Abstract
The ESHO protocol 3-85 is a multicentre randomized trial investigating the value of hyperthermia as an adjuvant to radiotherapy in treatment of malignant melanoma. A total of 134 metastatic of recurrent malignant melanoma lesions in 70 patients were randomized to receive radiotherapy alone (3 fractions in 8 days) or each fraction followed by hyperthermia (aimed for 43°C for 60 min). Radiation was given with high voltage photons or electrons. Tumours were stratified according to institution and size (above or below 4 cm) and randomly assigned to a total radiation dose of either 24 or 27 Gy to be given with or without hyperthermia. The endpoint was persistent complete response in the treated area. A number of 128 tumours in 68 patients were evaluable, with an observation time between 3 and 72 months. Sixty-five tumours were randomized to radiation alone and 63 to radiation + heat. Sixty received 24 Gy and 68 tumours received 27 Gy, respectively. Size was ≤4 cm in 81 and >4 cm in 47 tumours. Overall the 2-year actuarial local tumour control was 37%. Univariate analysis showed prognostic influence of hyperthermia (rad alone 28% vs. rad + heat 46%, p = 0.008) and radiation dose (24 Gy 25% vs. 27 Gy 56%, p = 0.02), but not of tumour size (small 42% vs. large 29%, p = 0.21). A Cox multivariate regression analysis showed the most important prognostic parameters to be: hyperthermia (odds ratio: 1.73 (1.07-2.78), p = 0.02), tumour size (odds ratio: 0.91 (0.85-0.99), p = 0.05) and radiation dose (odds ratio: 1.17 (1.01—1.36), p = 0.05). Analysis of the heating quality showed a significant relationship between the extent of heating and local tumour response. Addition of heat did not significantly increase the acute or late radiation reactions. The overall 5-year survival rate of the patients was 19%, but 38% in patients if all known disease was controlled, compared to 8% in the patients with persistent active disease.
Introduction
The use of heat as a method to improve radiation response in tumours was clinically introduced in 1910 by Miiller who described the potential of using local diathermia as an adjuvant to radiotherapy Citation[1]. For many years thereafter sporadic reports enthusiastically demonstrated a potential improvement of radiotherapy but it was not until the mid-70's that the concept was explored more scientifically. This subsequently led to numerous uncontrolled studies which strongly suggested that adjuvant hyperthermia may increase the probability of controlling tumours by radiotherapy Citation[2], Citation[3], Citation[4].
The biological rationale for combining hyperthermia with radiation has been well explored and a detailed discussion of the rationale and strategies to apply heat and radiation has been given elsewhere Citation[4], Citation[5], Citation[6].
A major problem with clinical hyperthermia has been the ability to provide a homogeneous heating to a given tumour area and despite intense research in this area, the problem is far from solved. Nevertheless there has been an abundant clinical experience in uncontrolled studies where combined heat and radiation has been applied and in general they have all pointed towards a significant benefit of combining heat with radiation relative to radiation alone Citation[7], Citation[4]. Furthermore, these early clinical studies have indicated that a significant improvement in local control could be obtained without increasing normal tissue morbidity if either a relatively long interval between the two modalities was allowed for or if the tumours were heated (semi)selectively. From the early studies it appeared that especially three tumour sites have been the subject of combined heat and radiation therapy, namely advanced neck nodes, recurrent or advanced breast tumours, and malignant melanoma Citation[5], Citation[8], Citation[9], Citation[10], Citation[11], Citation[3], Citation[12]. Part of the reason for this was the superficial nature of these tumours, but accumulating evidence from early clinical trials indicates that a significant improvement in local control was likely to be obtained without increasing normal tissue morbidity. Thus dose-response analyses of available data indicated a thermal enhancement ratio in the order of 1.5 Citation[4].
The fact that malignant melanoma has been found to respond well to radiotherapy given in large doses per fraction Citation[13], and that the response apparently can be significantly improved by adding hyperthermia Citation[2], Citation[5], Citation[14], Citation[15], Citation[16], Citation[17], Citation[18], Citation[19], Citation[20], makes this tumour type one of the most suitable clinical models for investigations of the interaction between radiotherapy and hyperthermia. The few fractions which can be spaced with relatively long intervals (several days) apparently without influencing the tumour control probability Citation[21] allow treatment with hyperthermia in association with each radiation fraction without having the treatment compromised by the problems of thermotolerance Citation[22], Citation[23]. Thus a pilot study clearly indicated the feasibility of such strategy and several studies have indicated that a 3-fraction radiation schedule using a dose of 8–9 Gy per fraction appears to be one of the most suitable radiotherapy schemes for this disease Citation[24].
On this basis the European Society for Hyperthermic Oncology in January 1986 initiated a multicentre randomized clinical trial (ESHO protocol 3-85) with the aim to assess the efficacy of local hyperthermia given as an adjuvant to radiotherapy in the treatment of advanced malignant melanoma lesions, to evaluate the tumour response and local control probability, assess early and late tolerance in normal tissues, and evaluate the feasibility of various heating techniques Citation[12].
