Abstract
Purpose: Evaluation of the efficacy and toxicity of a new setup of thermographically controlled water-filtered infra-red-A (wIRA) superficial hyperthermia (HT) combined with hypofractionated re-irradiation (re-RT) to treat large-sized breast cancer recurrences.
Methods: Records of 73 heavily pre-irradiated patients with 103 treatment regions, treated from September 2009 to July 2015 were retrospectively analysed. Sixty-four patients with macroscopic disease were treated with 94 regions including 46 patients with lymphangiosis carcinomatosa. Hypofractionated RT consisted of 4 Gy once per week up to a total dose of 20 Gy delivered within 1–4 min after wIRA-HT. Heating of tumour nodules and diffusely spreading cancer lesions was performed under real-time thermographic temperature monitoring, maintaining the maximum skin temperature in the ROI between 42 °C and 43 °C, achieving intratumoural temperatures up to a depth of 2 cm between 39.5 °C and 42 °C. Seventeen patients received re-re-irradiation (re-re-RT) using the same HT/RT-treatment schedule.
Results: Response rates in patients with macroscopic disease: 61% CR, 33% PR, 5% NC and 1% PD. Local control throughout life time after CR of macroscopic disease: 59%. All nine patients with microscopic disease had CR and local control throughout lifetime. Only grade 1 toxicities were observed.
Conclusions: Application of thermographically controlled wIRA-HT combined with extremely low-dose re-irradiation provides good local control throughout lifetime of heavily pre-treated breast cancer recurrences. The twin wIRA radiator provides a sufficiently homogeneous heat deposition for the treatment of larger areas. The time lag between HT and re-RT is substantially reduced. The possibility of re-re-RT opens new therapeutic options for the future.
Introduction
Locally recurrent breast cancer after initial RT is often a challenging clinical situation and considerably affects quality of life. When previous RT considerably limits the level of re-irradiation, the combination with superficial HT offers the possibility of achieving local control even with lower radiation doses, as recommended by current guidelines (e.g. [Citation1–3]).
In combined treatment schedules, including hyperthermia, temperature control is mandatory (a) for the adaptation of heat application to changes in tumour volume, and (b) to avoid overheating of normal tissues, and thus minimising the risk of side effects.
The technical design of hyperthermia devices for the effective treatment of large-sized, irregularly shaped, diffuse, superficial tumour lesions has been described as a major problem. There are considerable limitations using the currently available microwave techniques [Citation4]. Such lesions have required multiple sequential treatments of smaller regions (“patchwork technique”) [Citation5] or a multiple array antenna system. There have been numerous efforts to develop larger conformal microwave array (CMA) applicators [Citation6]. A contact free heating technique may have general advantages for the delivery of thermal energy to large superficial areas as well as for the patients’ compliance, especially in cases of touch-sensitive, ulcerated and/or bleeding lesions.
A well-established wIRA heating technique routinely used for various medical indications [Citation7] has been adapted for combined HT and RT in recurrent breast cancer using a thermographically controlled twin radiator system. This novel technique is (a) less complicated, (b) allows for sufficiently homogeneous and most compliant heat deposition, (c) guarantees adaptable treatment fields using a contact-free heating source, and (d) uses a feedback control system to avoid any risk of burning. The time interval between HT and RT has been reduced to only a few minutes. This latter condition is of crucial importance [Citation8,Citation9].
Using a retrospective analysis, we report here on the clinical outcome of wIRA-irradiation for superficial HT combined with low dose re-RT. The primary endpoints of this retrospective analysis were response, local control (LC) and toxicity.
Materials and methods
Patients
Inclusion criteria of this retrospective analysis were as follows: hormone- and chemotherapy-resistant and incompletely or non-resectable recurrent invasive-ductal or lobular breast cancer in previously irradiated areas with a depth up to 2 cm. From September 2009 to July 2015, 73 patients were consecutively treated of which 71 were referred from external Radiooncology/Oncology Departments. Sixty-four patients had macroscopic disease, including 46 patients with lymphangiosis carcinomatosa. summarises baseline patient and tumour characteristics at the time of first diagnosis. lists patient and tumour characteristics at the onset of HT combined with re-RT. and provide the evaluation of tumour response and LC.
Table 1. Baseline patient and breast cancer characteristics at the time of first diagnosis.
Table 2. Patient and tumour characteristics at the onset of HT combined with re-RT.
Table 3. Evaluation of tumour response upon termination of combined HT + re-RT treatment.
