Abstract
Tumour ablation is defined as the direct application of chemical or thermal therapy to eradicate or substantially destroy a tumour. Currently, minimally invasive ablation techniques are available for the local destruction of focal tumours in multiple organ sites. Microwave ablation (MWA) is premised on the biological response of solid tumours to tissue hyperthermia, and it is a relatively low-risk procedure. Due to several advantages of MWA, including higher thermal efficiency, higher capability for coagulating blood vessels, faster ablation time and the simultaneous application of multiple antennae, MWA could be a promising minimally invasive ablation technique for the treatment of solid tumours. Therefore, the use of MWA has developed rapidly in China during the last decade. Many successful studies have been performed, and widespread use has been achieved for multiple types of tumours in China, especially for liver cancer. This review will describe the state-of-the-art of MWA in China, including the development of MWA equipment and its application in the treatment of multiple types of tumours.
Image-guided minimally invasive ablation techniques have achieved widespread expansion as clinical treatment options for tumours during the past two decades. Due to advancements in both the imaging guidance modalities and the percutaneous devices used to delivery energy into tumour tissue, various thermal energy sources, such as radiofrequency, microwave, high intensity focussed ultrasound, cryoablation and laser, have established themselves as viable treatment options for the eradication of solid tumours in multiple organ sites. Microwave coagulation was initially developed in the early 1980s to achieve haemostasis along the plane of transection during hepatic resections [Citation1]. The microwave technique was first adopted for tumour ablation by Saitsu et al. [Citation2] from Japan to treat small liver cancers and has achieved wider application over the past two decades, particularly in China. Although radiofrequency ablation (RFA) remains the most widely used ablation technique worldwide, with the improvement of microwave ablation (MWA) equipment and treatment strategies, now MWA has expanded its clinical applications in many institutions in Far East countries and parts of Western countries, from small hepatocellular carcinomas (HCC) to large liver cancers greater than 5 cm and multiple other solid tumours including those in the kidney, adrenal, spleen, thyroid, lung, breast, abdominal wall and uterus [Citation3–10]. The MWA technique has developed rapidly over the past decade in China and has been used clinically in more than 500 hospitals in China. MWA is another promising local thermal ablation technique that has undergone tremendous progress and expanded clinical applications because of its favourable therapeutic efficacy. In this review, we summarise the status and the advancement of MWA in China, especially its application in solid tumour treatment.
Mechanism and equipment development
Microwave energy is a high-frequency electromagnetic wave that exerts its function by inducing frictional heat from its interaction with polar water molecules [Citation11,Citation12]. The water molecules oscillate between 2 and 5 billion times to align themselves in the field of microwave energy. This rapid molecular rotation generates heat which leads to cell death through coagulation necrosis which is instantaneous and continuous until the energy is stopped. Another less important mechanism of the heat generation is the ionic polarisation which occurs when the ions move in response to the applied microwave electric field.
All MWA systems contain three basic elements—a microwave generator, a low-loss flexible coaxial cable and a microwave antenna. The microwave is generated by the magnetron. The antenna is connected via a low-loss coaxial cable to the microwave equipment and delivers the microwave energy from the magnetron to the tissue. The design of the antenna is vital to its therapeutic efficacy. Currently, there are five commercially available MWA devices with frequencies of 915 MHz and 2450 MHz in China (), all of which were invented and produced by Chinese institutes. The device designers have focussed on a needle-like, thin, internally cooled, coaxial-based interstitial antenna [Citation13,Citation14], to achieve a larger ablation zone and to allow for percutaneous use. The diameter of the antennas range from 1.6 to 2.15 mm (14–16 gauge), with different active-tip length designs for different tumour types. The Kang-you MWA machine (Kangyou Medical, Nanjing, China) is also equipped with a thermal monitoring system which can continuously measure the temperature in real time during ablation. The thermal monitoring needle can be classified into thermocouple and thermistor-type with diameters of 0.7–0.9 mm (20–22 gauge); the needle is introduced into the tumour parenchyma through a non-conducting needle trocar. In the near future, a new type of MWA equipment with function of ablation protocol planning finished by a three-dimensional (3D) visualisation software will enter the Chinese clinical market, which will focus on promoting the development of precision ablation.
Table 1. Commercial MWA system and antenna in China currently.
The status of MWA application in China and other regions
Currently, there are more than ten different brands of MWA systems that are clinically available in more than 10 countries; the majority of them are from China or the USA, with only one each from the UK and Italy [Citation15]. Most of the MWA antennae used in the USA are shaft cooling (saline or CO2), except for the MedWaves AveCure system (non-cooled). The antenna diameter of the USA systems range from 12 to 17 gauge. Similar to the Chinese systems, the USA systems also use frequencies of 2450 MHz or 915 MHz and are equipped with a temperature monitoring unit. MWA is most widely used in China, compared with other regions, and it has been applied for the treatment of lesions in the liver, kidney, adrenal gland, lung, thyroid, parathyroid, uterus, bone and breast, all of which will be introduced in the following sections in detail. After China, the USA is the country with the second highest application of MWA. The application of MWA in the USA has been used mainly for the treatment of liver cancer, with pilot studies in lung and renal cancers [Citation16,Citation17]. Italy and Germany are two other countries with a relatively high number of MWA reports, behind China and the USA. Italian investigators have trialled MWA therapy in the liver, kidney, lung, pancreas and lymph nodes. With regard to liver cancer therapy, China, USA, UK and Italy have all published multicentre study results of MWA [Citation18–20], but China has examined the largest patient samples and reported the most long-term survival data. Most of the studies from Germany have focussed on the treatment of thyroid cancer [Citation21]. In addition, researchers in Japan and Korea have also performed some preliminary studies on the use of MWA for the treatment of liver and lung cancer, but only with very limited literatures.