A report of the trial focusing on the local control data has previously been published Citation[25], but the present study contains a more detailed analysis of parameters related to the outcome of the study, describes the quality of the heat treatment and the impact of local control on survival.
Patients and methods
Patients
This multicentre randomized trial included patients with advanced, recurrent, or metastatic lesions of non-lentiginous malignant melanoma who were estimated to be candidates for radiotherapy. The patients should have a life-expectancy >3 months and should not be subjected to other cancer therapy (especially chemotherapy) concurrently with the protocolized treatment. The treated areas should not previously have been treated with radiotherapy. All lesions included should be considered feasible for heating with the available equipment.
The protocol has been performed according to the guidelines laid down in the Helsinki Declaration II. In addition, the protocol was adapted and approved by all relevant national or lccal ethical committees.
Trial design
Since patients with this disease may frequently have multiple lesions and the treatment applied was local, the protocol was designed to allow inclusion of multiple lesions in the same patients. Thus randomization was performed based on individual lesions rather than on patients.
Tumours were stratified according to institution and size (≤4 cm vs. >4 cm in largest diameter) and randomized to one of the following schedules:
Radiotherapy alone (24-27 Gy tumour dose to gross tumour). Radiation was randomized to be given in 3 fractions of either 8 or 9 Gy with 4 days' interval between fractions (i.e. Monday–Friday–Tuesday or Thursday–Monday–Friday); or
Radiation as above (randomized to either 8 or 9 Gy per fraction) with each fraction followed within 30 min by hyperthermia. The heat session should aim for a minimal tumour treatment temperature of 43.0°C in 60 min.
Randomization
Randomization was performed centrally by telephone or fax. In patients with multiple tumours, each tumour was given a number prior to randomization. The same radiation dose was given to both lesions and if the first was randomized to radiation, the second would be given combined treatment or vice versa. Patients with 3 tumours were considered as patients with 2 plus 1 tumour, etc.
Treatment
Radiation was applied with either electrons or high-voltage photons through one or multiple portals. Radiation therapy doses were as specified in ICRU report 29, Citation[26].
Hyperthermia was applied with microwave or radiofrequency equipment. There was no limitations to the equipment used except that it should be likely to provide a tumour temperature of 43.0°C. Active skin cooling was allowed. The heat treatment should be applied after each of the radiation fractions and should be started within 30 min. Efforts should be made to avoid heating of normal tissue or to apply appropriate skin cooling. Hyperthermia and thermometry (with multiple measurement points in both tumour and normal tissue) were performed and recorded in accordance with the ESHO quality assurance guidelines previously published Citation[27]. In addition to the actual temperatures and times recorded, these values were also calculated as equivalent minutes at 43°C Citation[28], Citation[29].
Follow-up and evaluation
Patients were seen 2 and 4 weeks after completion of therapy, then monthly for the next 3 months. At each follow-up normal tissue reaction and tumour response were recorded. The end-point of the study was complete response (at 3 months) and persistent local control, acute and late normal tissue damage. Tumour response was defined according to WHO Citation[18]. Early and late radiation damage was recorded as previously described Citation[25]. All time estimates used the date of randomization as initial value.
Statistical considerations
The known dose-response relationship for both radiation alone and combined radiation and hyperthermia found in malignant melanoma suggested that after addition of hyperthermia a 30% improvement in local control may be expected. If the true frequency of tumour control is changed by 30% (from 50 to 80%) the likelihood that a significant difference is observed (p < 0.05) is >90% when 120 evaluable lesions are included. The trial was therefore designed to be closed after 120 evaluable lesions had reached the 3-month follow-up.
The primary endpoint was persistent local control, which was estimated by Kaplan–Meier analysis using the Mantel–Cox test for comparison. Frequencies were compared by chi-square test. Multivariate analyses of local control was performed by the Cox proportional hazards model using the BMDP 2L programme. Multivariate analysis of complete response at 3 months was performed by logistic regression analysis using the BMDP LR programme. The treatment effect was evaluated using ‘the intention to treat’principle and evaluable lesions were included in the randomization group irrespectively of whether or not they had completed the planned treatment. Analysis of thermometry data and other non-normal distributed comparison was performed by the Spearman rank correlation test, and comparison of paired data analysed by McNemar's test. In addition to the analysis of the randomized tumours the influence on patient survival of local tumour response and other prognostic parameters were investigated.
Results
Patients and tumours
One hundred and thirty-four lesions in 70 patients were randomized between January 1986 and May 1992. Among these 128 lesions in 68 patients were evaluable for analysis. One patient had two lesions removed by surgery before follow-up (one radiation alone, one combined treatment). One patient refused any participation and follow-up after randomization (one lesion randomized to radiation alone), and three lesions were never evaluated due to death before first follow-up (two combined treatment, one radiation alone).
Of the 68 patients, 33 were females. Thirty-seven patients had a single tumour whereas 31 patients had multiple (2 to 7) treated lesions. The median age was 58 years ranging from 19 to 88 years. The median observation time were 11 months (range: 3–73 months).