Table 4. Clinical outcome of combined HT + re-RT treatment.
Due to different histologies, another three patients with hemangio-endothelial sarcoma after standard breast conserving therapy were deliberately excluded in this report.
Localised wIRA-hyperthermia (wIRA-HT)
wIRA was generated by water-filtering of the radiation emitted by a halogen lamp of high colour temperature (type HPL, Ushio, Tokyo, Japan). The thickness of the water layer used for filtering is 7 mm [Citation10]. As shown in , this feature significantly reduces the spectral output within the bands of maximum water-absorbance with central wavelengths of 970, 1200 and 1400 nm and even eliminates the contributions of IR-B (1.4–3.0 μm) and of IR-C (3 μm–1 mm) [Citation11,Citation12]. Without water filtering, absorption of emitted IR radiation energy by the water molecules in the most superficial skin layers would result in overheating and tissue dehydration. The equipment used (hydrosun®750, Hydrosun Medizintechnik, Müllheim/Baden, Germany) allows irradiances of up to about 150–200 mW/cm2. In contrast, conventional, unfiltered IR-radiation only allows irradiances <60 mW/cm2 (IR-C), and <100 mW/cm2 (halogen IR-radiator of high colour temperature) due to the early generation of heat pain and thus limited patient compliance [Citation10,Citation13,Citation14].
Figure 1. Relative spectral irradiance of a halogen lamp (correlated colour temperature CCT =3000 K) as a function of wavelength, before (curve 1) and after passing a water-filter and a cut-off filter (BTE, Elsoff, Germany) (curve 2). Measurements by using a double monochromator spectroradiometer (Spectro 320 D, Instrument Systems, Munich, Germany) in the spectral range 0.4–1.7 μm) and calculation of data according to Planck’s law of radiation in the spectral range 1.7–4.5 μm. VIS: visible light spectrum
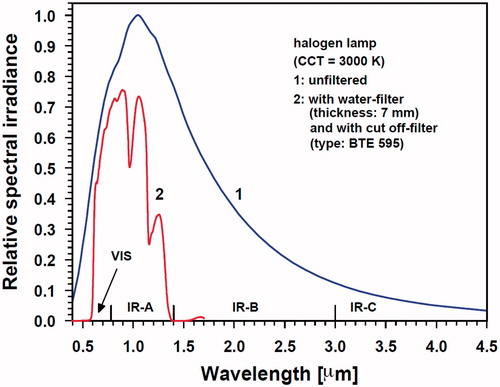
In order to achieve therapeutically relevant tissue temperatures in the oncologic setting (39 –42 °C, [Citation15]), a distance between the wIRA-radiator and the skin surface was set at 34 cm (distance rod), yielding an irradiance of about 150 mW/cm2 wIRA-irradiation which varies by less than ±10% within a skin area of 8 cm in radius, whereas the decrease of irradiance to 40% of the maximum (defining the limit of regularity according to DIN 5050–1 [Citation16]) was measured at a radius of 13 cm around the centre. The use of two wIRA-radiators enlarges the field accordingly and further allows adaptation to the individual body contour. As a rule, steady-state hyperthermia levels (42–43 °C maximum surface skin temperature, ROI) were reached within 5–10 min of exposure [Citation17].
Since the Bunsen–Roscoe law is not applicable to IR-induced tissue heating, a “conventional dose” cannot be defined. Therefore, the exposure is characterised by the definition of incident irradiance, exposure time (45–60 min), and the size of the treated area of exposure (see above) [Citation10,Citation18].
Thermography
In order to avoid skin burns in heavily pre-treated tumour regions and to guarantee an optimum and safe heat energy input, a thermographically controlled feedback system was used. This system consists of an infra-red thermography camera (VarioCAM® high resolution, Jenoptik, Jena, Germany), and a computer-controlled feedback system based on a special software (Heatcontrol®, InfraMedic, Mörfelden, Germany). This control unit registers the detailed temperature distribution of the treated skin area, whereby a ROI can be defined. The programme provides a real-time control of the wIRA radiation unit by switching off and on in a defined range of maximum temperature (42–43 °C) [Citation17,Citation19]. Treatment data were documented and stored. Visual inspections of the colour-coded temperature images were additionally used to guide the correct adjustment of the wIRA-radiator position, and to avoid the potential occurrence of hot spots. Hots spots, which chiefly occur in scar tissue with poor perfusion, were temporally shielded against further wIRA radiation as needed.
