apoptosis induced by HT Citation[29]. In addition, some studies reported that some ROS generators can enhance heat-induced apoptosis Citation[6–9],Citation[30]. In this study, we found that sanazole can induce intracellular superoxide and H2O2 generation, which were significantly increased in the combined treatment. Although sanazole alone was not capable of inducing apoptosis, the combined treatment can enhance apoptosis significantly compared with HT alone. Therefore sanazole could be suitable as a chemical sensitizer for such heat-induced apoptosis.
Caspases, a family of intracellular cysteine proteases, are the central executioners of apoptosis. Two pathways leading to caspase activation have been characterised Citation[31],Citation[32]. The extrinsic pathway is due to the direct interaction of cell surface receptors, such as Fas, with caspase-8, that in turn activates downstream effector caspases. On the other hand, the intrinsic pathway requires disruption of the mitochondrial membrane and the release of mitochondrial proteins including cytochrome c. Cytochrome c functions with Apaf-1 to induce activation of caspase-9, thereby initiating the apoptotic caspase cascade Citation[32]. These two apoptotic pathways could be interconnected by caspase-8-mediated cleavage of Bid, which triggers the activation of the mitochondrial pathway. In this study, an increase in the fractions of cells showing loss of MMP, release of cytochrome c to cytosol, and activation of capase-8 and caspase-3, were observed. These results indicate that the enhancement of HT-induced apoptosis by sanazole is due to activation of the caspase and mitochondrial-dependent pathway.
The Bcl-2 family of proteins is involved in pro- or anti-apoptotic processes by interacting with the mitochondria Citation[21]. Among them, Bcl-2 and Bcl-XL are known to be anti-apoptotic while Bax and Bid are known to be pro-apoptotic. In this study, the expression of Bcl-2 and Bax were unchanged as shown by Western blot analysis. However, the expression of Bid was significantly decreased by the combination treatment. Bid can be cleaved by caspase-8 and the cleaved Bid as the carboxyl-terminal fragment translocates to the mitochondria to induce the release of cytochrome c Citation[33]. The present data suggest that the activation of Bid by active caspase-8 may be one of the mechanisms contributing to the activation of mitochondrial pathways during the enhancement of apoptosis.
According to previous report, the activation of Fas is involved in the induction of ROS related apoptosis Citation[34]. And Fas/TNF-R1 is able to trigger apoptosis via a direct activation of the caspase cascade, or via mitochondria by activating caspase-8 and Bid Citation[35],Citation[36]. In this study we found an increase in ROS generation, externalization of Fas and activation of caspase-8 and Bid after the combined treatment of HT. These findings indicate that mitochondrial dysfunction is responsible for the contribution of Fas to the enhancement of apoptosis. The activation of Fas has been known to be associated with the down regulation of Hsp70 expression Citation[37]. At the post-mitochondrial level, Hsp70 prevents the recruitment of procaspase-9 to apoptosome by binding to Apaf-1, thereby inhibits the activation of caspase-3 Citation[22],Citation[23]. In this study, sanazole has significantly attenuated the expression of Hsp70 induced by HT. Therefore, down-regulation of Hsp70 appears to be involved in enhancing the apoptosis.
We have previously shown that an elevation of [Ca2+]i is involved in the enhancement of HT-induced apoptosis by some agents which are associated with increase in the ROS formation Citation[6–8],Citation[30]. It has been reported the oxidative stress causes a rise in [Ca2+]i in cytoplasm, which induces Ca2+ influx into mitochondria and nuclei to control apoptosis in chondrocytes Citation[38]. Judging from the present findings that ROS generation and increasing in [Ca2+]i was observed immediately after the combined treatment, the enhancement of apoptosis by sanazole is associated with the activation of a Ca2+ dependent pathway of apoptosis.