MWA in China for the treatment of liver cancer
The use of MWA for the treatment of liver cancer was first reported in China in 2001 by Lu et al. [Citation22]. Their study included 50 HCC patients with 107 lesions (mean size 2.7 cm). Each ablation was performed at 60 W for 300 s. The results revealed that the complete ablation rate was 94.4% and the 3-year survival rate was 73%. After that, the MWA technique was reported in numerous studies with larger samples of HCC patients and liver metastases patients. The largest series involving the use of MWA for HCC in a single institution was reported by Liang et al. [Citation23]; the study involved 288 patients with 477 tumours. The 1-, 3- and 5-year cumulative survival rates were 93%, 72% and 51%, respectively. Local tumour progression was observed in 8% of the patients. Patients with a single tumour ≤4.0 cm and child A cirrhosis had a higher probability of long-term survival [Citation23]. Because MWA was most widely used for the treatment of HCC, comparison studies of MWA with RFA and surgery show an increasing tendency. Most of the studies were retrospective and employed cool-tip applicators. The studies’ results revealed that MWA and RFA could achieve comparable results [Citation24–26] in small HCC tumours. However, only two retrospective studies were performed by Chinese doctors comparing the treatment of HCC with MWA vs. surgery conforming to Milan criteria, but the studies yielded different results. Wang et al. found similar results between MWA and surgery [Citation27] but Shi et al. detected a lower disease-free survival in patients treated with MWA [Citation28]. There are fewer reports on the impact of MWA on liver metastases than there are for HCC (). The largest series was also performed by Liang’s team, and they reported 1-, 3- and 5-year cumulative survival rates of 90.9%, 51.1% and 31.9%, respectively [Citation29].
Table 2. Major researches of MWA in primary and secondary liver malignancies treatment.
For the treatment of malignancies, MWA has achieved great progress beyond its initial use in small HCC tumours. It has now been successfully applied in larger tumours and tumours in risky locations (adjacent to the gallbladder, gastrointestinal tract, large vessels, hilum, diaphragm and marginal angle) by using it in combination with transcatheter arterial chemoembolization, percutaneous ethanol injection, artificial ascites and artificial pleural effusion [Citation30–39]. As for its safety, the MWA technique is a safe and feasible liver cancer therapy with relatively low major complication rates that range from 2.2 to 3.9%, based on several large-scale studies of MWA in the liver [Citation40–42].
Although promising single-centre reports have demonstrated MWA is safe and effective [Citation22–24,Citation43–51] as shown in , it was necessary to evaluate this modality in a prospective multicentre study. The first multicentre study with a large sample was performed in China, and the investigators documented that the 1007 patients with primary liver cancer treated with MWA achieved 1-, 3- and 5-year survival rates of 91.2%, 72.5% and 59.8%, respectively [Citation52]. There were three subsequent multicentre MWA studies from the United States and Europe that demonstrated low morbidity rates for MWA treatment of liver tumours, but they did not provide the long-term survival and recurrence data [Citation18–20]. Because of the advanced techniques and widespread application of MWA in China, the Society of Chinese Interventional Ultrasound (US) outlined the first clinical practice guideline for percutaneous MWA therapy for hepatic malignancy [Citation42] to provide assistance to physicians in: (1) evaluating patients with hepatic malignancy who may be candidates to undergo percutaneous MWA; (2) providing relevant and updated technique information for performing this treatment, including the mechanism, technical advantages, equipment information and detailed procedures; (3) understanding the consequences of this therapy and its use in combination with other modalities. With the help of this guideline, more and more patients with primary and secondary hepatic malignancies will benefit from the favourable effects of this thermal technique. In addition, MWA has been used by Chinese doctors to treat benign focal liver lesions including haemangiomas, focal nodular hyperplasia, inflammatory pseudotumors, solitary necrotic nodules and hepatic adenomas with the strict indications administration [Citation53].
MWA in the kidney
Cancer of the kidney accounts for approximately 3.5% of all malignancies and it is the third most common cancer of the urinary tract. Renal cell carcinoma (RCC) is the most lethal of all the genitourinary tumours [Citation54]. The standard of care for clinically localised RCC remains surgical resection due to the favourable outcomes after surgery and the relative ineffectiveness of systemic therapy. Because of the increased detection of small RCC, minimally invasive ablative technologies have emerged as promising treatment options for clinically localised RCC to reduce procedural morbidity while maintaining equivalent oncologic effectiveness with surgery. Effective renal cryoablation and RFA have been achieved by open and laparoscopic approaches, as well as by percutaneous image-guided techniques [Citation55,Citation56]. As one of the most recent and exciting advances in the field of thermoablative techniques, MWA now has a major role in the treatment of liver cancer. In contrast, research in the effect of MWA in the treatment of small RCC is in its preliminary stages, with studies involving small numbers of patients in the world and in China.