Technical specifications of the infra-red thermography camera used are as follows: spectral range: 7.5–14.0 μm, optical resolution: 640 × 480 pixels, NETD: < 30 mK at 30 °C, and pixel pitch: 35 μm. These data describe the relative accuracy of the camera. The absolute accuracy of temperature measurement – commonly specified as ±2%/2 K – was enhanced by the manufacturer to ±1.5%/1.5 K due to an enhanced calibration procedure for the relevant temperature range.
In this context, it is of relevance that one can rely on the accuracy and repeatability of the absolute temperature measured by the IR camera. The basic equation for temperature measurements with thermographic systems, brought into line with our arrangement of patient, wIRA-radiator and IR camera during therapy is
where Φ (Tskin) is the radiation power from the skin as a function of skin temperature, Tskin is the skin temperature, ΦM is the radiation power measured in the camera, ε is the emission coefficient of the skin and Φ (Tsur) is the radiation power of the surroundings (objects in the ambiance).
The temperature of the skin, Tskin, is given by the inverse function, Φ -1(Tskin), which is captured by the camera software converting the measured radiation power into temperature. The term for the ambient temperature can be determined by an IR image of the surrounding objects with preset emission coefficient of 1 in the camera.
According to the above equation, the key parameter for accurate absolute temperature measurement with thermographic cameras is the emission coefficient ε of the skin which has to be entered into the camera software in order to adapt the internal temperature calculation to the given conditions. This is done by varying the preset ε in the software until the thermographic temperature matches the absolute temperature.
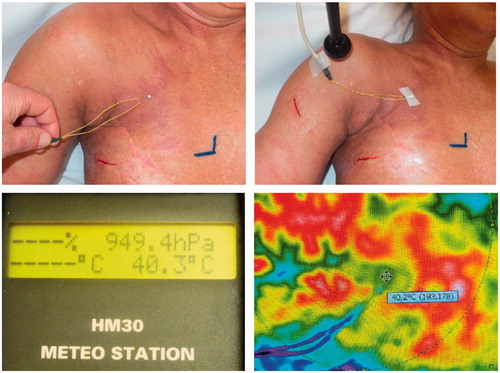
Absolute temperature measurements were performed with contact electrodes type PT 100 (Meteo Station HM 30 Type Mod. 00B. Revue Thommen, Waldheim, Switzerland) taped to the skin (. The emission coefficient in the camera was preset to 0.98, which is reliably reported as the mean value for healthy human skin [Citation20].
Figure 2. Measurement of surface temperature with contact electrodes (type PT100). Placement of sensor (upper left panel), fixation (upper right panel) and absolute temperature reading (lower left panel). Corresponding thermography (lower right panel).
In exemplary cases it was found, that the difference between absolute and IR camera temperature values in the upper relevant temperature interval was in the range of 0.1 K, corresponding to a deviation of the real local emission coefficient as compared with the preinstalled value of 0.98 at the camera. Additionally, basic calibration of the camera was performed monthly with a black body (988 ISOTECH Blackbody Source, Isothermal Technology Ltd., Southport, UK) designed for the temperature range of 20–45 °C (resolution: 0.01 K) with a combined accuracy/stability of ±0.2 K according to the data sheet of the manufacturer.
Invasive temperature measurements
In two selected cases, invasive temperature measurements were performed at defined depths (0.1–2.0 cm) before and during seven wIRA-HT treatments, using a commercially available invasive temperature measurement unit (TempSens®, OP-Sens® Inc. Quebec, Canada) with fibre optic sensors. The sensors were inserted under local anaesthesia directly into tumour nodules. Insertion needles resp. catheters were shielded from wIRA-irradiation to exclude direct heating falsifying the measurements. The sensors were then placed along the inserted catheter at defined depths (surface, 1 mm, 5 mm, 10 mm and 20 mm) and their temperature data were compared with the surface temperatures assessed by thermography.
Radiotherapy
Patients were treated with 4–12 MeV electrons. Hypofractionated RT consisted of 4 Gy once per week, up to a median total dose of 20 Gy. Electron beam energy was chosen according to tissue thickness, calculated on TPS and prescribed at 80% of maximum dose.
Treatment set-up
Following wIRA-exposure for 45 min – 60 min, patients were immediately thermally shielded with a preheated blanket and transferred to the linear accelerator. After immediate application of a 45 °C-preheated bolus of 3–5 mm, according to the applied electron energy, RT started within 1–4 min after completion of the HT-session.