The tumour characteristics are given in showing the two treatment groups to be comparable with regard to tumour volume, radiation dose, numbers and site of tumours, months to recurrence of the lesion in question, and observation time. Also the sex and age of the patients showed no difference.
Table I. Characteristics of 128 evaluable tumours.
Tumour response
Overall the treatment yielded a high response rate () and resulted in a significant palliative effect in most patients, irrespectively of the treatment arm. One hundred and three (80%) of the tumours obtained a complete or partial response which in most patients was persistent. Only 16 tumours regrew after a median response time of 10 months. This figure was independent of whether the patient obtained partial or complete response and was not influenced by the addition of hyperthermia. The combined heat and radiation treatment had, however, a significantly higher complete response rate than tumours treated with radiation alone (). The response rate was also significantly better in patients given 27 Gy compared to 24 Gy. Although small tumours had a 54% complete response rate vs. 38% in tumours >4 cm, this difference was not statistically significant in a univariate analysis. A multivariate logistic regression analysis using complete response as endpoint () did show that hyperthermia resulted in a highly significant benefit of treatment and also tumour size when evaluated as largest diameter, radiation dose, and sex were of significant influence. This is also seen when analysing the outcome in the two stratification parameters () where a dose-response relationship was found for the two treatment arms. A logit analysis performed on the complete response data yields a similar enhancement ratio of 1.12 (95% confidence limits; 1.02–1.24). Also the tumour volume was of importance and when comparing the effect of hyperthermia in small or large tumours it was apparent that especially tumours <4 cm which received combined treatment had a very impressive response (). In large tumours this difference was not found to the same extent.
Figure 1. Complete response in malignant melanoma treated with radiation alone or combined with hyperthermia as a function of radiation dose (left) and tumour volume (right).
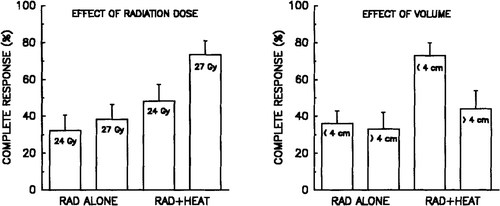
Table II. Initial tumour response in 128 evaluable tumours.
Table III. Univariate analysis of initial complete response and 2-year actuarial local control value as a function of treatment or stratification group.
Table IV. Final multivariate analysis of tumour response.
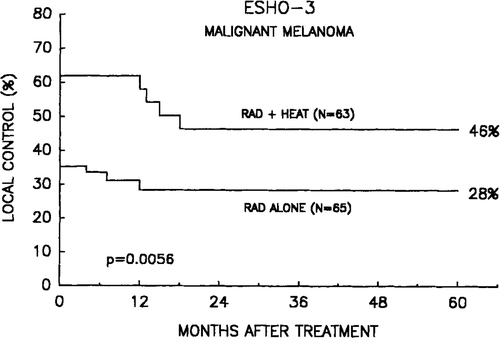
Although the survival time in many of these patients is limited, the persistent local control was also evaluated using the actuarial 2-year control rate as endpoint. As seen in , the difference in tumour control between radiation alone and radiation + heat is persisting in accordance with the low number of failures. A similar univariate analysis () shows also a significant difference as a function of dose, whereas size evaluated as above or below 4 cm was not significantly different. A Cox multi-variate analysis () revealed that additional hyperthermia, small tumour size, and high dose all were good independent prognostic parameters. In this analysis sex was not significant (p = 0.15). Neither did any of the multivariate analyses show significant influence of whether the patients had a single or multiple tumours, of the time from primary tumour to the recurrence of the treated lesion, or whether it was a lymph node or cutaneous lesion.
Figure 2. Tumour control probability after treatment with radiation alone or combined with hyperthermia.
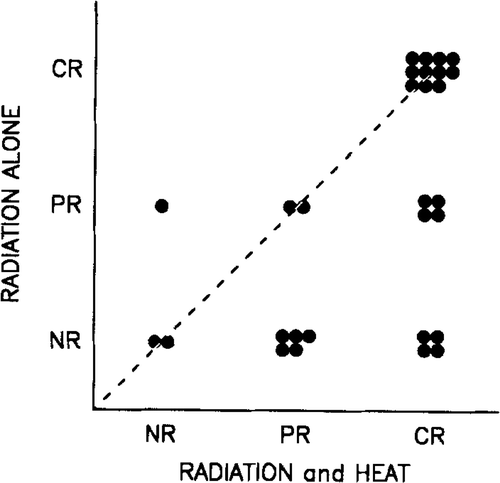
Since there may be an individual variation in the radiation sensitivity for malignant melanoma Citation[21], an analysis was performed on patients with multiple comparable lesions. shows the response in paired lesions from the same patients. The paired tumours were selected to be of same strata (below or above 4 cm) and were given same radiation dose (24 Gy or 27 Gy) but with or without hyperthermia. As seen in , all the heated lesions with one exception responded better or equally to the lesions given radiation alone, resulting in a highly significant benefit of hyperthermia (p = 0.014; McNemar's test).