A Chinese research group directed by Liang performed preclinical studies to evaluate the morphology, size and histologic features of the ablated areas in animal renal cancer models [Citation57], and estimated the amount of energy that could be deposited in tissue. On the basis of this experimental research, Liang et al. reported the first use of MWA for the treatment of small RCCs with a mean size less than 3 cm [Citation58]. They observed no residual tumour or recurrence at a median follow-up of 11 months. Following this report, there have been several recent studies assessing the safety and efficacy of MWA techniques for the treatment of small renal tumours in China () [Citation3,Citation58–65]. Liang et al. also reported their experience with US-guided MWA treatment of RCC with the largest patient volume and longest follow-up period, to our knowledge [Citation65]. The results shown in demonstrate that MWA can safely and quickly generate large ablation zones with uniform tissue necrosis. Successful complete ablation was achieved in 93.8–100% of RCC patients, with the majority of studies showing no recurrences during a median follow-up of 10–25.8 months and major complication rates that ranged from 0 to 2.5%. Two researchers also performed preliminary studies of MWA for the treatment of large benign renal tumours and showed that the technique can provide an effective alternative to nephrectomy for patients with benign tumours; however, one case resulted in a fistula of the descending colon and one case developed urinary leakage, which suggested that tumours near the colon and collecting system needed to be ablated with particular care. The effectiveness of MWA for the treatment of relatively large renal tumours requires further confirmation with larger samples.
Table 3. Major researches of MWA in renal tumours treatment.
As a new modality for the treatment of renal cancer, the difference between MWA and traditional surgery awaits clarification. Guan et al. performed a prospective randomised trial comparing MWA with partial nephrectomy in a cohort of 48 patients [Citation61]. Yu et al. reported their experiences in retrospective comparative studies between MWA vs. open radical nephrectomy in 65 patients, and MWA vs. retroperitoneal laparoscopic radial nephrectomy in 98 patients with small RCC [Citation62,Citation65]. They both concluded that MWA and surgery could achieve comparable oncologic outcomes in RCC patients. However, MWA resulted in lower estimated blood loss, renal function injury and postoperative hospitalisations. To date, there have been no other comparative studies between MWA and other modalities including RFA, and cryoablation for the treatment of RCC. In summary, MWA appears to be a safe and effective technique for the management of RCC, particularly small RCC in selected patients with less tumour invasion. The outcomes of prospective clinical studies that compare MWA with other ablation technique will determine the specific opportunities for the development of MWA for this indication.
MWA in the lung
Lung cancer is the most common cancer diagnosed worldwide with 1.3 million new cases every year. However, only 20% of all diagnosed lung tumours are resectable. MWA for the treatment of lung cancer was first attempted in 2002 in China, using computed tomography (CT) guided imaging. US guidance is only feasible for lung cancers in peripheral locations that can be visualised with ultrasound. A few other groups have tried to apply MWA for the treatment of lung tumours (). The rate of complete necrosis observed in lung tumours ranged from 56.3 to 84.8% [Citation5,Citation66–72]. Because MWA was mainly used to treat inoperable pulmonary malignancies, the LTP after MWA treatment of larger tumours was still a major problem. Most of the studies did not achieve good long-term survival results for this initial application of MWA for lung cancer. The most common complication was pneumothorax with an incidence rate of 1.5–20.6% [Citation5,Citation66–72]. Other common complications after MWA include chest pain, haemoptysis, skin burns, fever, pleural effusion and pulmonitis. Severe complications were rare.
Table 4. Results of Chinese clinical studies regarding MWA of lung tumours.
Compared with traditional pneumonectomy surgery, MWA represents a potentially safe, effective and less invasive percutaneous technique for the treatment of lung malignancies. With more widespread application and clinical experience, MWA may become a valid treatment option in lung malignancies and it may improve survival in patients who are not appropriate for surgery.
MWA in the thyroid
Publications on the use of MWA for benign and malignant thyroid nodules are limited; the first studies were reported by Chinese researchers. Feng et al. first demonstrated the feasibility of MWA to treat benign thyroid nodules and reported a mean volume reduction rate of 45.9% (from 1 to 9 month) [Citation6]. Yue et al. achieved better results in a larger study of 477 benign nodules, and reported a mean volume reduction of 65% at 6-month follow-up [Citation73]. Yue et al. also tried to treat solitary thyroid microcarcinoma and recurrent papillary thyroid carcinoma with MWA [Citation74,Citation75]. Their results demonstrated complete necrosis of all lesions without recurrence and suggested that MWA may be an alternative treatment option for malignant lesions in selected patients. The possible major complications of oesophageal perforation, tracheal injury, abscess, permanent hypothyroidism and deep haematomas that have been reported sporadically with laser ablation and RFA have not been reported with MWA. The incidence of vocal cord paresis was 3.6% after MWA; most of the symptoms were transient and most patients completely recovered their vocal cord function, either spontaneously or after active drug treatment [Citation73]. The moving shot technique, the fluid dissection technique and the use of the modified small-bore antenna may decrease the risk of nerve injury and haemorrhage.