Treatment endpoints
Local tumour response (CR, PR, NC and PD) was assessed between 4 and 8 weeks after HT/re-RT according to WHO criteria (CR = complete clinical disappearance of all detectable disease in the treatment fields observed, PR = decrease >50%, NC = decrease <50% or increase <25%, PD = increase >25%). Local control was defined as absence of new local tumour progression during lifetime after CR. Re-recurrences were defined as a new local tumour progression infield, on the border or outside of the former treated region after CR. Acute and chronic toxicity was registered according to RTOG/EORTC criteria and to corresponding burning grades for hyperthermia-related side effects.
Results
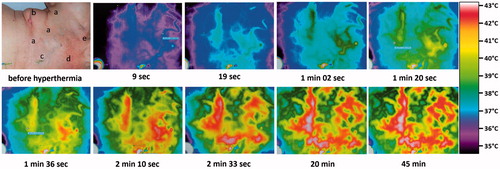
The prescribed maximum surface temperature of 42.5–43.2 °C was generally achieved in tumour nodules and tissues with lymphovascular invasion. As expected, the superficial temperature of areas in the ROI that were tumour-free (“normal tissue”) was often significantly lower (down to approx. 38.5 °C) in spite of the same IR energy input, whereas maximum temperatures were seen in the areas of tumour lesion and scars (see .
Figure 3. Thermographic control of wIRA-hyperthermia in a patient with inflammatory breast cancer recurrence. Before start of heating: (a) visible inflammatory disease, (b) scar of the former tumourectomy, (c) field-mark for RT, (d) subcutaneous venous drainage, and (e) sebaceous retention cyst. Immediately after start of hyperthermia and within 19 s the venous drainage increases and transports heat away (cooling effect). After a few minutes this effect is clearly visible, then a breakdown of blood flow can be seen (after 2.5 min). These latter effects disappear after 3 min. Inflammatory recurrence and/or scarred tissue are heated up to 43 °C after 2.5 min and stay at this higher temperature level during the whole treatment period (see the bottom line at 20 and 45 min).
Temperature profiles within tumour nodules were assessed in two patients during several treatments using wIRA-HT (). At mean surface temperatures of 42.0 °C, the steady-state tissue temperatures were 41.9–42.0 °C at 1 mm depth, 41.3–41.5 °C at 5 mm, 40.9 –41.1 °C at 10 mm and 39.6–40.1 °C at 20 mm (red triangles in .
Figure 4. Inflammatory recurrent breast cancer in a 51-years old patient. Former conservative therapy for triple negative breast cancer consisting in tumourectomy, sentinel lymphadenectomy, adjuvant chemotherapy and radiotherapy with 50 Gy whole breast and boost up to 60 Gy. First recurrence treated with salvage surgery, chemotherapy followed by breast reconstruction. Top: Situation before start of re-RT and wIRA-HT (21.6.2011, patient in treatment position). Several biopsies on both breasts confirmed the inflammatory recurrence. Middle: First thermographically controlled wIRA-HT of both chest wall regions. Bottom: 4 years after completion of hypofractionated re-RT with 5 × 4 Gy and wIRA-HT (NED, 21.9.2015, patient in sitting position). Teleangiectasias are evident especially in the décolleté and the former irradiated region
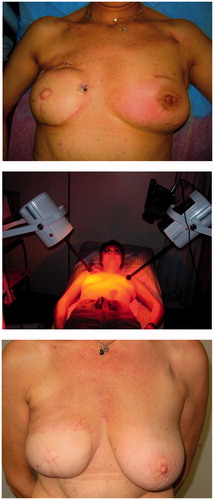
Figure 5. Mean temperature profiles within tissues during wIRA-exposure under steady state conditions. Curve 1: Calculated by averaging measured data in normal tissues (Hellige in Ref. [Citation11], open squares), tumour tissues (Seegenschmiedt et al. [Citation31], circles) and in recurrent breast cancers (Notter et al., current report, red triangles). Curve 2: temperature profile in untreated skin and subcutis, i.e., no wIRA exposure (Hellige in Ref. [Citation11], filled squares).
