Abstract
Purpose: To determine the effectiveness of thermography to control the distribution of abdominal temperature in the development of a closed chemohyperthermia model.
Materials and methods: For thermographic analysis, we divided the abdominopelvic cavity into nine regions according to a modification of carcinomatosis peritoneal index. A difference of 2.5 °C between and within the quadrants, and thermographic colours, were used as asymmetric criteria. Preclinical study:· Rats Model: Six athymic nude rats, male, rnu/rnu. They were treated with closed technique and open technique. Porcine Model: 12 female large white pigs. Four were treated with open technique and eight with closed recirculation CO2 technique. Clinical Pilot Study, EUDRACT 2011-006319-69: 18 patients with ovarian cancer were treated with cytoreductive surgery and hyperthermia intraperitoneal chemotherapy, HIPEC, with a closed recirculating CO2 system. Thermographic control and intra-abdominal temperature assessment was performed at the baseline, when outflow temperature reached 41 °C, and at 30´.
Results: The thermographic images showed a higher homogeneity of the intra-abdominal temperature in the closed model respect to the open technique. The thermogram showed a temperature distribution homogeneity when starting the circulation of chemotherapy. There was correlation between the temperature thermographic map in the closed porcine model and pilot study, and reached inflow and outflow temperatures, at half time of HIPEC, of 42/41.4 °C and 42 ± 0.2/41 ± 0.8 °C, respectively. There was no significant impact to the core temperature of patients after reaching the homogeneous temperature distribution.
Conclusions: To control homogeneity of temperature distribution is feasible using infra-red digital images in a closed HIPEC with CO2 recirculation.
Introduction
Hyperthermic intra-abdominal chemotherapy,HIPEC, is an effective treatment after cytoreductive surgery in selected patients with peritoneal carcinomatosis. It can be performed using open or closed access. Intra-abdominal chemotherapy with closed access helps avoid toxic exposure for people in the operating room, and it reduces heat loss. However, it is unknown if hyperthermic dextrose and chemotherapeutics agents are distributed homogeneously in the abdominal cavity when using the closed technique. The development of a closed system for HIPEC with recirculating CO2 allows homogeneous intra-abdominal heat distribution and achieves an optimal cytotoxicity model [Citation1].
Intra-abdominal hyperthermic with chemotherapy is not a replacement for standard therapy in malignant diseases. It improves the development of the disease by means of its interaction with some drugs and their intrinsic cytotoxic activity.
Adding hyperthermia to certain drugs improves their pharmacokinetic and pharmacodynamic properties. It increases the intracellular concentration of the drug and leads to protein damage acceleration and better distribution, metabolism, and excretion of the drug. At temperatures of 41–42 °C, there are direct cytotoxic effects from alterations in the cell membrane inducing apoptosis and alterations in intracellular proteins and nucleic acids. It is essential for optimal treatment that drugs be used at temperatures of 41–43 °C and be homogeneously distributed throughout the abdominal cavity to reach equal cytotoxic characteristics in all abdominopelvic areas [Citation2–10]. Infra-red thermography is the recording of the temperature distribution of a body using the infra-red radiation that surface of that body emits. In humans we use infra-red cameras to calculate a thermogram of the distribution of temperatures of the skin [Citation11–14].
The aim is to describe the effectiveness of thermography for controlling the distribution of abdominal temperature during development and implementation of a closed intra-abdominal chemohyperthermia model during the preclinical development and clinical phase.
Materials and methods
Murine model
The study tried to perform controlling temperature distribution in open and closed HIPEC techniques without using drugs to control the effectiveness and safety of distribution of hyperthermic dextrose. This study was approved by the Committee on Animal Experimentation at the hospital.
Animals
The experimental animal model included athymic nude rats, six male rnu/rnu rats (Harlan Laboratories, Indianapolis, IN), five weeks old, weighing 150–200 grams. The rats were randomised into two groups with three rats in each group. The rats were housed following the requirements associated with current regulations for experimental animals. All experiments were carried out in accordance with the EU Directive for animals experiments and were designed and approved by our Animal Experimental Ethical Committee. Studies were always performed at the same time. All animals were healthy and did not receive pre-treatment. The rats were maintained with food and water ad hoc, with a cycle of 12 h of light and 12 h of darkness and a room temperature of 20 °C ± 2 °C with a relative humidity of 50%–70%. Animals were housed for seven days before starting the experimental procedure.