Although initial studies determined that MWA was a safe and effective alternative technique for benign nodules and small cancers of the thyroid, prospective randomised large studies comparing MWA with other techniques such as surgery, RFA and laser ablation are necessary to determine the efficacy, safety, cost/benefit balance and quality of life outcomes.
MWA in the spleen
Classic splenectomy is regarded as a first-line choice for hypersplenism and splenic tumours. The spleen is an important immune organ that defends against infection. Preservation of 25–50% of the spleen can ensure adequate physiological function, especially immune function [Citation76]. Chinese researchers have evaluated MWA as a technique to preserve part of the spleen in the treatment of hypersplenism and splenic tumours. For hypersplenism, the higher the splenic volumes that were ablated (over 40–50%), the more the levels of leukocytes, platelets and peripheral lymphocyte subsets improved [Citation77–79]. Jiang et al. [Citation79] completed the latest study with the largest patient sample and evaluated the effects of MWA in 38 patients with secondary hypersplenism caused by hepatic cirrhosis. The ratio of ablation volume ranged from 20 to 40%. The leukocyte and platelet levels started to increase after the 1st week, and 45% of the patients peaked at 3 months, 32% at 6 months, 13% at 12 months, and 10% peaked at 24 months after ablation. The clinical symptoms of fatigue, bleeding gums and skin ecchymosis were definitely improved.
Tumours of the spleen, whether benign or malignant, are relatively rare entities. Thus, data on the use of ablation for splenic tumours are rather limited. To date, only two papers have reported on the use of MWA for splenic tumours, and both were performed by Liang’s team [Citation7,Citation80]. In their latest study, they evaluated the feasibility and effects of MWA in 7 patients with 2 benign splenic nodules (diameter range, 5.9–6.3 cm) and 6 malignant nodules (diameter range, 1.3–2.9 cm). All 8 of the nodules showed no progression for 4–48 months and no complications occurred; intraperitoneal haemorrhage was the major risk.
These limited studies have suggested that MWA is feasible and effective for the treatment of splenic diseases. Although there are no reports on the comparative efficacy of MWA, RFA and HIFU for splenic diseases, due to the higher thermal efficacy of MWA, there is more potential advantage of MWA in the spleen due to its rich blood supply. The application of this new ablation technique in the spleen merits further exploration and discussion.
MWA in the uterus
Several treatments are commonly used to relieve the symptoms of patients suffering from adenomyosis and uterine fibroids. Hysterectomy ensures permanent relief of fibroid or adenomyosis related symptoms, but it is associated with significant morbidity and guarantees infertility. Many patients would prefer alternative modalities for the permanent alleviation of the symptoms of this benign disease rather than a surgical radical hysterectomy. Cheng et al. first reported on transvaginal MWA as a minimally invasive treatment for pedunculated submucosal fibroids in the vagina in 1997 [Citation81]. Zhang et al. first reported on US-guided percutaneous MWA for symptomatic uterine fibroid in 2008 [Citation82]. Since then, MWA has developed rapidly as a minimally invasive management technique for uterine fibroids within the last several years in China, with a reported 1-year volume reduction of 93.1% [Citation83]. The use of MWA for adenomyosis was first reported in 2011 by Zhang et al. [Citation84]. Their group evaluated the largest sample of MWA therapy for adenomyosis in 142 patients [Citation85]. All of the patients tolerated the therapy well and reported significant clinical improvements during the follow-up period, with the 1-year symptoms severity score improving 99.3%. MWA of uterine fibroids can eliminate or effectively relieve the clinical symptoms and improve quality of life by shrinking the fibroids and eradicating adenomyosis tissue. The advantages of this use of MWA are that the procedure is easy to perform, minimally invasive, safe and it preserves the uterus. However, because this is a relatively new method for treating disorders of the uterus, comparative studies with other established minimally invasive techniques such as uterine artery embolisation are warranted.
MWA in the adrenal gland
Metastatic lesions, functional adrenal neoplasms and larger benign adrenal neoplasm are the indications for the treatment of adrenal lesions. Because of the relatively high complication rates with adrenalectomy, ablation is a promising technique for the percutaneous treatment of adrenal tumours. There are only three published reports on MWA in adrenal tumours in China, with a total of 47 patients [Citation4,Citation86,Citation87]. According to Ren et al. who studied 33 patients for a follow-up period of 24 months, technical success was achieved in all cases. The rate of local tumour progression was 15.2% for all of the patients, and there was a significant difference noted between tumours ≤5 cm and >5 cm (p < 0.01). For the seven cases of metabolically active adrenal tumours, all of the patients demonstrated normalisation of the abnormal biochemical markers after the ablation. One patient with a pheochromocytoma experienced a hypertensive crisis during MWA treatment that was relieved after the ablation was paused and an intravenous drug was administered. Percutaneous MWA of adrenal tumours appears to be a feasible technique for local adrenal tumour control and to treat hormonally active tumours with clinical syndromes, especially for tumours that are ≤5 cm. Larger multi-centre studies with long-term follow-up are required to further examine the clinical efficacy of this therapy.