![Figure 5. Mean temperature profiles within tissues during wIRA-exposure under steady state conditions. Curve 1: Calculated by averaging measured data in normal tissues (Hellige in Ref. [Citation11], open squares), tumour tissues (Seegenschmiedt et al. [Citation31], circles) and in recurrent breast cancers (Notter et al., current report, red triangles). Curve 2: temperature profile in untreated skin and subcutis, i.e., no wIRA exposure (Hellige in Ref. [Citation11], filled squares).](/cms/asset/c86adc7d-48f8-475e-9ee3-b32473c80553/ihyt_a_1235731_f0005_c.jpg)
All 73 patients started with a stringent HT/RT-procedure related to dose and fractionation, time interval and sequencing of HT and RT. Six patients failed to complete the intended treatment schedule, due to deterioration of general condition.
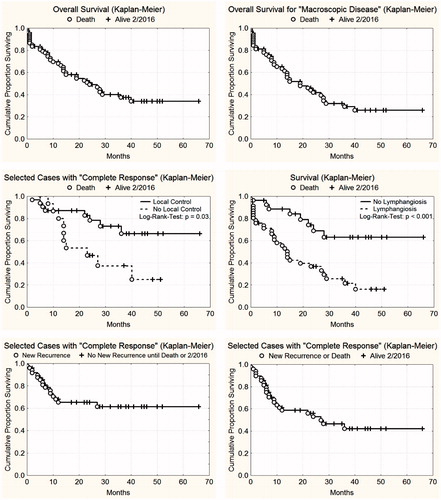
summarises the response rates of patients and treated regions. Response rates of macroscopic disease: 39 patients (61%) with CR, 21 patients (33%) with PR, three patients (5%) with NC and one (2%) patient with PD. Patients presenting with lymphangiosis carcinomatosa had a lower CR rate (52%). All patients with microscopic disease achieved a CR (for further details see ).
By February 2016, 39 out of 64 patients with macroscopic disease had died, 25 were still alive, including nine with NED. Nine patients with microscopic disease were all locally controlled, including eight with NED. Local control throughout lifetime after CR of macroscopic disease was noted in 23/39 patients (59%), 16/39 patients (41%) showed re-recurrences (), occurring after 0–27 months (median: 6 months). All of them were related to extended lymphangiosis carcinomatosa (). A more detailed analysis of re-recurrences reveals that three (11%) were infield, 12 (44.5%) at the field border and 12 (44.5%) outside the treatment fields. Seventeen patients with re-recurrent lymphangiosis carcinomatosa were re-treated in 47 regions using the same HT- and RT-treatment schedule (“re-re-irradiation”), achieving 33 CR, 11 PR, two NC and one PD. Metastasis was present in 33 patients (45%) at onset of combined re-RT + HT, another 12 patients developed first metastasis after combined re-RT + HT ().
Overall survival and survival of locally controlled vs. uncontrolled recurrences are indicated in . CR and local control during lifetime had a positive influence on survival in the log-rank test (p = .03), whereas the presence of lymphangiosis carcinomatosa had a negative influence (p < .001). In addition, Kaplan–Meier analyses of local progression free survival are presented (, lower panels).
Figure 6. Top left: Overall survival of 73 patients with locally recurrent breast cancer treated with re-irradiation and wIRA-hyperthermia. Top right: Overall survival of 64 patients (macroscopic disease) with locally recurrent breast cancer treated with re-irradiation and wIRA-hyperthermia. Middle left: Influence of local control on survival after CR. 32 patients had local control throughout life time (upper curve), 16 patients presented with re-recurrences (lower curve). Difference is statistically significant (log rank test p = .03). Middle right: Influence of lymphangiosis carcinomatosa on survival. Forty-six patients had lymphangiosis carcinomatosa (lower curve), 18 patients presented with macroscopic disease without lymphangiosis (upper curve). Difference is statistically significant (log rank test p < .001). Bottom left: Local progression-free survival until re-recurrence after CR (n = 48). Bottom right: Local progression-free survival until re-recurrence or patient death after CR (n = 48).
The combined HT/RT-treatments were well tolerated. The observed side effects cannot be clearly separated in RT- or HT-related side effects. No acute grade 2–4 toxicities have been observed, especially no burning with blistering. Three additional new moderate teleangiectasias occurred after 1–2 years at follow up (). Tissue transfer (reconstructions, skin transplants, mesh grafts) in 20 patients including contralateral prothesis in three patients did not show any additional toxicity.
Table 5. Side effects upon HT + re-RT of recurrent breast cancers.
Discussion
The usual approach for the majority of heavily pre-treated patients is palliative due to far progressed disease and distant failures. Nevertheless, local control during lifetime is always the main therapeutic objective due to the extreme suffering caused by a visible disease requiring extensive care procedures and that may lead to social isolation.