Group 1: Experimental group received hyperthermic carrier solution over 41 °C and simulating HIPEC with closed technique.
Group 2: Experimental group received hyperthermic carrier solution over 41 °C and simulating HIPEC performed by open surgery.
Anaesthesia and surgical procedure
Anaesthesia
After preoperative weight control, rats were anaesthetised using Xylazine (Indianapolis, IN) (10 mg/kg) and ketamine (75 mg/kg).
Surgical technique
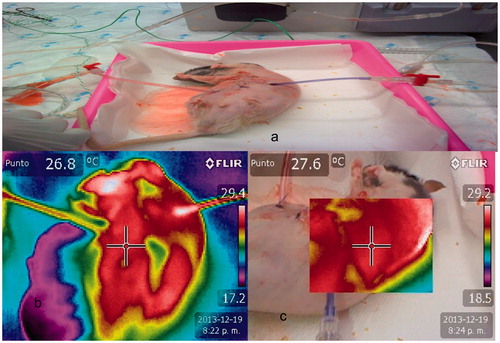
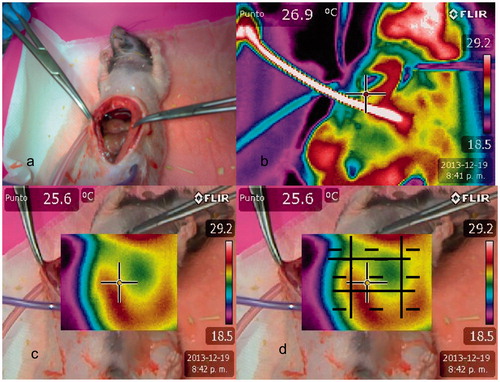
A subxiphoid laparotomy was performed of about three inches with cytoreductive surgery simulation in both groups. In Group 1, two multi-perforated catheters of 5 mm in diameter were used and were connected to a digital thermometer. They were placed in the right subcostal region (inflow) and left iliac fossa to pelvic region (outflow). Wall closure by planes was performed with Polisorb 4/0 (Minneapolis, MN). After recirculation and drainage for 60 min of 1.5% hyperthermic dextrose, inlet and outlet temperature control was performed at 30 min and a thermographic map to control temperature distribution in two groups. In Group 2, the closure of the abdominal wall was not performed, as a open technique described by Sugarbaker [Citation10].
Perfusion system
The study used the infusion system GST Combat PRS® (Galmaz SL ). This system allows perfusion of a 1.5% dextrose solution at 70 cc/min (up to 200 cc/min) through inlet and outlet catheters. They were located in the upper abdomen with intra-abdominal pelvic orientation. The intraperitoneal temperature was maintained at 41–42 °C with a flow of 70 cc/min for 60 min. Body surface area was calculated using the Dubois formula, Body Surface Area (BSA) (m2) = bodyweight0.425 (kg)×bodyheight0.725 (cm) × 0.007184, to control instilled volume and mimic drug use in this volume.
Thermography
For the thermographic analysis in the different models, the study divided into nine regions as a modified abdominopelvic peritoneal carcinomatosis index. As quantitative thermographic asymmetry criteria, the study used the existence of different temperature gradients between and within the quadrants of 2.5 °C or higher. Temperature values were obtained by surface control in each quadrant using the thermographic sensor of the camera. If there is that difference between a quadrant and other that is located just beside, the score for that quadrants is 0. If there is a different temperature gradient over 2.5 °C within the quadrant, the value is 0.
Uptake of thermographic images and surface temperatures were performed by the thermographic camera, FLIR E40BX characterised by accuracy, ±2 °C or ±2% of reading, thermal sensitivity, <0.045 °C, resolution, 160× 120 pixels, emissivity correction that is variable from 0.01 to 1, or selected from materials list, (FLIR Systems Ltd., Kent, UK).
The study also realised a qualitative analysis using a system of colours. A red image was considered maximum temperature, followed by yellow, green, and blue images. The blue image is considered the coldest area, according to a decrease in the amount of infra-red radiation emitted. Homogeneity in the cases was defined as the existence of red images in all quadrants and the existence of red images attached to a yellow quadrant. In these cases, all quadrants are scored as one. Temperature difference was considered excessive if there was discontinuity of values between quadrants, and a red image was attached to green or blue images. In those cases, the temperature distribution was considered heterogeneous, and the values per quadrant were zero. If there is heterogeneity of colours within a quadrant the score is 0.Scores ranged from zero to nine. The unique method to control a deep distribution of heated solution was performing a lateral view. The study performed a lateral qualitative temperature distribution control, using the system of colours and considering the lateral plane as an unique quadrant, between retroperitoneum to laparotomy, to diagnosis the distribution of heated solution over lateral abdominal areas, that included right paracolic gutter, left paracolic gutter, right diaphragm, left diaphragm, right pelvic peritoneum and left pelvic peritoneum. After the procedure, the rats were sacrificed using intravenous overdoses of KCl in the anaesthetised rats.