The present results have shown a synergism between sanazole and HT to cause apoptosis in human lymphoma U937 cells. Sanazole can enhance the apoptosis induced by HT through the Fas-caspase-8- and [Ca2+]i-dependent pathways. The down regulation of Hsp70 also contributes to the enhancement. Sanazole could be used as a chemical sensitizer for apoptosis induced by HT. For a better verification of the effectiveness of sanazole as a heat sensitizer, various cancer cells need to be tested in the future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 1999; 15: 79–107
- Dahl O, Mella O. Referee: Hyperthermia alone or combined with cisplatin in addition to radiotherapy for advanced uterine cervical cancer. Int J Hyperthermia 2002; 18: 25–30
- Prosnitz L, Jones E. Counterpoint: Test the value of hyperthermia in patients with carcinoma of the cervix being treated with concurrent chemotherapy and radiation. Int J Hyperthermia 2002; 18: 13–18
- Salganik RI. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 2001; 20: 464S–472S, Discussion 473S–475S
- Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 2000; 28: 1387–1404
- Arai Y, Kondo T, Tanabe K, Zhao QL, Li FJ, Ogawa R, Li M, Kasuya M. Enhancement of hyperthermia-induced apoptosis by local anesthetics on human histiocytic lymphoma U937 cells. J Biol Chem 2002; 277: 18986–18993
- Cui ZG, Kondo T, Matsumoto H. Enhancement of apoptosis by nitric oxide released from alpha-phenyl-tert-butyl nitrone under hyperthermic conditions. J Cell Physiol 2006; 206: 468–476
- Zhao QL, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 2006; 40: 1131–1143
- Wada S, Cui ZG, Kondo T, Zhao QL, Ogawa R, Shoji M, Arai T, Makino K, Furuta I. A hydrogen peroxide-generating agent, 6-formylpterin, enhances heat-induced apoptosis. Int J Hyperthermia 2005; 21: 231–246
- Shibamoto Y, Sakano K, Kimura R, Nishidai T, Nishimoto S, Ono K, Kagiya T, Abe M. Radiosensitization in vitro and in vivo by 3-nitrotriazoles. Int J Radiat Oncol Biol Phys 1986; 12: 1063–1066
- Dobrowsky W, Huigol NG, Jayatilake RS, Kizilbash NI, Okkan S, Kagiya VT, Tatsuzaki H. AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: Results of an IAEA multicentre randomised trial. Radiother Oncol 2007; 82: 24–29
- Schepetkin IA, Cherdyntseva NV, Kagiya VT. Sanazole as substrate of xanthine oxidase and microsomal NADPH/cytochrome P450 reductase. Pathophysiology 2001; 8: 119–127
- Osinsky SP, Bubnovskaya LN, Ganusevich II. In vivo termochemosensitizing activity and pathophysiological effects of hypoxic radiosensitizer AK-2123. Exp Oncol 1994; 16: 61–65
- Rao BS, Devi PU. Multimodality treatment using AK-2123, hydralazine, radiation & hyperthermia on a transplantable mouse tumour. Indian J Med Res 1996; 104: 182–189
- Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 1987; 139: 3199–3206
- van Heerde WL, de Groot PG, Reutelingsperger CP. The complexity of the phospholipid binding protein Annexin V. Thromb Haemost 1995; 73: 172–179
- Gorman A, McGowan A, Cotter TG. Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett 1997; 404: 27–33
- Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys 1993; 302: 348–355
- Datta R, Kojima H, Yoshida K, Kufe D. Caspase-3-mediated cleavage of protein kinase C theta in induction of apoptosis. J Biol Chem 1997; 272: 20317–20320
- Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998; 17: 3237–3245
- Tsujimoto Y, Shimizu S. Bcl-2 family: Life-or-death switch. FEBS Lett 2000; 466: 6–10
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000; 2: 469–475
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2000; 2: 476–483
- Skibba JL, Quebbeman EJ, Kalbfleisch JH. Nitrogen metabolism and lipid peroxidation during hyperthermic perfusion of human livers with cancer. Cancer Res 1986; 46: 6000–6003
- Yoshikawa T, Kokura S, Tainaka K, Itani K, Oyamada H, Kaneko T, Naito Y, Kondo M. The role of active oxygen species and lipid peroxidation in the antitumor effect of hyperthermia. Cancer Res 1993; 53: 2326–2329
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 1996; 93: 5116–5121
- Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Lett 1998; 431: 285–286
- Frank J, Kelleher DK, Pompella A, Thews O, Biesalski HK, Vaupel P. Enhancement of oxidative cell injury and antitumor effects of localized 44°C hyperthermia upon combination with respiratory hyperoxia and xanthine oxidase. Cancer Res 1998; 58: 2693–2698
- Katschinski DM, Boos K, Schindler SG, Fandrey J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J Biol Chem 2000; 275: 21094–21098
- Yu DY, Matsuya Y, Zhao QL, Ahmed K, Wei ZL, Hori T, Nemoto H, Kondo T. Enhancement of hyperthermia-induced apoptosis by a new synthesized class of benzocycloalkene compounds. Apoptosis 2008; 13: 448–461
- Green DR. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000; 102: 1–4
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15: 2922–2933
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501
- Nitobe J, Yamaguchi S, Okuyama M, Nozaki N, Sata M, Miyamoto T, Takeishi Y, Kubota I, Tomoike H. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res 2003; 57: 119–128
- Strasser A. Newton K. FADD/MORT1, a signal transducer that can promote cell death or cell growth. Int J Biochem Cell Biol 1999; 31: 533–537
- Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res 2000; 10: 161–167
- Schett G, Steiner CW, Groger M, Winkler S, Graninger W, Smolen J, Xu Q, Steiner G. Activation of Fas inhibits heat-induced activation of HSF1 and up-regulation of hsp70. Faseb J 1999; 13: 833–842
- Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes: Involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 2001; 50: 19–23