Recent reviews of randomised studies and retrospective analyses confirm the additional benefit of combined RT/HT for pre-irradiated recurrent breast cancer [Citation21–24]. Inflammatory disease is reported to have a worse prognosis and significantly lower CR-rates [Citation25,Citation26].
includes studies with pre-irradiated patients and studies where the data of those patients could be separately extracted. Skin toxicity data are restricted to available information on hyperthermia-related skin toxicity ≥ grade 2 (blisters, ulcers and necrosis). The study designs vary considerably, as well as the results. Despite larger tumour sizes (median tumour size: 480 cm2, mean tumour size: 819 cm2) and high percentage of lymphangiosis, the CR-rate of 61% (including microscopic disease: 66%) of patients in our analysis is comparable with recent studies (e.g. [Citation27,Citation28]).
Table 6. Combination of RT and HT in pre-irradiated, recurrent breast cancer: Review of available data.
Preclinical and clinical studies showed a much better effect of combined RT/HT with simultaneous application [Citation8,Citation9,Citation29], achieving a thermal enhancement ratio (TER) from 1.5 to about 2 [Citation8]. Our solution to achieve a nearly simultaneous combination of HT and RT is to begin with wIRA-HT followed by RT within 1–4 min while maintaining the elevated temperature by using a pre-warmed coat during the transport of the patient to the accelerator and a preheated bolus at the electron application. This bolus is only used for a few minutes during electron beam radiotherapy and not as a main part of hyperthermia. It just maintains the elevated temperatures of the skin during irradiation. Keeping time intervals between hyperthermia and re-irradiation as short as possible is crucial for obtaining the local control achieved by the extremely low dose re-irradiation.
The use of wIRA may be a more feasible treatment modality especially for diffusely spreading inflammatory recurrences, whereas in cases of nodular manifestations with a thickness of more than 2 cm, microwave hyperthermia can achieve a better depth effect.
The wIRA technique allows the use of real-time thermography to assess and control surface temperatures instead of punctual skin temperature measurements as currently used with microwave techniques that require the feedback of the patient concerning any discomfort. Patients with sensory disturbances of the skin can barely give such feedback, hence running a higher risk of skin burns [Citation22,Citation30]. Using thermography-controlled wIRA, tissue transfer is, therefore, no contraindication. The same holds for prothesis, metallic implants, ports and pacemakers. Requirements for hygiene and disinfection are low. Moreover, the real-time thermographic temperature monitoring with its high-temperature resolution enables the observation of dynamic developments in the often quite heterogeneous target area during the HT session.
A more detailed discussion of thermographic analysis and wIRA properties in the preclinical and clinical setting can be found in the Supplement material.
Promising clinical results and intratumoural temperature measurements with the use of wIRA for superficial hyperthermia in the oncologic setting obtained in a multicenter phase I/II study were first reported by Seegenschmiedt et al. [Citation31].
As mentioned above, HT may have the benefit of doubling the effect of radiotherapy. Therefore, we initially chose approx. 50% of an accepted “effective dosage” and kept this schedule unchanged due to the promising clinical results from the very start. Moreover, the low dosage keeps the therapeutic window open even for repeated re-irradiation (“re-re-irradiation”) with the same schedule and dosage.
An experimental study using the same fractionation and RT dosage combined with wIRA exposure on human breast cancer cell spheroids has shown a similar radiosensitizing effect of this regimen [Citation32], therefore, supporting the clinical observation. Non-thermal radiosensitizing effects of wIRA in vitro were described by Heselich et al. [Citation33]. Whether such effects play any clinical role in the action of wIRA-HT has to be investigated in future experiments. In addition, the observed therapeutic effect of wIRA-HT in combination with RT, especially in ulcerating or bleeding tumour manifestations, might be supported by improved wound-healing effects of wIRA reported in randomised trials and reviewed by Hoffmann [Citation7].
Conclusion and outlook
This observation study presents an innovative schedule of hyperthermia combined with re-irradiation using an extremely low radiation dose and a so far not routinely used heating technique. Despite large tumour load, the quasi-simultaneous application of thermography-controlled wIRA-HT and re-RT has shown high efficacy even in patients with high total doses of pre-irradiation. Extremely short time intervals between HT and RT seem to be crucial. The remarkably low toxicity allows the re-use of the same therapeutic schedule in new re-recurrences. Hypofractionated low-dose re-irradiation with 5 × 4 Gy 1×/week combined with superficial hyperthermia seems to be a good palliative option and should be prospectively tested against standard schedules.