MWA in other organs
MWA has also been trialled to treat solid tumours in multiple other organs including the bone, breast, abdominal wall and bladder [Citation8,Citation9,Citation88–95]. Published reports have been very scarce, and the technique has not been widely used in these organs, as in the liver, kidney and lung, but the preliminary results were encouraging. By combining MWA with osteoplasty, technical success and pain relief were achieved in all 33 patients who suffered from extraspinal bone metastases and 11 patients with osteosarcoma invading the epiphysis [Citation88,Citation89]. Because MWA seemed to provide adequate local tumour control, it made it possible to salvage more native joints and bone. For breast cancer therapy, MWA has fewer published reports than RFA. The only two trials were from Zhou et al. [Citation9,Citation90] in China; they showed that 97.3% of tumours achieved complete coagulation with cancer up to 3.0 cm in the largest diameter in 41 patients and 100% technique success was achieved in 12 patients with cancer up to 2.0 cm in the largest diameter. By combining MWA with neoadjuvant chemotherapy, an alternative method was made available for small breast cancer patients without surgical options, but the long-term outcomes need to be determined in the future. Sun et al. and Qi et al.[Citation91,Citation8] also attempted to treat bladder cancer and abdominal wall metastases with MWA; they reported comparable results to surgery and thus provided another treatment option for these patients. Some preliminary MWA studies have been performed in prostate cancer, endometrial and vessel therapy [Citation92–95] which have laid the foundation for future clinical therapy.
Precision ablation in China
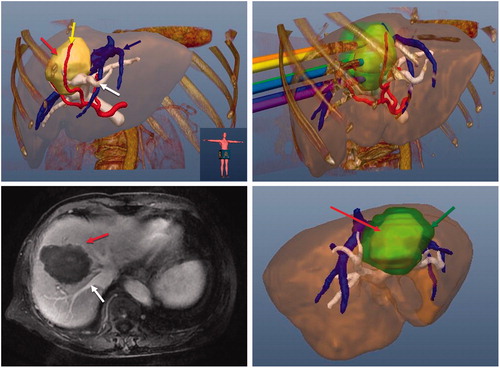
Precision ablation involves achieving complete and conformal necrosis of a tumour in a 3D space without injuring other adjacent tissues. Now that MWA is a mature technique in China, some experienced researchers have begun to pursue precision ablation in recent years. Liang’s group first tried to achieve precision MWA in liver tumours by using multimodal imaging fusion navigation and a computer-assisted 3D visualisation ablation planning system which was invented by their group [Citation96–99]. The development and clinical use of this ablation planning system are also leading research internationally. The 3D visualisation ablation planning system uses integrated electromagnetic tracking technology with guidance and planning software. The system has the function of loading the preprocedural images, performing offline tumour segmentation, creating an interactive puncture path planning, real time navigation during ablation procedure and ablation effect evaluation (). Pilot results showed that the system can achieve a higher success rate of first ablation and fewer sessions; further studies are ongoing and expected. Several other groups have performed studies on image fusion techniques for guiding ablation and assessing ablation margins in liver tumours [Citation100,Citation101]. In combination with advanced imaging techniques, tumour ablation will be established on a platform of precision, safety and efficiency.
Figure 1. Images of a 70-year-old female patient with a tumour at the border of the left and right lobe. (A) Preoperative 3D images visualised the spatial relationship of the tumour (red arrow) and the surrounding nutrient artery (yellow arrow), portal vein (blue arrow) and hepatic vein (white arrow) in multiple angles. (B) Ablation protocol was achieved through three-dimensional visualisation of the preoperative planning system, and six needles were needed to ablate the tumour completely. (C) The contrast-enhanced magnetic resonance imaging showed complete tumour necrosis (red arrow) one month after microwave ablation without hepatic vein (white arrow) injury. (D) Three-dimensional visualisation software system showed that the ablation zone (green arrow) covered the tumour (red arrow) completely one month after microwave ablation.
Technology and market share of MWA in China
Because many clinical uses of MWA have not been published yet, it is not accurate to illustrate the extent of the adoption of MWA in China based solely on published reports. Therefore, we performed a nationwide hospital survey and learned that MWA and RFA dominated 20% and 80% of the thermal ablation field for liver cancer 5–10 years ago, respectively. However, in the last 5 years, with the broad application of MWA techniques in multiple organs, MWA dominated approximately 50% of the thermal ablation field in liver, kidney, thyroid, lung and uterus lesions, and other ablation techniques dominated approximately 50% of ablation field. This may be related to the higher efficacy and shorter ablation time of MWA, which can provide a high efficiency to treat the large number of tumour patients in China.