Porcine model
Animals
The porcine experimental model included 12 female large white pigs weighing between 35 kg and 38 kg. Four pigs were included in the control group and received intraperitoneal chemotherapy using the open technique. Eight pigs were included in the closed group and received intraperitoneal chemotherapy using the experimental technique with CO2 closed agitation. The study was approved by the Animal Ethics Committee of the Veterinary University of Barcelona, Spain. We used this experimental model not only to analyse the intraperitoneal distribution of drug as the rat model, we also wanted to control the safety and effectiveness in management fluid status and hemodynamic parameters during closed hyperthermic intrabdominal chemotherapy with CO2 circulation.
Anaesthesia and perioperative management
Azaperone (0.4 ml/kg IM), ketamine (0.2 ml/kg IM), and morphine (0.4 mg/kg SC) were used for the initial pre-anesthetic phase. Midazolam (0.4 ml/10 kg IV) and atracurium (0.2 ml/10 kg IV) were used for the anaesthesia technique, and all pigs were maintained using inhaled oxygen (100%) and isoflurane (2.5%). Core temperature was monitored using an oesophageal thermometer.
Surgical technique
The study realised a midline laparotomy. A hysterectomy, bilateral oophorectomy, peritonectomy and pelvic-aortic lymphadenectomy were performed. HIPEC was performed using an open technique in four animals, as described by Sugarbaker [Citation10]. The drug containing solution was preheated and infused into the abdominal cavity at 2400 cc/min. The CO2-assisted closed technique was performed on eight animals. We tried to simulate surgical stress and drug toxicity as in human protocol.
Perfusion technology
The study used a closed-perfusion system (PRS Combat®, Galmaz Biotech SL, Madrid, Spain). A roller pump preheated and recirculated the solution. Roller pump B administered the chemotherapeutic drug and removed the perfusate solution. The system includes a gas exchanger located at the highest level of the abdominal wall. After closing the laparotomy, the drug containing solution (peritoneal dialysis solution containing 1.36% glucose and 25 mmol/l bicarbonate) was preheated to 42 °C and infused into the abdominal cavity at 2400 cc/min. The study applied paclitaxel at doses of 175 mg/m2 for 60 min with the closed abdominal technique. Turbulence of the perfusate was generated by the infusion of CO2 under controlled pressure to get an optimal hyperthermic solution distribution.
Temperature control
Temperatures were measured using inflow and outflow temperature probes and internally using six PicoLog temperature probes (Pico Technology, Cambridgeshire, UK). Recirculation of drug only started if temperature was 41 °C in inflow and outflow controls or higher, and thermographic images were homogeneous. Quantitative relation of intra-abdominal temperatures measured by temperature probes, respect to inflow and outflow temperatures was previously checked by our Group [Citation1].
Thermography
The study also used a thermographic camera (FLIR E4 0BX, FLIR Systems Ltd., Kent, UK) for temperature control. Images obtained were used to check the homogeneity of temperature distribution at 30 min after starting the procedure and related to inflow and outflow temperatures.
The study controlled hyperthermic perfusate and temperature distribution by scoring abdominal areas using a modified peritoneal carcinomatosis index system described in the rat model. It realised lateral view for controlling deep temperature distribution.
If there was a difference of temperature of more than 2.5 °C, controlled by superficial temperature control and confirmed by heterogeneity distribution of colours, it was considered a heterogeneous distribution of temperature. Animals were euthanized with intravenous sodium pentobarbital (100 mg/kg IV).
Pilot study
Study design and patients
A Pilot Clinical Study (EUDRACT 2011-006319-69) included 18 patients with diagnosis of ovarian cancer who were undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. The study was approved by the Local Ethics Committee and informed consent was obtained from all patients.
Operative technique
The study performed hysterectomy, bilateral oophorectomy, pelvic-aortic lymphadenectomy and peritonectomy. Visceral and parietal peritonectomy were performed to obtain a complete cytoreduction.