Acknowledgements
The authors thank Mr. M. Thomsen, The University of Sydney Medical School, Discipline of Pharmacology, Sydney/Australia for linguistic support, and Dr. K. Münch, Lindenhofspital Berne/Switzerland for statistical analyses.
Disclosure statement
The authors have no conflicts of interest to declare.
Funding
This study was supported by the Dr. med. h.c. Erwin Braun Foundation, Basel, Switzerland (M. N., H. P.). Peter Vaupel is a long-standing board member ad honorem of the Dr. med. h.c. Erwin Braun Foundation.
References
- German S3 guidelines. (2012). Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms; Langversion 3.0, 199.
- NCCN Clinical Practise Guidelines in Oncology (NCCN Guidelines) Breast Cancer Version 3 (2014) MS-57.
- Harms W, Budach W, Dunst J, et al. (2016). DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences. Strahlenther Onkol 192:199–208.
- Zagar TM, Vujaskovic Z, Formenti S, et al. (2014). Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer treated. Int J Hyperthermia 30:285–94.
- Engin K, Tupchong L, Waterman FM, et al. (1994). Multiple field hyperthermia combined with radiotherapy in advanced carcinoma of the breast. Int J Hyperthermia 10:587–603.
- Stauffer PR, Maccarini P, Arunachalam K, et al. (2010). Conformal microwave array (CMA) applicators for hyperthermia of diffuse chest wall recurrence. Int J Hyperthermia 26:686–98.
- Hoffmann G. (2009). Water-filtered infrared-A (wIRA) in acute and chronic wounds. GMS Krankenhhyg Interdiszip 4: Doc12.
- Horsman MR, Overgaard J. (2007). Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 19:418–26.
- Myerson RJ, Straube WL, Moros EG, et al. (1999). Simultaneous superficial hyperthermia and external radiotherapy: report of thermal dosimetry and tolerance to treatment. Int J Hyperthermia 15:251–66.
- Piazena H, Kelleher DK. (2010). Effects of infrared-A irradiation on skin: discrepancies in published data highlight the need for an exact consideration of physical and photobiological laws and appropriate experimental settings. Photochem Photobiol 86:687–705.
- Vaupel P, und Krüger W, (Hrsg.). (1992). Wärmetherapie mit wassergefilterter Infrarot-A-Strahlung: Grundlagen und Anwendungsmöglichkeiten, Hippokrates, Stuttgart, 1. Auflg.
- Vaupel P, Kelleher D, Krüger W. (1992). Water-filtered infrared-A radiation: a novel technique to heat superficial tumors . Strahlenther Onkol 168:633–9.
- Vaupel P, und Krüger W, (Hrsg.). (1995). Wärmetherapie mit wassergefilterter Infrarot-A-Strahlung: Grundlagen und Anwendungsmöglichkeiten, Hippokrates, Stuttgart, 2. Auflg.
- Vaupel P, Rzeznik J, Stofft E. (1995). Wassergefilterte Infrarot-A-Strahlung versus konventionelle Infrarotstrahlung: Temperaturprofile bei lokoregionaler Wärmetherapie. Phys Rehab Kur Med 5:77–81.
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. (2005). Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 21:779–90.
- DIN 5050-1. (1992). Solarien und Heimsonnen, Teil 1: Messverfahren, Typeneinteilung und -kennzeichnung. Beuth-Verlag, Berlin.
- Notter M, Germond JF, Wolf E, et al. (2011). Thermography guided irradiation using water-filtered infra-red-A (wIRA) and radiotherapy on recurrent breast cancer – first experiences and temperature analysis. Thermol Internat 21:47–53.
- Piazena H, Kelleher D. (2008). Comments on “Cellular response to infra-red radiation involves retrograde mitochondrial signaling”. Letter to the Editor. Free Radic Biol Med 44:1869.
- Dombrovsky LA, Timchenko V, Pathak C, et al. (2015). Radiative heating of superficial human tissues with the use of water-filtered infra-red-A radiation: a computational modelling. Int J Heat Mass Transfer 85:311–20.
- Villaseñor-Mora C, Sánchez-Marin FJ, Calixto-Carrera S. (2009). An indirect skin emissivity measurement in the infra-red thermal range through reflection of a CO2 laser beam. Rev Mex Fis 55:387–92.