When comparing MWA with other ablation techniques, the most powerful results are from the studies of HCC therapy. Most comparative studies have demonstrated that MWA can achieve similar results to RFA in the treatment of HCC. However, for the treatment of other tumours, there are no comparative studies to illustrate the differences between MWA and other techniques. According to our recent review papers on MWA therapy in the liver and kidney [Citation21,Citation102], MWA achieved the similar encouraging results in China compared with the results in other regions. For other types of tumours, however, there are less data to illustrate the regional differences.
Conclusion
MWA has proven to be a successful technology with the advantage of high thermal efficiency for the treatment of cancer. Many successful studies have been conducted in China, and more widespread use of MWA has been achieved in multiple types of cancer, particularly in liver cancer. The technical advantages of MWA have attracted more and more researchers from Europe and the USA to focus on it. However, it is still a relatively novel method, and its use is not yet comparable to that of surgery and RFA for treating tumours. With improvements in MWA equipment design and treatment strategies, the advantages of MWA should be recognised more widely because it has been proven to be effective. In conclusion, MWA is a promising method and will likely be used increasingly for the ablation of tumours in multiple organs.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Tabuse K, Katsumi M, Kobayashi Y, et al. (1985). Microwave surgery: hepatectomy using a microwave tissue coagulator. World J Surg 9:136–43.
- Saitsu H, Yoshida M, Taniwaki S, et al. (1991). Laparoscopic coagulo-necrotic therapy using microtase for small hepatocellular carcinoma. Jpn J Gastroenterol 88:2727.
- Yu J, Liang P, Yu XL, et al. (2012). US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263:900–08.
- Wang Y, Liang P, Yu X, et al. (2009). Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia 25:455–61.
- Yang X, Ye X, Zheng A, et al. (2014). Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 110:758–63.
- Feng B, Liang P, Cheng Z, et al. (2012). Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 166:1031–37.
- Yu J, Liang P, Yu X. (2011). Ultrasound-guided percutaneous microwave ablation of splenic metastasis: report of four cases and literature review. Int J Hyperthermia 27:517–22.
- Qi C, Yu XL, Liang P, et al. (2012). Ultrasound-guided microwave ablation for abdominal wall metastatic tumors: a preliminary study. World J Gastroenterol 18:3008–14.
- Zhou W, Jiang Y, Chen L, et al. (2014). Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol 83:1771–77.
- Hao Y, Zhang J, Han Z, et al. (2014). Follow-ups of mid-term and long-term outcomes for uterine intramural myomas after percutaneous microwave ablation therapy. Zhonghua Yi Xue Za Zhi 94:664–66.
- English NJ, Mac Elroy JM. (2003). Molecular dynamics simulations of microwave heating of water. J Chem Phys 118:1589–92.
- Diederich CJ. (2005). Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia 21:745–53.
- Bertram JM, Yang D, Converse MC, et al. (2006). A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit Rev Biomed Eng 34:187–213.
- Hoffmann R, Rempp H, Erhard L, et al. (2013). Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology 268:89–97.
- Dou JP, Liang P, Yu J. (2016). Microwave ablation for liver tumors. Abdom Radiol (NY) 41:650–58.
- Kitchin D, Lubner M, Ziemlewicz T, et al. (2014). Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 30:299–305.
- Wells SA, Wheeler KM, Mithqal A, et al. (2016). Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol (NY) 41:1203–11.
- Groeschl RT, Pilgrim CH, Hanna EM, et al. (2014). Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 259:1195–200.
- Livraghi T, Meloni F, Solbiati L, et al. Collaborative Italian Group using AMICA system. (2012). Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 35:868–74.
- Lloyd DM, Lau KN, Welsh F, et al. (2011). International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 13:579–85.
- Heck K, Happel C, Grünwald F, et al. (2015). Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia 31:560–67.
- Lu MD, Chen JW, Xie XY, et al. (2001). Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology 2001;221:167–72.
- Liang P, Dong BW, Yu XL, et al. (2005). Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 235:299–307.
- Ding J, Jing X, Liu J, et al. (2013). Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol 68:608–15.
- Ding J, Jing X, Liu J, et al. (2013). Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 82:1379–84.
- Qian GJ, Wang N, Shen Q, et al. (2012). Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 22:1983–90.
- Wang ZL, Liang P, Dong BW, et al. (2008). Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg 12:327–37.
- Shi J, Sun Q, Wang Y, et al. (2014). Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol 29:1500–7.
- Liang P, Dong BW, Yu XL, et al. (2006). Evaluation of long-term therapeutic effects of ultrasound-guided percutaneous microwave ablation of liver metastases. Zhonghua Yi Xue Za Zhi 86:806–10.
- Li M, Yu X, Liang P, et al. (2015). Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia 31:579–87.
- Huang H, Liang P, Yu XL, et al. (2015). Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia; 31:40–7.
- Zhang M, Liang P, Cheng ZG, et al. (2014). Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 30:134–41.
- Zhang D, Xie D, Wei X, et al. (2014). Microwave ablation of the liver abutting the stomach: insulating effect of a chitosan-based thermosensitive hydrogel. Int J Hyperthermia 30:126–33.