The abdominal cavities were closed after surgery. The carrier solution was preheated to 42 °C and infused at 2400 cc/min. After checking the carrier solution temperature, paclitaxel at doses of 175 mg/m2 was applied for 60 min with closed technique abdomen. During recirculation of the perfusate, turbulence was generated by infusion of CO2 that was added for creating turbulence inside the abdominal cavity to achieve an optimal distribution of chemotherapeutic agent. The CO2 exited the abdominal cavity via the gas exchanger.
Perfusion system technology
The study used a closed perfusion system (PRS Combat®, Galmaz Biotech SL, Madrid, Spain) for HIPEC that includes CO2 circulation. The system includes a gas exchanger that is located at the higher level of the abdominal wall, and it communicates with the abdominopelvic cavity. It allows a visual monitoring of complete filling of the abdominal cavity. The gas exchanger avoids an increase in abdominal pressure due to solution in the abdominal cavity. The intraperitoneal temperature was continuously monitored by two temperature probes placed at the inflow and outflow catheters.
Thermography
Recirculation only started if temperature was 41 °C in inflow and outflow controls or higher and thermographic images were homogeneous. At starting could not exist differences of 2.5 °C or higher between or within, PCI areas as modified distribution systems, or in colour images distributed between quadrants, as previously described. Lateral thermographic images had to be also homogeneous ().
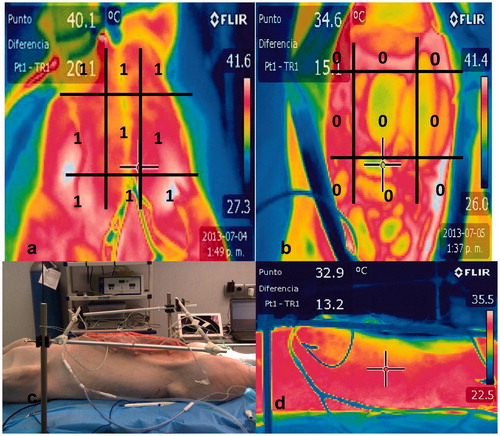
Figure 1. Thermographic images of closed chemohyperthermia in rats. There were homogeneity in temperature and thermal images. a. Closed recirculation in rat. b. Anterior thermal image during HIPEC. c. Lateral thermal infra-red image during HIPEC.
At 30 min after starting HIPEC (FLIR Systems Ltd., Kent, UK), the study realised inflow and outflow monitoring of temperatures. Central temperature was analysed at starting (t1), at 30 min after starting (t2), and at the end of the procedure (t3). A thermographic camera (FLIR E4 0BX, FLIR Systems Ltd., UK ) was used for measuring temperature and perfusate homogeneity distribution.
Statistical analysis
Continuous variables were expressed as mean, median, and standard deviation. The means of continuous variables were compared with the t test of students. For statistical analysis, the study used SPSS15.0® (Chicago, IL). Statistical significance was defined as p < .05.
Results
Murine model
In closed Group, at 30 min from starting recirculation, the group reached homogeneity in temperature distribution. Temperatures measured in specific surface controls distributed according to the system of quantification of images didn’t reach differences over 2.5 °C. Distribution of images was homogeneous in all quadrants. The outlet temperature was always above 41 °C (41.5°, 41.8°, and 41.9 °C) ).
In the open Group, there was a heterogeneous temperature distribution at 30 min. It was probably maintained by the exposure of the viscera in the open technique. The study measured temperature value differences over 2.5 °C between quadrants. There was asymmetry in the distribution of colour images. Temperatures didn't reach values above 41 °C in any of the rats ().
Figure 2. a. Open recirculation in rat. b. Anterior thermographic image of open chemohyperthermia in rats. c. Anterior termographic image that only included laparotomy. d. There were heterogeneity in temperature and thermal images in all quadrants. Total value after applying thermographic quadrants measurement system was 0.
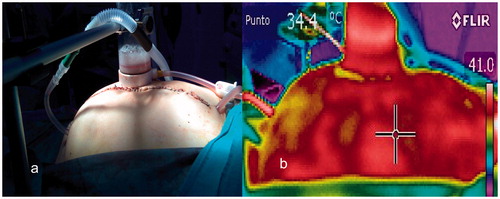
Porcine model
The inlet and outlet temperatures reached at half time of HIPEC were 42 °C in the closed Group and 41.4 °C in the open Group. In the group with laparotomy access, the outflow temperature did not reach 40 °C in any pig. Distribution of images was heterogeneous, probably because there was maintained environmental exposure. However, depth thermographic distribution was homogeneous. Prior to manual agitation of the carrier solution with the drug, temperature reached 41 °C heterogeneously in some locations. After agitation, intra-abdominal temperature didn’t reach 40 °C. There are temperature and colours heterogeneity in the pig open HIPEC Group. For this heterogeneity the values are 0 in quadrants ().