Compliance and complications
The compliance to radiation was good, and all patients received the planned treatment. The acute radiation toxicity was acceptable and not significantly different in the two treatment arms, neither did radiation induced late fibrosis differ in the two treatment arms ().
Table V. Acute and late radiation reaction in skin.
The hyperthermic treatment was more troublesome. The heating technique was a single applicator using electromagnetic heating with frequencies ranging from 144 to 915 MHz. Thermometry was performed as described in the ESHO quality assurance guidelines with thermocouples, thermistors or Luxtron probes. The number of temperature points in tumours range from 0 to 19 (median 4), and the number of points in surrounding normal tissue range from 0 to 100 (median 2). The lack of temperature measurements in some tumours and normal tissues were due to failure in the recording system. Only active probes have been recorded.
All but four of the tumours randomized to hyperthermia received all three treatments (one tumour was not heated at all, three received only two heat sessions). The hyperthermia was in general well-accepted and in 73% of the treatments, no pain nor discomfort were noted. Slight pain was observed in 13% of the heat sessions, moderate pain in 8%, and only in 6% of the treatments the pain was so severe that the treatment was interrupted or stopped.
Thermal parameters and quality of hyperthermia
One hundred and forty-two of the 170 treatments, in which some or all quality assurance data are available, completed the planned overall treatment time (84%). The reporting in this multicentre trial did not allow a detailed analysis of individual temperature data in the tumours. The only information available was for each heat treatment, the minimal (Tmin) and the maximal (Tmax) heating time estimated in equivalent minutes at 43°C. In the normal tissue the maximal temperature was recorded (Tnorm) and the number of temperature measurement points in both tumour and normal tissues were recorded. Tumour temperature data were achievable for 168 individual treatments, and normal tissue data were recorded in 90 treatments. The remaining data were not available either due to technical failure or loss of information in the computer.
shows an overview of the achieved temperatures and their mutual relationship. In 62% the maximal tumour temperature (Tmax) reached the planned level (>60 min Eq. 43°C) with a median of 68 min, range 0–1000). However, only in 9% the minimal measured tumour temperature was above that level, and in general the value was very low with a median of 9 min Eq. 43°C (range 0–219). Thus, by far most of the tumours did not obtain the planned treatment. The normal tissue was in general kept at the low temperature level, and only 17% of the treatments resulted in an equivalent temperature of above 43°C (median 7, range 0–312).
Figure 4. Relationship between the Tmax, Tmin and Tnorm, showing the quality and ability of the hyperthermic therapy.
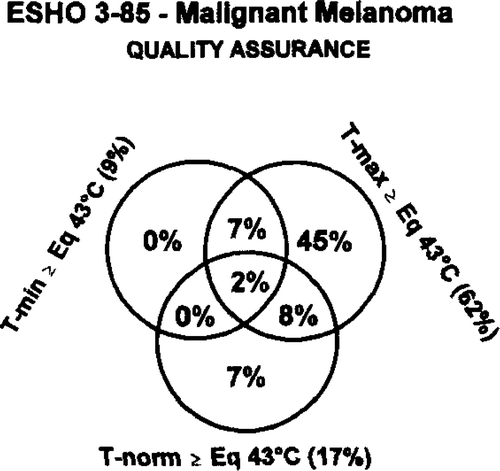
As indicated in , the quality of the heating was far below the aim put forward in the protocol. Thus, in only 9% of the heat treatments the therapeutic aim was achieved. In order to analyse the parameters responsible for this insufficiency a number of factors were analysed. shows the relationship between Tmax, Tmin, Tnorm, tumour size (greatest diameter), and tumour depth under surface. In addition, the relationship between the temperature parameters and number of heat fractions were analysed. As seen, there is a strong correlation between the maximal and minimal temperature, which is also apparent from where the detailed temperature information is shown. There is, however, no correlation between neither minimal nor maximal tumour temperature and the normal tissue temperature, probably because active skin cooling has been applied in a number of cases. The tumour size and its depth are of importance and high minimal temperature seems more likely to be achieved in small tumours, and especially in tumours close to the surface. This relationship was not observed between tumour size and maximal temperature. A significant relationship between number of temperature measurement points and Tmin, but not Tmax, was observed.
Figure 5. Relationship between the Tmax and Tmin in 168 heat treatments. The correlation is highly significant (p < 0.0001, Spearman). The dashed lines separates between treatments above or below 60 min Eq. 43 C, and indicate the number of sufficient and insufficient heat treatments.
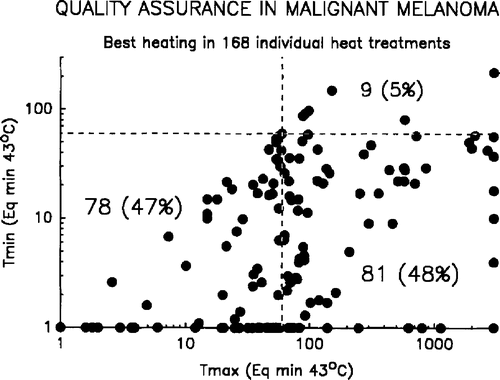
Table VI. Spearman rank correlation and statistical analysis of 168 heat treatments.