- Huang S, Yu J, Liang P, et al. (2014). Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol 83:552–58.
- Zhang D, Liang P, Yu X, et al. (2013). The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia 29:663–70.
- Li M, Yu XL, Liang P, et al. (2012). Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 28:218–26.
- Ren H, Liang P, Yu X, et al. (2011). Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: a pilot study. Int J Hyperthermia 27:249–54.
- Liu FY, Yu XL, Liang P, et al. (2010). Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 26:448–55.
- Ni JY, Sun HL, Chen YT, et al. (2014). Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol 20:17483–490.
- Yu J, Liang P, Yu XL, et al. (2012). Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 81:2495–99.
- Wang XH, Yu J, Liang P, et al. (2012). Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: analysis of major complications in 693 patients. Zhonghua Zhong Liu Za Zhi 34:945–99.
- Liang P, Wang Y, Yu X, et al. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology 251:933–40.
- Dong BW, Liang P, Yu XL, et al. (2006). Long-term results of percutaneous sonographically-guided microwave ablation therapy of early-stage hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 86:797–800.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna — experimental and clinical studies. Radiology 242:914–24.
- Jiao D, Qian L, Zhang Y, et al. (2010). Microwave ablation treatment of liver cancer with 2,450-MHz cooled-shaft antenna: an experimental and clinical study. J Cancer Res Clin Oncol 136:1507–16.
- Liu Y, Zheng Y, Li S, et al. (2013). Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol 68:21–26.
- Wang J, Liang P, Yu J, et al. (2014). Clinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastases. Oncol Lett 8:323–26.
- Zhang TT, Luo HC, Cui X, et al. (2015). Ultrasound-guided percutaneous microwave ablation treatment of initial recurrent hepatocellular carcinoma after hepatic resection: long-term outcomes. Ultrasound Med Biol 41:2391–99.
- Zhang NN, Lu W, Cheng XJ, et al. (2015). High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol 70:1237–43.
- Sun AX, Cheng ZL, Wu PP, et al. (2013). Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol 19:5430–38.
- Yu J, Liang P, Yu XL, et al. (2015). Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol 25:1119–26.
- Liang P, Yu J, Yu XL, et al. (2012). Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naïve lesions in 1007 patients in China. Gut 61:1100–1.
- Tang XY, Wang Z, Wang T, et al. (2015). Efficacy, safety and feasibility of ultrasound-guided percutaneous microwave ablation for large hepatic hemangioma. J Dig Dis 16:525–30.
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90.
- Tanagho YS, Roytman TM, Bhayani SB, Kim EH, et al. (2012). Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology 80:307–14.
- Estebanez Zarranz J, Artozki Morras E, Aguirreazaldegui García L, et al. (2009). Radiofrequency ablation of renal cell carcinoma. Actas Urol Esp 33:514–21.
- Zhang D, Dong B, Wang Y, et al. (2009). Percutaneous microwave ablation or nephrectomy for VX-2 carcinoma in rabbit kidney. J Urol 182:1588–93.
- Liang P, Wang Y, Zhang D, et al. (2008). Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 180:844–8. discussion 848.
- Bai J, Hu Z, Guan W, et al. (2010). Initial experience with retroperitoneoscopic microwave ablation of clinical T(1a) renal tumors. J Endourol 24:2017–22.
- Guan W, Bai J, Hu Z, et al. (2010). Retroperitoneoscopic microwave ablation of renal hamartoma: middle-term results. J Huazhong Univ Sci Technol Med Sci 30:669–71.
- Guan W, Bai J, Liu J, et al. (2012). Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol 106:316–21.
- Yu J, Liang P, Yu XL, et al. (2014). US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 270:880–7.
- Lin Y, Liang P, Yu XL, et al. (2014). Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology 83:357–63.
- Han ZY, Liang P, Yu XL, et al. (2015). Ultrasound-guided percutaneous microwave ablation of sporadic renal angiomyolipoma: preliminary results. Acta Radiol 56:56–62.
- Yu J, Zhang G, Liang P, et al. (2015). Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging 40:3248–56.
- Feng W, Liu W, Li C, et al. (2002). Percutaneous microwave coagulation therapy for lung cancer. Zhonghua Zhong Liu Za Zhi 24:388–90.
- He W, Hu XD, Wu DF, et al. (2006). Ultrasonography-guided percutaneous microwave ablation of peripheral lung cancer. Clin Imaging 30:234–41.
- Lu Q, Cao W, Huang L, et al. (2012). CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol 10:80.
- Wei Z, Ye X, Yang X, et al. (2015). Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol 38:135–42.
- Zheng A, Wang X, Yang X, et al. (2014). Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 98:243–8.
- Wei Z, Ye X, Yang X, et al. (2015). Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol 32:464.
- Sun YH, Song PY, Guo Y, et al. (2015). Computed tomography-guided percutaneous microwave ablation therapy for lung cancer. Genet Mol Res 14:4858–64.
- Yue W, Wang S, Wang B, et al. (2013)Ultrasound guided percutaneous microwave ablation of benign thyroid nodules safety and imaging follow-up in 222 patients. Eur J Radiol 82:e11–16.