Clinical phase
The Pilot Study (EUDRACT 2011-006319-69/NCT 02681432) included 18 female patients. The median age was 57 years old (42–84), with DM 4/18, 28.5%, hypertension, 10/18, 55.6%, BMI, 27 ± 4.5 kg/m2. Five patients were treated preoperatively with neoadjuvant therapy. Intraoperative urine output was 902 ± 399 cc. Mean intraoperative bleeding was 809 ± 714 cc. Temperature (T1) was 36 ± 1.2 °C. Temperature (T2) was 37 ± 0.7 °C. Temperature (T3) was 36.3 ± 0.9 °C. Inflow temperature values reached at half time of HIPEC were 42 ± 0.2°, and outflow temperature values were 41 ± 0.8 °C, respectively. There was no influence in core temperature of patients after reaching the homogeneous temperature distribution ().
Discussion
Thermography is the measurement of temperature using a non-contact method. The thermal images obtained show the temperature distribution on the surface and do not allow the measuring of internal temperatures. Properties and surface condition are fundamental because the emission of infra-red radiation depends on the temperature of the surface. In clinical thermography, it is possible to assess the skin surface temperature, which depends on the blood circulation in the skin. Underlying skin issues like neoplasms, inflammation, and tissue trauma influence the skin temperature and can be analysed by specific thermograms. Thermography has been applied in several benign diseases such as chronic lung disease, appendicitis, urinary tract infections, tonsillitis, and sinusitis. Breast cancer has been frequently used for the development of clinical thermography. Its pathogenesis is the generation of heat by the tumour respect to healthy tissue. Neoplastic tissue exhibits greater metabolism and angiogenesis than adjacent tissue [Citation11–16]. Infra-red thermal imaging has been even used as an screening method for breast carcinoma due to the continued improvements in thermal images technology [Citation15]. Other authors have used thermographic images to obtain information about the intratumoral temperature by magnetic nanoparticle hyperthermia in an experimental model [Citation16,Citation17].
The application for monitoring the distribution of chemohyperthermia after cytoreductive surgery has only been done in a closed model by Jäger et al. [Citation18], and our Group [Citation19]. Our group incorporated the use of thermography in the closed HIPEC system development with the recirculation of CO2 as a routine activity for the implementation of this technique [Citation19].
In experimental animal studies, the thermography has been used to monitor the presence and development of tumour implants with great sensitivity due to its disturbance in respect to vascularisation of normal tissue. It also has been used to identify the biological response to neoplastic therapy [Citation11].
At the beginning of the project, the goal was to identify heat losses and their distribution in a Murine model. When the study simulated the open technique, it failed to reach outlet temperatures of 41 °C or higher, but it reached that temperature with the closed technique. Thermograms were heterogeneous in colour, not reaching quantification in any rats of the maximum value of nine. On the other hand, the study reached scores of nine, in the three rats used in the closed system, probably due to a sustained visceral environmental exposure. The lateral thermograms were homogeneous in both groups. The study obtained an optimal depth distribution. In theory, cold fluids, which have a higher density, would distribute to deeper areas and therefore images would be green or blue, but this didn’t occur in the rats studied.
In the second model, it was considered that the closed system could better hold heat, and the study incorporated the recirculation system using CO2 to distribute the hyperthermic solution. In the experimental group, the anteroposterior and depth distribution was homogeneous at baseline and 30 min, reaching maximum values of up to nine after applying the thermographic quadrants measurement system. It allowed that at half of the HIPEC procedure, the inlet and outlet temperatures remained above 41°. In the control group, it was not possible. There was heterogeneity of anteroposterior thermograms in all animals but homogeneity in depth images. We didn’t reach quantification in any rat of the maximum value of nine.
After these results, a clinical translation occurred in the development of a pilot study to assess the implementation of a new HIPEC model [Citation1].
In conclusion, the use of thermographic images allowed participants to learn the distribution in quadrants and the depth of hyperthermic carrier solution prior to introducing the drug in any model for performing chemohyperthermia. It may be necessary to maintain the recirculation and preheated period.