In order to evaluate the quality of the heating and which temperature parameters may be most relevant for determining the outcome of treatment, an analysis has been performed in the tumours in which sufficient temperature data were available. This included 54 evaluable heated tumours which were analysed together with the 65 tumours treated with radiation alone. The following temperature parameters were recorded: the maximal and minimal temperature achieved in any of the three heat sessions (Tmax/any and Tmin/any). Also the average of these maximal and minimal temperatures in the three treatments were analysed (Av-Tmax, Av-Tmin). The parameters were included in the multivariate analysis, and the parameters were either included as continuous values or separated by their medians. shows the p-values for the various parameters when using complete response or local control as end-points. As seen in the table, the univariate analysis showed a significant influence of all the estimated parameters on the outcome of treatment. However, in the multivariate analysis, taking in all other parameters described in the previous analysis into account (), the dominating parameter is the average of the maximal temperatures achieved in all heatings (Av-Tmax). This importance of heating quality for the outcome is illustrated in where the heated tumours have been divided according to whether they are above or below the median Av-Tmax value. A significant dose-response relationship for the heat effect was observed, and tumours achieving the best hyperthermia showed a superior local control probability.
Figure 6. Effect of heating quality. Tumour control probability after treatment with radiation alone or combined with high or low dose hyperthermia above or below the median value of Av-Tmax. Data from 65 non-heated and 54 heated tumours where sufficient temperature data were available.
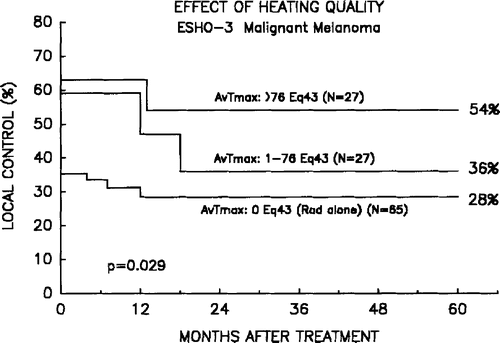
Table VII. Prognostic importance of various temperature parameters describing the heating quality. Univariate and final multivariate levels of significance are given for data analysed by stepwise logistic regression analysis using CR as endpoint and by Cox proportional hazard analysis using 2-years local control probability.
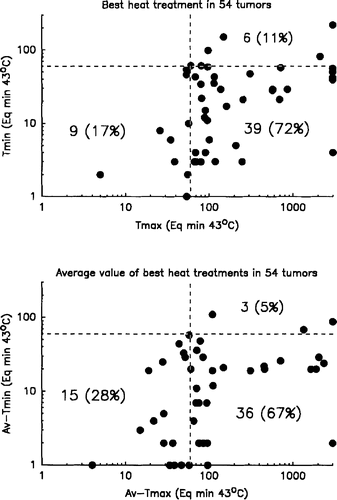
The values and relationship between the Tmax/any and Tmin/any, as well as between the Av-Tmax and Av-Tmin are shown in . Only in six respective three of the 54 heated tumours did the Tmin/any and Av-Tmin, respectively, reach the required values. Similarly was sufficient values of Tmax/any and Av-Tmax not obtained in nine and 15 tumours respectively. In fact, only two of the analysed tumours received a Tmin of >60 min Eq. 43°C in all three heat treatments, and only in 22 tumours was that true for values of Tmax.
Figure 7. The values and relationship between Tmax/any and Tmax/any (top) and between Av-Tmax and Av-Tmin (bottom) for 54 heated tumours with evaluable data. The dashed lines separate between treatments above or below 60 min Eq. 43°C, and indicate the number of sufficient and insufficient heat treatments.
Survival
The overall 5-year survival rate of the patients was 19% (). Local treatment of patients with advanced and eventually multiple metastatic lesions from malignant melanoma may seem redundant without major benefit for the patients. However, univariate analysis of patient survival indicated that if all known disease in the patients were treated, and if the outcome was persistent local control, the patient had a highly significant better survival probability than patients with known disseminated disease and/or lack of local control. shows that 38% of the patients have a 5-year survival probability if all known disease was controlled (independent of the treatment given), compared to 8% in the patients with persistent active disease (the last patient in this group died after 63 months) (p = 0.002). Also sex was of prognostic importance with a 5-year survival of 30% in women compared to 8% in men p = 0.008). Similarly, patients with single tumours survived significantly longer than patients with multiple lesions (5-year survival of 24 vs. 13%; p = 0.007), whereas the time to recurrence of lesions did not significantly influence the survival probability. These univariate observations were confirmed in a Cox multivariate analysis ().
Figure 8. Probability of crude survival in all patients with evaluable tumours (top left); in patients with or without persistent control of all known disease at the time of treatment (top right); as a function of number of recurrent or metastatic tumours (bottom left); and related to sex (bottom right).