- van der Zee J, de Bruijne M, Mens JW, et al. (2010). Reirradiation combined with hyperthermia in breast cancer recurrences: overview of experience in Erasmus MC. Int J Hyperthermia 26:638–48.
- Zagar TM, Oleson JR, Vujaskovic Z, et al. (2010). Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia 26:612–17.
- Kouloulias V, Triantopoulou S, Uzunoglou N, et al. (2015). Hyperthermia is now included in the NCCN Clinical Practice Guidelines for Breast Cancer Recurrences: an analysis of existing data. Breast Care (Basel) 10:109–16.
- Datta NR, Puric E, Klingbiel D, et al. (2016). Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 94:1073–87.
- Hofman P, Knol RGF, Lagendijk JW, Schipper J. (1989). Thermoradiotherapy of primary breast carcinoma. Int J Hyperthermia 5:1–11.
- Feyerabend T, Wiedemann GJ, Jäger B, et al. (2001). Local hyperthermia, radiation, and chemotherapy in recurrent breast cancer is feasible and effective except for inflammatory disease. Int J Radiat Oncol Biol Phys 49:1317–25.
- Datta NR, Puric E, Heuberger J, et al. (2015). Hyperthermia and reirradiation for locoregional recurrences in preirradiated breast cancers: a single institutional experience. Swiss Med Wkly 145:w14133. doi: 10.4414/smw.2015.14133.
- Oldenborg S, Griesdoorn V, van Os R, et al. (2015). Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: size matters. Radiother Oncol 117:223–8.
- Moros EG, Penagaricano J, Novak P, et al. (2010). Present and future technology for simultaneous superficial thermoradiotherapy of breast cancer. Int J Hyperthermia 26:699–709.
- Linthorst M, van Rhoon GC, van Geel AN, et al. (2012). The tolerance of reirradiation and hyperthermia in breast cancer patients with reconstructions. Int J Hyperthermia 28:267–77.
- Seegenschmiedt MH, Klautke G, Walther E, et al. (1996). Water filtered infra-red-A-hyperthermia combined with radiotherapy for advanced and recurrent tumours. Strahlenther Onkol 172:475–84.
- Thomsen AR, Aldrian C, Nanko N, et al. (2016). Fraktionierte Strahlentherapie von Mamma-Karzinom-Sphäroiden in Kombination mit wassergefilterter Infrarot-A-Hyperthermie (wIRA). Strahlenther Onkol 192 (Suppl.1):92.
- Heselich A, Frohns F, Frohns A, et al. (2012). Near-infrared exposure changes cellular responses to ionizing radiation. Photochem Photobiol 88:135–46.
- Vernon CC, Hand JW, Field SB, et al. (1996). Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 35:731–44.
- Jones EL, Oleson JR, Prosnitz LR, et al. (2005). Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 23:3079–85.
- Li RY, Lin SY, Zhang TZ. (1990). Assessment of combined thermoradiotherapy in recurrent of advanced carcinoma of the breast. Adv Exp Med Biol 267:521–3.
- Du Bois JB, Hay M, Bordure G. (1990). Superficial microwave-induced hyperthermia in the treatment of chest wall recurrences in breast cancer. Cancer 66:848–52.
- Phromratanapongse P, Steeves RA, Severson SB, Paliwal BR. (1991). Hyperthermia and irradiation for locally recurrent previously irradiated breast cancer. Strahlenther Onkol 167:93–7.
- van der Zee J, van der Holt B, Rietveld PJ, et al. (1999). Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer 79:483–90.
- Kouloulias VE, Dardoufas CE, Kouvaris JR, et al. (2002). Liposomal doxorubicin in conjunction with reirradiation and local hyperthermia treatment in recurrent breast cancer: a phase I/II trial. Clin Cancer Res 8:374–82.
- Ben-Yosef R, Vigler N, Inbar M, Vexler A. (2004). Hyperthermia combined with radiation therapy in the treatment of local recurrent breast cancer. Isr Med Assoc J 6:392–5.
- Li G, Mitsumori M, Ogura M, et al. (2004). Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma. Int J Clin Oncol 9:179–83.
- Wahl AO, Rademaker A, Kiel KD, et al. (2008). Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 70:477–84.
- Gabriele P, Ferrara T, Baiotto B, et al. (2009). Radio hyperthermia for re-treatment of superficial tumours. Int J Hyperthermia 25:189–98.
- Linthorst M, Baaijens M, Wiggenraad R, et al. (2015). Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: results in 248 patients. Radiother Oncol 117:217–22.