The thermographic images recorded during the development of chemohyperthermia can allow changes to the locations of intra-abdominal catheters and inlet temperature adjustments.
Temperatures that were identified digitally by the camera showed the body surface temperatures. They are lower because of environmental exposure than intra-abdominal temperatures evidenced with inlet and outlet thermometers. It is necessary to only use these temperatures as colour images to control abdominopelvic temperature distribution.
In conclusions the infra-red digital images application in the closed system have shown how the temperature is maintained during chemohyperthermia. Closed and high flow recirculation avoid heat loss and heterogeneity infra-red images. These findings confirm that inclusion of a CO2 recirculation system allows an optimal and homogeneous distribution of the carrier solution and hyperthermia by the abdominopelvic cavity.
Disclosure statement
The authors report no declaration of interest.
References
- Sanchez-Garcia S, Padilla-Valverde D, Villarejo-Campos P, et al. (2014). Experimental development of an intra-abdominal chemohyperthermia model with closed abdomen technique and CO2 recirculation system by means of PRS-1.0 COMBAT@. Surgery 155:719–25.
- Hildebrant B, Wust P, Ahlers O, et al. (2002). The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43:33–56.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002). Hyperthermia in combined treatment of cancer. Lancet Oncol 3:487–97.
- Issels RD. (2008). Hyperthermia adds to chemotherapy. Eur J Cancer 44:2546–54.
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. (2005). Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 21:779–90.
- Chichet A, Skowronek J, Kubaszewska M, Kanikowski M. (2007). Hyperthermia-description of a method and a review of clinical applications. Rep Pract Oncol Radiother 12:267–75.
- Lepock JR. (2005). How do cell cells respond to their thermal environment? Int J Hyperthermia 21:681–7.
- Beck WT, Dalton WS. (1997). Mechanisms of drug resistance. In: DeVita VTJ, Hellmann S, Rosenberg SA, eds. Cancer-principles and practise of oncology. Philadelhia (New York): Lippincott-Raven Publishers, 498–512.
- Hildebrandt B, Wust P. (2007). Interactions between hyperthermia and citotoxic drugs. In: Ceelen WP, ed. Peritoneal carcinomatosis. A multidisciplinary approach.. New York, USA: Springer, 185–93.
- Sugarbaker PH. (2007). Peritonectomy procedures. In: Ceelen WP, ed. Peritoneal carcinomatosis. A multidisciplinary approach. New York (USA): Springer, 247–64.
- Song CH, Appleyard V, Murray K, et al. (2007). Thermographic assessment of tumor growth in mouse xenografts. Int J Cancer 121:1055–8.
- Arora N, Martins D, Ruggerio D, et al. (2008). Effectiveness of a non-invasive digital infra-red thermal imaging system in the detection of breast cancer. Am J Surg 196:523–6.
- Ring EFJ, Ammer K. (2012). Infra-red thermal imaging in medicine. Physiol Meas 33:33–46.
- Kennedy DA, Lee T, Seely D. (2009). A comparative review of thermography as a breast cancer screening technique. Integr Cancer Ther 8:9–16.
- Rassiwala M, Mathur P, Mathur R, et al. (2014). Evaluation of digital infra-red thermal imaging as an adjunctive screening method for breast carcinoma: a pilot study. Int J Surg 12:1439–43.
- Ivkov R. (2013). Magnetic nanoparticle hyperthermia: a new frontier in biology and medicine?. Int J Hyperthermia29:703–5.
- Rodrigues HF, Mello FM, Branquinho LC, et al. (2013). Real-time infrared thermography detection of magnetic nanoparticle hyperthermia in a murine model under a non-uniform field configuration. Int J Hyperthermia 29:752–67.
- Jäger T, Dinnewitzer A, Augschöll C, et al. (2014). Infrared thermography monitoring in closed hyperthermic intraperitoneal chemotherapy: a novel technique to maintain therapeutic intraperitoneal temperature distribution. EJSO 40:S28.
- Sanchez-Garcia S, Villarejo-Campos P, Padilla-valverde D, et al. (2016). Intraperitoneal chemotherapy hyperthermia (HIPEC) for peritoneal carcinomatosis of ovarían cáncer origin by fluid and CO2 recirculation using the closed abdominal technique (PRS-1.0 Combat): a clinical pilot study. Int J Hyperthermia 32:488–95. doi: 10.3109/02656736.2016.1152515.