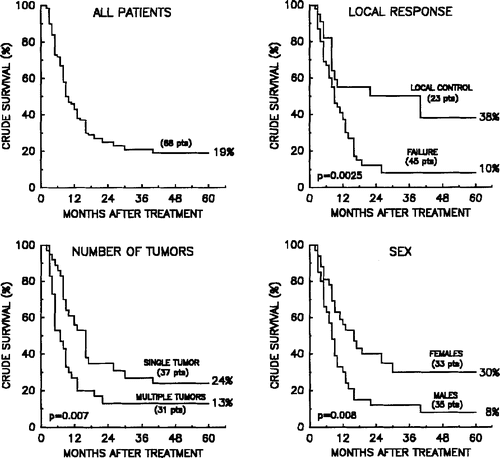
Table VIII. Cox proportional hazard analysis of 68 patients using death as endpoint.
Discussion
The ESHO 3-85 protocol, which is the first completed randomized study comparing the effect of adjuvant hyperthermia to radiotherapy in a single histopathological tumour type, confirmed that hyperthermia significantly enhances the effect of radiation, both evaluated as complete response and persistent local control. The benefit of the combined treatment was in the same order as that previously described in uncontrolled studies Citation[2], Citation[14], Citation[15], Citation[16], Citation[17], Citation[18], Citation[24]. The trial also confirms that total dose of radiation and tumour volume were other prominent prognostic factors Citation[21], Citation[5]. In addition, we observed that female sex was beneficial for complete response, whereas the number of tumours in each patient, the type of lesions and the time between primary tumour and the development of the recurrence treated here appeared to be unimportant.
As seen in other clinical trials with hyperthermia, the heating technique is the Achilles heel also in the present study. All patients achieved the planned radio-therapeutic treatment but a sufficient dose description of hyperthermia was impossible to obtain. Thus, only 9% of the heat treatments were in accordance with the protocol requirement. Nevertheless, the quality of the heating is of the same magnitude as that described in previous studies where these parameters have been recorded Citation[9], Citation[30]. The philosophy behind the European Society for Hyperthermic Oncology's clinical trials Citation[8], Citation[12] has been to challenge the heating techniques which in previous non-controlled studies have indicated a potential benefit Citation[4], Citation[6] in a phase III randomized study. Certainly, such policy has its shortcomings, but rather than continuing to optimize the heating ability while constantly being subjected to uncontrolled claims of efficiency, we have felt a need for conducting a randomized trial which would evaluate the clinical ability of the current heating equipment. This does imply that the efforts towards obtaining better heating of tumours should be ceased, but such development can only take place if it is supported by biological and clinical data which can provide a useful rationale and indication for a better quality of hyperthermic therapy.
Since the various thermal parameters are related () it is obvious that when one parameter (Av-Tmax) was allowed to dominate in a multivariate analysis, it must take place at the expense of the others. In fact, if Av-Tmin was forced into the model, this also became significant and the Av-Tmax would loose its power. This relationship is supported by the strong correlation described in . It is interesting to note that the Av-Tmax is a stronger prognostic parameter for outcome than information of whether or not the tumour has been heated, and therefore points towards the importance of heating quality as the dominating factor. shows this tendency. In this figure the heated tumours have been divided according to whether they are above or below the median Av-Tmax value. It is seen that there appears to be a dose-response relationship for the heat effect, and that tumours achieving the best hyperthermia appears to have the best tumour control probability. This agrees with the result from a uncontrolled study in malignant melanoma Citation[9], and which is also supported by the data with hyperthermic brain implants where a hyperthermic dose-response relationship was observed Citation[31].
Many thermal parameters and characteristics have in non-controlled studies been observed to be of prognostic value Citation[32], Citation[6], Citation[33], and the dominating thermal parameter may very well be strongly influenced by the temperature measurement techniques. However, all studies stress the importance of adequate heating, as also demonstrated in the current trial.
The volume effect was interesting to the extent that smaller tumours did respond better than larger, but the effect of hyperthermia was especially seen in tumours <4 cm in diameter. This finding is in accordance with the findings in the RTOG 81-4 study, where it was observed that especially the small tumours (<3 cm) had the benefit of combining hyperthermia with radiation, probably due to a better ability to heat these lesions Citation[30], Citation[34]. The same situation may exist in the ESHO 3-85 protocol, since the heating quality (Tmin) was significantly poorer in large tumours ().
Of interest is the significant relationship between number of temperature measurement points and minimal tumour temperature. This parameter is decreasing with increasing number of temperature points, whereas the maximal temperature is independent of this parameter. This relationship is of course understandable since an increasing number of temperature measurements are likely to detect a cold spot, whereas the maximal temperature normally occurs in the centre of the tumour where there normally always is placed a temperature probe. These data clearly demonstrate that in order to obtain comparable temperature quality data the thermometry must be standardized. There was no correlation between the number of heat fractions and the outcome of treatment, and an anticipated hypothesis that the first treatment tends to break down the blood circulation, allowing a better heating in the subsequent sessions, could not be confirmed in this study.
The complications to treatment were minor and acceptable and despite most heat treatments being given within half an hour after radiation, this did not result in any substantial enhanced radiation damage, probably due to the use of active skin cooling in most cases.