- Yue W, Wang S, Yu S, et al. (2014). Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 30:150–7.
- Yue W, Chen L, Wang S, et al. (2015). Locoregional control of recurrent papillary thyroid carcinoma by ultrasound-guided percutaneous microwave ablation: a prospective study. Int J Hyperthermia 31:403–8.
- Witte MH, Witte CL, Van Wyck DB, et al. (1983). Preservation of the spleen. Lymphology 16:128–37.
- Liang P, Gao Y, Zhang H, et al. (2011). Microwave ablation in the spleen for treatment of secondary hypersplenism: a preliminary study. AJR Am J Roentgenol 196:692–6.
- Duan YQ, Gao YY, Ni XX, et al. (2007). Changes in peripheral lymphocyte subsets in patients after partial microwave ablation of the spleen for secondary splenomegaly and hypersplenism: a preliminary study. Int J Hyperthermia 23:467–72.
- Jiang X, Gao F, Ma Y, et al. (2016). Percutaneous microwave ablation in the spleen for treatment of hypersplenism in cirrhosis patients. Dig Dis Sci 61:287–92.
- Yu J, Liang P, Yu X, et al. (2015). Clinical evaluation of ultrasound-guided percutaneous microwave ablation of splenic tumors. Nan Fang Yi Ke Da Xue Xue Bao 35:333–7.
- Xiangyun C, Qidong L. (1997). Microwave in therapy of submucous myoma of uterus into vagina. Chin J Obstet and Gynecol 32:30.
- Zhang J, Dong B, Feng L, et al. (2008). Ultrasound-guided percutaneous microwave ablation for management of uterine fibroid-a case report. Chin J Ultrasongry 17:326.
- Zhang J, Feng L, Zhang B, et al. (2011). Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment – a clinical study. Int J Hyperthermia 27:311–13.
- Zhang J, Han ZY, Feng L, et al. (2011). Ultrasound-guided percutaneous microwave ablation in the treatment of diffuse adenomyosis. Zhonghua Yi Xue Za Zhi 91:2749–52.
- Yang Y, Zhang J, Han ZY, et al. (2015). Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep 5:10034
- Li X, Fan W, Zhang L, et al. (2011). CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer 117:5182–8.
- Ren C, Liang P, Yu XL, et al. (2016). Percutaneous microwave ablation of adrenal tumours under ultrasound guidance in 33 patients with 35 tumours: a single-centre experience. Int J Hyperthermia May 5:1–7.
- Wei Z, Zhang K, Ye X, et al. (2015). Computed tomography-guided percutaneous microwave ablation combined with osteoplasty for palliative treatment of painful extraspinal bone metastases from lung cancer. Skeletal Radiol 44:1485–90.
- Li J, Guo Z, Wang Z, et al. (2015). Does microwave ablation of the tumor edge allow for joint-sparing surgery in patients with osteosarcoma of the proximal tibia? Clin Orthop Relat Res 473:3204–11.
- Zhou W, Zha X, Liu X, et al. (2012). US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 263:364–73.
- Sun Y, Xu C, Qian S, et al. (2001). Transurethral microwave needle ablation for bladder cancer. Chin Med J (Engl) 114:546–7.
- Li ZC, Zhang J, Hu DM, et al. (2011). Ultrasound-guided transrectal microwave ablation of the prostate in dogs. Zhonghua Nan Ke Xue 17:813–16.
- Zhang G, Dong L, Tai Y, et al. (2014). Contrast-enhanced sonographically guided percutaneous 915-MHz microwave ablation therapy compared to local hemostatic drug injection in a renal artery injury model. J Ultrasound Med 33:611–21.
- Yang L, Wang XP, Su WJ, et al.. (2013). Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg 46:473–9.
- Zhu Y, Yang J. (2013). Microwave endometrial ablation for endometrial protection in women with breast cancer on adjuvant tamoxifen. J Obstet Gynaecol Res 39:1411–14.
- Liu F, Yu X, Liang P, et al. (2011). Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia 27:555–62.
- Liu FY, Yu XL, Liang P, et al. (2012). Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol 81:1455–9.
- Yu X, Liu F, Liang P, et al. (2011). Microwave ablation assisted by a computerised tomography-ultrasonography fusion imaging system for liver lesions: an ex vivo experimental study. Int J Hyperthermia 27:172–9.
- Liu F, Liang P, Yu X, et al. (2013). A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: a preliminary clinical application. Int J Hyperthermia 29:671–7.
- Xu HX, Yin XY, Lu MD, et al. (2003). Usefulness of three-dimensional sonography in procedures of ablation for liver cancers: initial experience. J Ultrasound Med 22:1239–47.
- Yuan CH, Xiu DR, Ge HY, et al. (2013). Ultrasound guided ablation therapy of hepatic colorectal metastases: initial experience of real time virtual sonography navigation system. Beijing Da Xue Xue Bao 45:956–9.
- Feng B, Liang P. (2012). Local thermal ablation of renal cell carcinoma. Eur J Radiol 81:437–40.