Despite a collective effort by numerous European institutions, it took about 6 years to recruit the required number in the present trial and almost half of the patients were included from two institutions. This is an illustration of the problems with hyperthermic oncology Citation[8], Citation[6–8], Citation[5], namely that rather than to perform a joint effort in making a proper evaluation of this biologically promising modality, many institutions conduct their activities independently, which in general has led to non-conclusive results. It is our hope that the current results in addition to its clinical results also will be recognized for demonstrating the usefulness of international collaboration.
Most of the initial European collaborative trials have now be completed. In addition to the ESHO 3-85 study on malignant melanoma hyperthermia has also been found to significantly enhance the effect of radiotherapy in the treatment of recurrent breast carcinoma in the chestwall, and in advanced pelvic tumours Citation[36], Citation[37].
Conclusion
Overall the treatment yielded a high response rate and resulted in a significant palliative effect in most patients, irrespective of the treatment schedules. Univariate and multivariate analysis both showed that adjuvant hyperthermia significantly improved local tumour control when applied in association with few large radiation fractions in the treatment of malignant melanoma. Also tumour size and dose of radiation were found to be of significant prognostic importance. The effect of adjuvant hyperthermia was related to the extent of heating achieved. Hyperthermia did not enhance the early or late radiation morbidity (due to active skin cooling). Compliance and tolerance to hyperthermia was good, but the quality of the heating was poor, and most tumours did not receive the prescribed heat dose.
Univariate and multivariate analysis both showed that good survival probability was associated with female sex, number of malignant lesions and most importantly, persistent control of all known disease at time of treatment. In other words, successful local treatment to patients with a single or a few metastatic malignant melanoma lesions may be curative in approximately half of these patients.
Acknowledgements
The following departments have participated in the ESHO 3-85 protocol: Radiotherapy Department, Academisch Medisch Centrum, Amsterdam, The Netherlands (D. González González). Department of Oncology, Aarhus University Hospital, Denmark (J. Overgaard). G. Porfiri Oncology Center, Latina, Italy (G. Arcangeli). Department of Oncology, Haukeland Hospital, Bergen, Norway (O. Dahl) Div. Oncologica Medica, Osp. S. Maria delle Croci, Ravenna, Italy (G. Cruciani). Istituto Regina Elena, Rome, Italy (F. Di Filippo). Department of Radiotherapy, Institute of Oncology, Warsaw, Poland (J. Fijuth). Institut Curie, Paris, France (C. Jaullery). MRC Cyclotron Unit, Hammersmith Hospital, London, UK (C. C. Vernon). The Christie Hospital, Manchester, UK (R. D. James). Department of Radiotherapy, University of Freiburg, Germany (R. Engelhardt).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Müller C. Eine neüe Behandlungsmethode bösartiger Geschwülste. Münchener Medizinische Wochenschrift 1910; 28: 1490–1493
- Arcangeli G, Benassi M, Cividalli A, Lovisolo GA, Mauro F. Radiotherapy and hyperthermia. Analysis of clinical results and identification of prognostic variables. Cancer 1987; 60: 950–956
- Meyer JL, Kapp DS, Fessenden P, Hahn GH. Hyperthermic oncology: Current biology, physics and clinical results. Pharmacology and Therapeutics 1989; 42: 251–288
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int. J Radiation Oncology, Biology, and Physics 1989; 16: 535–549
- Overgaard J. The rationale for clinical trials. Practical aspects of clinical hyperthermia, SB Field, JW Hand. Taylor and Francis, London and Philadelphia 1990; 213–241
- Overgaard J, Bach Andersen J. Hyperthermia. Oxford Textbook of Oncology, M Peckham, H Pinedo, U Veronesi. Oxford University Press, Oxford 1995; 823–835
- Hofman P, Knol RGF, Lagendijk JJW, Schipper J. Thermoradiotherapy of primary breast carcinoma. International Journal of Hyperthermia 1989; 5: 1–12
- Kapp DS. Site and disease selection for hyperthermic clinical trials. International Journal of Hyperthermia 1986; 2: 139–156
- Overgaard J. Hyperthermia as an adjuvant to radiotherapy. Review of the randomized multicenter studies of the European Society for Hyperthermic Oncology. Strahlentherapie und Onkologie 1987; 163: 453–457
- Arcangeli G, Overgaard J, Gonzalez Gonzalez D, Shrivastava PM. Hyperthermia trials. International Journal of Radiation Oncology, Biology, and Physics 1988; 14: S93–Sl09
- Ben-Yosef R, Kapp DS. Prognostic factors in metastatic malignant melanoma treated with combined radiation therapy and hyperthermia. International Journal of Hyperthermia 1993; 9: 767–781
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. International Journal of Hyperthermia 1994; 10: 457–483
- Overgaard J. The role of radiotherapy in recurrent and metastatic malignant melanoma: A clinical radiobiological study. International Journal of Radiation Oncology, Biology, and Physics 1986; 12: 867–872
- Overgaard J. Fractionated radiation and hyperthermia. Experimental and clinical studies. Cancer 1981; 48: 1116–1123
- Overgaard J. Thermoradiotherapy of malignant melanoma. Medical Radiology. Thermoradiotherapy and Thermochemotherapy Vol. 2. Clinical Applications, MH Seegenschmiedt, P Fessenden, C Vernon. Springer-Verlag, Berlin 1995; 69–83
- Gonzalez Gonzalez D, van Dijk JDP, Blank LECM, Rumke P. Combined treatment with radiation and hyperthermia in metastatic malignant melanoma. Radiotherapy and Oncology 1986; 6: 105–113
- Emami B, Perez CA, Konefal J, Pilepich MV, Leybovich L, Straube W, Vongerichten D, Hederman MA. Thermoradiotherapy of malignant melanoma. International Journal of Hyperthermia 1988; 4: 373–381
- Overgaard J, Bentzen SM. Radiotherapy alone or combined with hyperthermia in the treatment of advanced malignant melanoma. Pigment Cell. Vol. 10. Therapy of advanced melanoma, P Rümke. Karger, Basel 1990; 14–35
- Shidnia H, Hornback NB, Shen RN, Shupe RE, Yune M. An overview of the role of radiation therapy and hyperthermia in treatment of malignant melanoma. Advances in Experimental Medicine and Biology 1990; 267: 531–545
- Engin K, Tupchong L, Phil D, Waterman FM, Moylan DJ, Nerlinger RE, Leeper DB. Hyperthermia and radiation in advanced malignant melanoma. International Journal of Radiation Oncology, Biology, and Physics 1992; 25: 87–94
- Bentzen SM, Overgaard J, Thames HD, Overgaard M, Hansen PV, von der Maase H, Meder J. Clinical radiobiology of malignant melanoma. Radiotherapy and Oncology 1989; 16: 169–182
- Overgaard J, Nielsen OS. The importance of thermotolerance for the clinical treatment with hyperthermia. Radiotherapy and Oncology 1983; 1: 167–178
- Sapareto SA. Thermal isoeffect dose: Addressing the problem of thermotolerance. International Journal of Hyperthermia 1987; 3: 297–305
- Overgaard J, Overgaard M. Hyperthermia as an adjuvant to radiotherapy in the treatment of malignant melanoma. Znterlzational Journal of Hyperthermia 1987; 3: 483–501
- Overgaard J, Gonzalez Gonzalez D, Hume SP, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995a; 345: 540–543
- Hand JW, Lagendijk JJW, Bach Andersen J, Bolomey JC. Quality assurance guidelines for ESHO protocols. International Journal of Hyperthermia 1989; 5: 421–428
- Field SB, Morris CC. The relationship between heating time and temperature: Its relevance to clinical hyperthermia. Radiotherapy and Oncology 1983; 1: 179–186
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–214
- Perez CA, Gillespie B, Pajak T, Hornback NB, Emami BN, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the Radiation Therapy Oncology Group. International Journal of Radiation Oncology, Biology, and Physics 1989; 16: 551–558
- Sneed PK, Larson DA, Gutin PH. Brachytherapy and hyperthermia for malignant astrocytomas. Seminars in Oncology 1994; 21: 186–197
- Valdagni R, Liu F-F, Kapp DS. Important prognostic factors influencing outcome of combined radiation and hyperthermia. International Journal of Radiation Oncology, Biology, and Physics 1988; 15: 959–972
- Cox RS, Kapp DS. Correlation of thermal parameters with outcome in combined radiation therapy-hyperthermia trials. International Journal of Hyperthermia 1992; 8: 719–732
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. American Journal of Clinical Oncology 1991; 14: 133–141
- Curran WJ, Goodman RL. Hyperthermia 1991: A critical review. Radiation Research: Q Twentieth-Century Perspective, WC Dewey, M Edington, RJ Fry, EJ Hall, GF Whitmore. Academic, San Diego 1992; 883–888
- Dewhirst MW, Griffin TW, Smith AR, Parker RG, Hanks GE, Brady LW. Intersociety council on radiation oncology essay on the introduction of new medical treatments into practice. Journal of the National Cancer Institute 1993; 85: 951–957
- Overgaard J, van der Zee C, Vernon C, Gonzalez Gonzalez D, Arcangeli G. Thermoradiotherapy of malignant tumors. European randomized multicenter trials evaluating the effect of adjuvant hyperthermia in radiotherapy. Progress in Radio-Oncology V, HD Kogelnik. Monduzzi Editore, Bologna 1995b; 507–513
- ICRU Report 29, 1978, Dose specification for reporting external beam therapy with phofons and electrons, Bethesda, Maryland, USA: International Commission on Radiation Units and Measurements; 1978
- International Collaborative Hyperthermia Group, 1995, Hyperthermia in the treatment of superficial localized primary and recurrent breast cancer - results from five randomized controlled trials. Submitted for publication
- Kim JH, Hahn EW, Ahmed SA. Combination hyperthermia and radiation therapy for malignant melanoma. Cancer 1982; 50: 478–482